Abstract
Diabetes affects American Indians disproportionately compared with other racial/ethnic groups in the United States and is almost exclusively type 2 diabetes. Much of our knowledge about diabetes in American Indians comes from studies in a few tribes. The most extensively studied American Indians are the Pima Indians from the Gila River Indian Community in Arizona, who participated in a longitudinal study of diabetes and its complications between 1965 and 2007. They have one of the highest reported incidence and prevalence of type 2 diabetes in the world, and kidney disease attributable to diabetes is a major cause of morbidity and mortality. In this article, we examine the course, determinants, and trends of diabetic kidney disease in American Indians, with special emphasis on studies conducted in the Pima Indians. We also review therapeutic strategies for managing diabetic kidney disease.
Introduction
American Indians are a diverse group of over 3 million people from 561 federally recognized tribes in the United States. They often live on reservations and in rural communities, mostly in the western United States and in Alaska. In general, American Indians face widespread economic, educational, and social disadvantage.
Diabetes affects American Indians disproportionately compared with other racial/ethnic populations and is almost exclusively type 2 diabetes. Because diabetes often develops at a younger age in American Indians than in other populations [1•], the major complications of diabetes also appear at a younger age, further exacerbating socioeconomic disparities and leading to increased levels of disability and reduced longevity. Most of the information about the impact of diabetes in American Indian communities comes from longitudinal studies in the Pima Indians who live in the Gila River Indian Community in south central Arizona. Systematic testing for diabetes and its complications in the Pima Indians over the past 43 years permits the onset and duration of type 2 diabetes and diabetic kidney disease to be known with greater certainty than in other populations. In this article, we examine the course and determinants of diabetic kidney disease in American Indians, with a special emphasis on findings in the Pima Indians.
Clinical Course
Albuminuria
The earliest indicator of diabetic nephropathy (DN) is the appearance of elevated urinary albumin excretion, which is divided arbitrarily into microalbuminuria (albumin-to-creatinine ratio [ACR] between 30 and 300 mg/g or an albumin excretion rate of 30–300 mg/24 hours) and macro-albuminuria (ACR ≥ 300 mg/g or an albumin excretion rate of ≥ 300 mg/24 hours). Microalbuminuria is characterized by stable kidney function and a greater risk for developing progressive DN than ACR less than 30 mg/g. Elevated albuminuria is also an established marker of more generalized endothelial cell dysfunction associated with fatal and nonfatal cardiovascular events independently of other cardiovascular risk factors [2]. Although the mechanism of this association remains largely unknown, elevated albuminuria is independently associated with left ventricular systolic and diastolic dysfunction in American Indians with type 2 diabetes [3], suggesting that the vascular changes that lead to kidney disease may also be present in the systemic and coronary vasculature.
In diabetic American Indians 45 to 74 years old from Arizona, Oklahoma, the Dakotas [4], or northern Minnesota [5], the prevalence of microalbuminuria and macroalbuminuria varies between 35% and 65%. Eighteen percent develop elevated albuminuria (ACR ≥ 30 mg/g) within 4 years of diabetes onset [6]. Higher American Indian heritage (ie, low genetic admixture with other populations), age, duration of diabetes, and fasting plasma glucose concentrations are positively associated with albuminuria in these tribes. The prevalence of elevated albuminuria among Zuni Indians is higher than among American Indians from Oklahoma and the Dakotas but lower than in those from Arizona [7]. In contrast with diabetic Pima Indians, in whom intercapillary glomerulosclerosis is by far the predominant form of kidney disease, in some tribes, such as the Navajo and Zuni Indians, diabetic individuals have a high frequency of hematuria, reflecting the high frequency of diabetes and its common association with nondiabetic pathologies that occur with greater frequency in these tribes (ie, IgA-positive mesangiopathic glomerulonephritis, focal sclerosing glomerulonephritis, and membranous glomerulopathy) [7].
Early kidney disease is particularly common in the Pima, Maricopa, and Tohono O’odham Indians of central Arizona, and its prevalence increases with increasing duration of diabetes. Elevated albumin excretion, defined by a urine ACR ≥ 30 mg/g, was found in 8% of Pima Indians with normal glucose tolerance, in 15% with impaired glucose tolerance, and in 47% of those with diabetes [8]. Similarly, in the Inter-Tribal Heart Project, which includes American Indians residing on the Menominee reservation in central Wisconsin and the Red Lake and White Earth Chippewa reservations in Minnesota, impaired fasting glucose and hypertension were associated with a twofold higher prevalence of microalbuminuria than among individuals without these traits [9]. The presence of abnormal albuminuria in those with impaired glucose regulation suggests that in some individuals hyperglycemia is associated with kidney abnormalities even at levels below those diagnostic of diabetes.
