To the Editor:
Cystic fibrosis (CF) is a lethal autosomal recessive inherited disease caused by the loss or dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride (Cl−) channel activity resulting from mutations (1, 2). More than 1,600 mutations, which can be broadly grouped into six classes, have been identified in the CFTR gene (3). The incidence of CF and the frequency of specific mutations have been found to vary among ethnic populations (4, 5).