Once macroalbuminuria develops, progression to kidney failure is common in diabetic Pima Indians; half of those with macroalbuminuria progress to end-stage kidney disease (ESKD) within 10 years. Whereas Caucasians with diabetes and proteinuria tend to die of cardiovascular disease (CVD) before they develop kidney failure [10], Pima Indians often progress to kidney failure before dying from CVD [11]. Pima Indians are also on average younger at onset of diabetes and less likely to have fatal CVD than Caucasians. Thus, the lower rate of kidney failure in Caucasians than in Pima Indians with type 2 diabetes is due in part to CVD morbidity and mortality outweighing the risk for kidney disease progression. A study in the Veterans Administration health care system confirmed that American Indians are less likely to have CVD, hypertension, stroke, depression, and chronic obstructive pulmonary disease, and more likely to have early DN (adjusted OR, 1.5; 95% CI, 1.1–2.1) and diabetic ESKD (adjusted OR, 1.9; 95% CI, 1.5–2.3) than Caucasians [12].
Glomerular hemodynamics
Changes in kidney function during the DN course have been studied extensively in the Pima Indians, in whom the onset and duration of diabetes are known with greater certainty than in other populations with type 2 diabetes, and in whom nearly all kidney disease is attributable to diabetes. During a 4-year follow-up, the glomerular filtration rate (GFR) increased on average by 14% in individuals with impaired glucose tolerance, and continued to increase after onset of diabetes, reaching a plateau in individuals with microalbuminuria. GFR remained high but stable in diabetic individuals with normoalbuminuria or microalbuminuria, even with longstanding diabetes [13]. Higher GFR is also described in Caucasians with type 1 diabetes, particularly in the presence of uncontrolled hyperglycemia, but elevated GFR is less well documented in Caucasians with type 2 diabetes, possibly because of their older age and associated renovascular disease. Insulin treatment significantly depresses elevated GFR in both types of diabetes, suggesting that some of the early hemodynamic changes may be related to the level of glycemic control.
Hyperfiltration declines progressively in Pima Indians after onset of macroalbuminuria. The rate of GFR loss in the Pima Indians is 13.8 mL/min/1.73 m2 per year compared with 3 to 5.3 mL/min/1.73 m2 per year in European populations with type 2 diabetes [14]. Given that Pima Indians have larger glomeruli than Caucasians, a greater filtration surface area is lost for each glomerulus that is lost to fibrosis, leading to a more rapid GFR decline [14]. Higher baseline albuminuria predicts the change in GFR, whereas baseline GFR in those with normoalbuminuria or microalbuminuria does not predict higher albumin excretion, suggesting that enhanced transglomerular passage of protein, and not hyperfiltration, progressively injures the glomeruli and determines DN progression [13]. Enhanced transglomerular trafficking of protein may contribute to a progressive decline in GFR by stimulating glomerular cells to produce extracellular matrix, which eventually obliterates the glomerular capillaries [13]. Alternatively, exposure to high concentrations of proteins may induce the production of proinflammatory and profibrotic factors by tubular cells, leading to tubular atrophy and interstitial fibrosis [15]. Perhaps both mechanisms are involved in progressive glomerular injury.
Glomerular structure
Podocyte injury appears to play an essential role in the development and progression of DN. With glomerular hypertrophy that occurs with the onset of diabetes, the podocytes, which have a limited to absent proliferative potential [16], extend their foot processes to maintain coverage of the expanded glomerular basement membrane, a compensatory mechanism believed to influence their functional integrity [17]. Sustained mechanical stress, increased passage of plasma proteins across the glomerular filtration barrier, and glomerular hypertension may ultimately lead to podocyte detachment. In Pima Indians, microalbuminuria is associated with 20% and macroalbuminuria with 40% podocyte loss relative to normal albuminuria [17]. Moreover, microalbuminuric individuals followed up for 4 years had a 35% decline in the number of podocytes per glomerulus, and half of them progressed to macroalbuminuria during follow-up [18]. Because lost podocytes are not replaced, significant damage to the podocytes is a potential starting point for irreversible glomerular injury.
Risk Factors
In addition to elevated albuminuria and changes in hemodynamic function, several other risk factors contribute to the development and progression of DN. These factors include, but are not limited to, hyperglycemia, hypertension, diabetes duration, various intrauterine exposures, and smoking. The greater burden of chronic kidney disease among American Indians than in other populations may reflect the higher frequency of these risk factors. Strong familial aggregation of DN suggests that genetic factors may play an important etiologic role and may also be involved in the ethnic disparities in kidney disease frequency. Several of the risk factors are particularly relevant to American Indians.
Genetics
Heredity is a major determinant of DN. Among Pima Indians, proteinuria occurred in 14% of the diabetic offspring of diabetic parents if neither parent had proteinuria, in 23% if one parent had proteinuria, and in 46% if both parents had proteinuria. Segregation analysis in this population was consistent with a major genetic effect on DN prevalence after accounting for duration of diabetes, suggesting that familial aggregation of DN may be explained largely by the action of a single or a few genes [19]. In addition, DN in parents is a risk factor for diabetes in the offspring, implying that parents with diabetes and DN may have greater genetic susceptibility that increases the risk of diabetes in the offspring and the risk of nephropathy once diabetes has developed [20]. Combining high-density single nucleotide polymorphism microarrays with a pooled genomic DNA design in Pima Indians with diabetes provided the first evidence supporting a potential role for variants in the PVT1 gene in susceptibility to ESKD [21•]. Although Pima Indians are not represented in the HapMap, surveys of linkage disequilibrium in American Indian populations suggest that they are similar to other non-Africans in this respect [22]. Replication of these results in other populations will help to clarify the role of PVT1 variants in the development of ESKD in diabetic patients.
The FIND, the largest DN genetic study to date, collected DNA and cell lines from European American, African American, American Indian, and Hispanic American families with ESKD or advanced DN. For all ethnicities combined, the strongest evidence for linkage to DN was on chromosomes 7q21.3, 10p5.3, 14q23.1, and 18q22.3, to ACR was on chromosomes 2q14.1, 7q21.1, and 15q26.3, and to GFR was on chromosomes 1q43, 7q36.1, and 8q13.3. Additionally, the evidence for linkage was different among the different ethnic groups, suggesting that the relative importance of these loci may differ across ethnic groups. [23,24].
Intrauterine factors
Exposure to a diabetic intrauterine environment is a strong risk factor for kidney disease, perhaps as a consequence of damage to the developing nephrons [25]. In Pima Indians, who have a higher frequency of such exposure than in other populations, offspring of diabetic mothers had nearly four times the odds of elevated albuminuria later in life than the offspring of nondiabetic or prediabetic mothers. This association persisted even when adjusted for the effects of parental hypertension and proteinuria. Evidence of increased glomerular and tubular apoptosis in the offspring of diabetic mice may provide an explanation for the reduced nephron mass postulated in the Pima Indians [26]. Tight glycemic control from mid to late gestational periods may reduce the adverse effects of maternal diabetes on kidney development [27].
A reduction in maternal fuels manifested by low birth weight is also associated with an increased DN risk, presumably by reducing nephron formation [28]. Increasing evidence exists that individuals with reduced nephron endowment are prone to develop hypertension, kidney failure, and CVD later in life.
Hyperglycemia
Hyperglycemia is a strong risk factor for the occurrence and progression of microalbuminuria, but has a lesser impact on progression at more advanced stages of kidney disease, at which hypertension, hypercholesterolemia, and genetic factors play a greater role in shaping the outcome. In Pima Indians, 2-hour plasma glucose concentration, fasting plasma glucose concentration, and hemoglobin A1c each predict elevated albuminuria, defined as an ACR ≥ 30 mg/g, after adjusting for age, sex, and duration of diabetes [29].
Blood pressure
Hypertension is one of the most common comorbidities in patients with diabetes. The onset of hypertension and macrovascular disease generally precedes DN in type 2 diabetes and is often associated with obesity. A family history of hypertension increases the risk for developing DN. In Pima Indians with type 2 diabetes, the prevalence of proteinuria was similar if neither parent or only one parent had hypertension (8.9% and 9.4%, respectively), but was significantly higher if both parents had hypertension (18.8%). When both parents had hypertension, the odds for proteinuria in the offspring were two times that if only one parent had hypertension. This association remained even when controlled for age, sex, duration of diabetes, 2-hour postload plasma glucose concentration, mean arterial pressure, and its treatment in the offspring and for diabetes in the parents [30]. In addition, higher blood pressure before the onset of type 2 diabetes is related to a higher prevalence of elevated albuminuria after the onset of diabetes, suggesting that blood pressure plays a causal role in DN development [31].
Obesity
Obesity is a major risk factor for diabetes, hypertension, and CVD, which in turn increase the risk for DN. The increasing prevalence of obesity in Pima Indian youth combined with a nearly fourfold increase in the frequency of exposure to diabetes in utero has shifted the onset of diabetes to younger ages [32]. Even though the proportion of youth developing diabetes among the Pima Indians and in the general US population is small, the current epidemic of obesity in this age group is already associated with an increasing incidence of diabetes in childhood and adolescence [33].
Diabetes onset during youth leads to substantially increased ESKD rates in midlife [34•]. Duration-specific incidence rates of proteinuria were similar in Pima Indians with diabetes onset before and after 20 years of age. By contrast, the frequency of retinopathy was lower in youth-than adult-onset type 2 diabetes, suggesting that youth does not offer the same protection from progressive DN as it does from diabetic retinopathy [35]. The age-sex–adjusted incidence of diabetic ESKD in Pima Indians diagnosed with type 2 diabetes before 20 years of age was nearly five times as high as in those of the same age but with older-onset diabetes. The longer duration of diabetes by middle age in those diagnosed during childhood or adolescence largely accounted for the higher ESKD incidence (Fig. 1). Although susceptibility to DN differs by ethnicity, the rise in childhood type 2 diabetes is likely to increase the frequency of kidney disease. Furthermore, the higher DN rates in young and middle-aged adults with youth-onset diabetes may contribute to a rise in cardiovascular complications in these age groups. These complications have a significant economic and public health impact because they will affect those with youth-onset diabetes at the height of their productive years.
Figure 1.
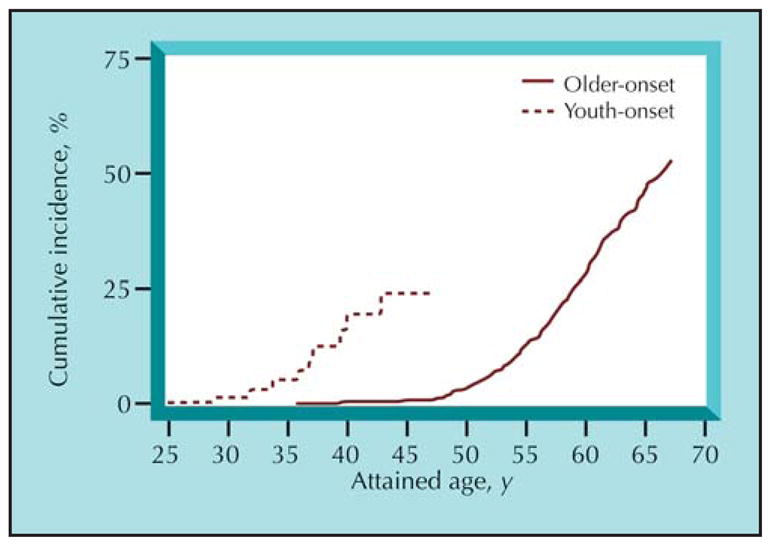
Cumulative incidence of diabetic end-stage kidney disease (ESKD) in Pima Indians with type 2 diabetes. At any attained age, participants with youth-onset type 2 diabetes, defi ned as onset of diabetes before 20 years of age, have a longer duration of diabetes, and thus a higher risk of diabetic ESKD than those with older-onset diabetes mellitus. (Adapted from Pavkov et al. [34•].)
Periodontal disease
Periodontal disease, a chronic infection of the tissue surrounding the teeth, is a frequent complication of diabetes and is associated with systemic elevation of inflammatory biomarkers and endothelial dysfunction. Severity of periodontitis and being edentulous predict macroalbuminuria and ESKD in a dose-dependent manner among diabetic Pima Indian adults [36]. Although experimental and epidemiologic studies suggest a link between periodontal disease and chronic kidney disease, the mechanism by which periodontitis impacts the kidneys remains to be elucidated. Moreover, the effect of periodontal disease treatment in reducing the risk of kidney disease in persons with diabetes is not known.
Trends in DN
Recent data found that American Indians were the only group in the United States with a significant decline in age-adjusted diabetic ESKD incidence despite their apparent increasing diabetes prevalence [37]. Efficacious treatments and practices to reduce risk factors for diabetic ESKD may have contributed to the decline [38]. Other ethnic groups experienced a slower increase or a plateau in the incidence of diabetic ESKD. Perhaps differences in access to care or physicians’ awareness of increased risk in minority populations may, in part, explain this difference. Similarly, the decline in the age-sex–adjusted incidence of ESKD among diabetic Pima Indians coincided with the introduction and widespread use within this community of new hypoglycemic medicines and medicines that block the renin-angiotensin system (RAS) [39]. The decline in diabetic ESKD incidence in the Pima Indians occurred primarily in those ≥ 45 years old. The lack of decline in ESKD in younger diabetic Pima Indians may be due to less aggressive management of DN in younger patients or lower death rates from competing causes of death, such as CVD, or both. Conversely, despite improvements in blood pressure, glycemic control, and cholesterol control, the age-sex–adjusted incidence of proteinuria increased from 24.3 cases/1000 person-years in 1967 to 1978 to 38.9 cases/1000 person-years in 1991 to 2002. The incidence of proteinuria remained largely unchanged, however, when those of similar duration of diabetes were compared, indicating that the incidence increased because of an increasing average duration of diabetes in the population [39]. Thus, newer DN treatments may slow its progression but not its onset.
A substantial proportion of persons with diabetes and microalbuminuria spontaneously regress to normoalbuminuria, implying that microalbuminuria may represent an initial phase of dynamic and reversible kidney injury rather than the onset of an inevitable progression to ESKD. The proportion of diabetic Pima Indians who regressed from microalbuminuria to normoalbuminuria during a median follow-up of 2.4 years was 24%, whereas 19% progressed to macroalbuminuria [40]. With more advanced kidney disease, fewer persons regressed; most individuals with macroalbuminuria remained in this category (85%) at the second ACR measurement, whereas 15% regressed to microalbuminuria or normoalbuminuria [40]. Nevertheless, although past measurements predicted progression to ESKD, they did not add to the predictive power when the current measurement was considered. A low current ACR value is associated with good prognosis, regardless of whether earlier values were higher, the same, or lower [40].
Treatment
Studies in the Pima Indians offer insight into the course and determinants of diabetic kidney disease, but much less is known about the efficacy and safety of various treatments for kidney disease in Pima Indians and in other American Indian populations, as they are often not included in clinical trials. In this section, we review the management of diabetic kidney disease based on studies conducted in other racial and ethnic groups.
Glycemic control
The UKPDS [41] demonstrated that improved glycemic control significantly reduces the risk of microvascular complications proportional to the level of control. Intensive treatment reduced the risk of albuminuria by 35% in patients with newly diagnosed type 2 diabetes, and was accompanied by a 67% reduction in the proportion of patients who had a doubling in plasma creatinine, suggesting that treatment of hyperglycemia may slow progression of established kidney disease.
Thiazolidinediones appear to reduce microalbuminuria and slow the progression of DN [42]. This reduction in albuminuria was associated with improved metabolic control and decreased circulating adipocytokine levels (tumor necrosis factor-α, adiponectin, and free fatty acids). However, long-term studies in a large patient population are necessary to determine thiazolidinediones’ benefit in preventing DN.
ADVANCE, the world’s largest diabetes randomized clinical trial yet reported, enrolling 11,140 patients with type 2 diabetes, confirmed that achieving a mean hemoglobin A1c of 6.5% while not affecting the risk for CVD, significantly reduces the risk of nephropathy [43]. Intensive treatment most significantly reduced the development of macroalbuminuria, with no effect on the doubling of serum creatinine level, as reflected by only a slight trend toward a reduction in kidney failure or death from kidney disease.
Hypoglycemia is a major concern for persons with decreased kidney function (chronic kidney disease stages 3–5) because of impaired kidney clearance of insulin and some of the oral hypoglycemic agents. In addition, gluconeogenesis decreases with reduced kidney mass, potentially increasing the likelihood of hypoglycemia. Intensive glycemic control, although not preventing further deterioration in kidney function in these patients, may still prevent or slow the progression of retinopathy, neuropathy, and macrovascular disease and therefore improve survival.
Blood pressure control
RAS inhibitors slow the increase in albumin excretion and delay the progression from microalbuminuria to macroalbuminuria, thus reducing the incidence of albuminuria and ESKD. These effects are secondary to blood pressure lowering and may also reflect blood pressure–independent effects on the kidney [44]. By acting at the receptor level, angiotensin receptor blockers (ARBs) provide more complete blockade of RAS than angiotensin-converting enzyme (ACE) inhibitors, without potentiating bradykinins, which are thought to mediate the ACE inhibitor–induced cough. The superiority of RAS inhibitors over other antihypertensive agents is less well established in the primary prevention of kidney disease in patients with type 2 diabetes [45]. A study of kidney biopsies in normotensive, normoalbuminuric type 1 diabetic patients with normal GFR found no changes in glomerular mesangial fractional volume or other structural characteristics and a similar progression of DN with RAS inhibitors compared with placebo [46]. Although RAS inhibitors reduced progression of retinopathy, the lack of benefit on kidney structural outcomes suggests that they may not be similarly protective early in kidney disease, or that a much longer treatment is required to attain measurable effects.
In patients with type 2 diabetes, normal kidney function, and hypertension, ACE inhibitors decrease the incidence rate of microalbuminuria relative to nondihydropyridine calcium channel blockers, suggesting that ACE inhibitors are the medication of choice for controlling blood pressure in these patients [47].
In patients with type 2 diabetes and more advanced kidney disease (ie, macroalbuminuria and reduced GFR), ARBs are more effective than other antihypertensive classes in reducing albuminuria and slowing progression of kidney disease [48]. Although similar studies are not available for ACE inhibitors in persons with type 2 diabetes, ARBs appeared similar to ACE inhibitors in their ability to reduce proteinuria [49]. The largest comparative noninferiority trial to date showed no additional advantage from the combination of full doses of ARB and ACE inhibitors compared with ACE inhibitors alone [50]. Moreover, the combination regimen significantly increased the risk of renal dysfunction requiring dialysis. When RAS inhibitors are used for extended periods of time, a reactive increase in plasma renin occurs, which limits the benefit of RAS inhibitors on blood pressure and albuminuria. The first trial of a direct inhibitor of renin found additional benefit in reducing albuminuria; whether this additional blockade of RAS is superior to currently available agents in terms of preventing the decline in kidney function and CVD outcomes is unknown [51].
Other interventions to reduce albuminuria include reducing dietary protein intake, cigarette smoking cessation, and weight loss. Intensive multifactorial interventions, such as the approach described in the Steno-2 study, should be the goal of therapy because growing evidence exists for the long-term sustained benefits on microvascular and CVD outcomes of this approach [52].
Conclusions
Over the past 43 years, the overall incidence of early DN increased in Pima Indians, largely in response to the increasing duration of diabetes. The widespread use of newer medicines to control blood pressure, reduce hyperglycemia, hyperlipidemia, and block the RAS has led to improvements in these risk factors among diabetic Pima Indians and a slower progression to ESKD. More recently, the same trends were observed in the overall American Indian population. However, a continued increase in the incidence of type 2 diabetes in youth threatens to reverse this trend. Given the extraordinary rate of diabetic kidney disease in American Indians, future clinical trials of potential therapies should include these populations to ensure that the efficacy of these therapies are assessed directly in the populations that need them the most.
Acknowledgments
We thank the thousands of volunteers from the Gila River Indian Community for participating in many of the studies described here.
Clinical Trial Acronyms
- ADVANCE
Action in Diabetes and Vascular disease: Preterax and Diamicron MR Controlled Evaluation
- FIND
Family Investigation of Nephropathy and Diabetes
- UKPDS
United Kingdom Prospective Diabetes Study
Footnotes
Disclaimer
The findings and conclusions in this presentation have not been formally disseminated by the US Centers for Disease Control and Prevention and should not be construed to represent any agency determination or policy.
Disclosures
This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. No other potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1•.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. The data are an update of diabetes prevalence estimates last reported 2 years ago and now published in the 2007 National Diabetes Fact Sheet. It shows an increase in diabetes prevalence to nearly 8% of the US population. [Google Scholar]
- 2.Xu J, Knowler WC, Devereux RB, et al. Albuminuria within the “normal” range and risk of cardiovascular disease and death in American Indians: the Strong Heart Study. Am J Kidney Dis. 2007;49:208–216. doi: 10.1053/j.ajkd.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Bella JN, Roman MJ, Fabsitz R, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes. The Strong Heart Study. J Am Coll Cardiol. 2003;41:2022–2028. doi: 10.1016/s0735-1097(03)00403-0. [DOI] [PubMed] [Google Scholar]
- 4.Robbins DC, Knowler WC, Lee ET, et al. Regional differences in albuminuria among American Indians: an epidemic of renal disease. Kidney Int. 1996;49:557–563. doi: 10.1038/ki.1996.79. [DOI] [PubMed] [Google Scholar]
- 5.Hirata-Dulas CA, Rith-Najarian SJ, McIntyre MC, et al. Risk factors for nephropathy and cardiovascular disease in diabetic Northern Minnesota American Indians. Clin Nephrol. 1996;46:92–98. [PubMed] [Google Scholar]
- 6.Sosenko JM, Dongsheng H, Welty T, et al. Albuminuria in recent-onset type 2 diabetes. The Strong Heart Study. Diabetes Care. 2002;51:1078–1084. doi: 10.2337/diacare.25.6.1078. [DOI] [PubMed] [Google Scholar]
- 7.Scavini M, Shah VO, Stidley CA, et al. Kidney disease among the Zuni Indians: the Zuni Kidney Project. Kidney Int Suppl. 2005;68:S126–S131. doi: 10.1111/j.1523-1755.2005.09721.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson RG, Kunzelman CL, Pettitt DJ, et al. Albuminuria in type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in Pima Indians. Diabetologia. 1989;32:870–876. doi: 10.1007/BF00297452. [DOI] [PubMed] [Google Scholar]
- 9.Hoehner CM, Greenlund KJ, Rith-Najarian S, et al. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic Native Americans. The Inter-Tribal Heart Project. J Am Soc Nephrol. 2002;13:1626–1634. doi: 10.1097/01.asn.0000015762.92814.85. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Renal Data System. USRDS 2007 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 11.Pavkov ME, Bennett PH, Sievers ML, et al. Predominant effect of kidney disease on mortality in Pima Indians with or without type 2 diabetes. Kidney Int. 2005;68:1267–1274. doi: 10.1111/j.1523-1755.2005.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of Veterans. Diabetes Care. 2003;26:2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335:1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 14.Lemley KV. A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int. 2003;63(Suppl 83):S38–S42. doi: 10.1046/j.1523-1755.63.s83.9.x. [DOI] [PubMed] [Google Scholar]
- 15.Eddy AA. Proteinuria and interstitial injury. Nephrol Dial Transplant. 2004;19:277–281. doi: 10.1093/ndt/gfg533. [DOI] [PubMed] [Google Scholar]
- 16.Becker JU, Hoerning A, Schmid KW, Hoyer PF. Immigrating progenitor cells contribute to human podocyte turnover. Kidney Int. 2007;72:1468–1473. doi: 10.1038/sj.ki.5002524. [DOI] [PubMed] [Google Scholar]
- 17.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemley KV, Abdullah I, Myers BD, et al. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney Int. 2000;58:1228–1237. doi: 10.1046/j.1523-1755.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanson RL, Elston RC, Pettitt DJ, et al. Segregation analysis of non-insulin-dependent diabetes mellitus in Pima Indians: evidence for a major-gene effect. Am J Hum Genet. 1995;57:160–170. [PMC free article] [PubMed] [Google Scholar]
- 20.McCance DR, Hanson RL, Pettitt DJ, et al. Diabetic nephropathy: a risk factor for diabetes mellitus in offspring. Diabetologia. 1995;38:221–226. doi: 10.1007/BF00400098. [DOI] [PubMed] [Google Scholar]
- 21•.Hanson RL, Craig DW, Millis MP, et al. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes. 2007;56:975–983. doi: 10.2337/db06-1072. Study combining high-density single nucleotide polymorphism microarrays with a pooled genomic DNA design to identify novel loci for genes predisposing to end-stage renal disease in Pima Indians with type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 22.Conrad DF, Jakobsson M, Coop G, et al. A worldwide survey of haplotype variation and linkage disequilibrium in the human genome. Nat Genet. 2006;38:1251–1260. doi: 10.1038/ng1911. [DOI] [PubMed] [Google Scholar]
- 23.Iyengar SK, Abboud HE, Goddard KAB, et al. Genome-wide scans for diabetic nephropathy and albuminuria in multi-ethnic populations: the Family Investigation of Nephropathy and Diabetes (FIND) Diabetes. 2007;56:1577–1585. doi: 10.2337/db06-1154. [DOI] [PubMed] [Google Scholar]
- 24.Schelling JR, Abboud HE, Nicholas SB, et al. Genome-wide scan for estimated GFR in multi-ethnic diabetic populations: The Family Investigation of Nephropathy and Diabetes. Diabetes. 2008;57:235–243. doi: 10.2337/db07-0313. [DOI] [PubMed] [Google Scholar]
- 25.Nelson RG. Intrauterine determinants of diabetic kidney disease in disadvantaged populations. Kidney Int. 2003;83:S13–S16. doi: 10.1046/j.1523-1755.63.s83.4.x. [DOI] [PubMed] [Google Scholar]
- 26.Tran S, Chen YW, Chenier I, et al. Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol. 2008;19:943–952. doi: 10.1681/ASN.2007080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahamson DR, Steenhard BM. Perinatal nephron programming is not so sweet in maternal diabetes. J Am Soc Nephrol. 2008;19:837–839. doi: 10.1681/ASN.2008030280. [DOI] [PubMed] [Google Scholar]
- 28.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int. 2005;68:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RG, Knowler WC, Pettitt DJ, et al. Incidence and determinants of elevated urinary albumin excretion in Pima Indians with NIDDM. Diabetes Care. 1995;18:182–187. doi: 10.2337/diacare.18.2.182. [DOI] [PubMed] [Google Scholar]
- 30.Nelson RG, Pettitt DJ, de Courten MP, et al. Parental hypertension and proteinuria in Pima Indians with NIDDM. Diabetologia. 1996;39:433–438. doi: 10.1007/BF00400674. [DOI] [PubMed] [Google Scholar]
- 31.Nelson RG, Pettitt DJ, Baird HR, et al. Pre-diabetic blood pressure predicts urinary albumin excretion after the onset of type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia. 1993;36:998–1001. doi: 10.1007/BF02374490. [DOI] [PubMed] [Google Scholar]
- 32.Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990;6:1–27. doi: 10.1002/dmr.5610060101. [DOI] [PubMed] [Google Scholar]
- 33.Pavkov ME, Hanson RL, Knowler WC, et al. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care. 2007;30:1758–1763. doi: 10.2337/dc06-2010. [DOI] [PubMed] [Google Scholar]
- 34•.Pavkov ME, Bennett PH, Knowler WC, et al. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–426. doi: 10.1001/jama.296.4.421. Compares incidence of diabetic end-stage renal disease and mortality in Pima Indians with onset of diabetes before and after 20 years of age. [DOI] [PubMed] [Google Scholar]
- 35.Krakoff J, Lindsay RS, Looker HC, et al. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care. 2003;26:76–81. doi: 10.2337/diacare.26.1.76. [DOI] [PubMed] [Google Scholar]
- 36.Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–311. doi: 10.2337/dc06-1184. [DOI] [PubMed] [Google Scholar]
- 37.Burrows NR, Li Y, Williams DE. Racial and ethnic differences in trends of end-stage renal disease: United States, 1995 to 2005. Adv Chronic Kidney Dis. 2008;15:147–152. doi: 10.1053/j.ackd.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Narva AS. Reducing the burden of chronic kidney disease among American Indians. Adv Chronic Kidney Dis. 2008;15:168–173. doi: 10.1053/j.ackd.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Pavkov ME, Knowler WC, Bennett PH, et al. Increasing incidence of proteinuria and declining incidence of end-stage renal disease in diabetic Pima Indians. Kidney Int. 2006;70:1840–1846. doi: 10.1038/sj.ki.5001882. [DOI] [PubMed] [Google Scholar]
- 40.Pavkov ME, Knowler WC, Hanson RL, et al. Predictive power of sequential measures of albuminuria for progression to ESRD or death in Pima Indians with type 2 diabetes. Am J Kidney Dis. 2008;51:759–766. doi: 10.1053/j.ajkd.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [no authors listed] Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 42.Miyazaki Y, Cersosimo E, Triplitt C, DeFronzo RA. Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int. 2007;72:1367–1373. doi: 10.1038/sj.ki.5002516. [DOI] [PubMed] [Google Scholar]
- 43.Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The ADVANCE Collaborative Group [no authors listed] N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 44.Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators. Circulation. 2002;106:672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- 45.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 46.Mauer M, Zinman B, Gardiner R, et al. The renin-angiotensin system study (RASS): effects of enalapril and losartan on diabetic nephropathy and diabetic retinopathy in nor-motensive, normoalbuminuric patients with type 1 diabetes mellitus. Presented at the American Diabetes Association 68th Scientific Sessions; San Francisco, CA. June 6–10, 2008. [Google Scholar]
- 47.Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing micro-albuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 48.Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 49.Mogensen CE, Neldam S, Takkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321:1440–1444. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Telmisartan Ramipril or both in patients at high risk for vascular events. The ONTARGET Investigators. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 51.Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 52.Gæde P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]


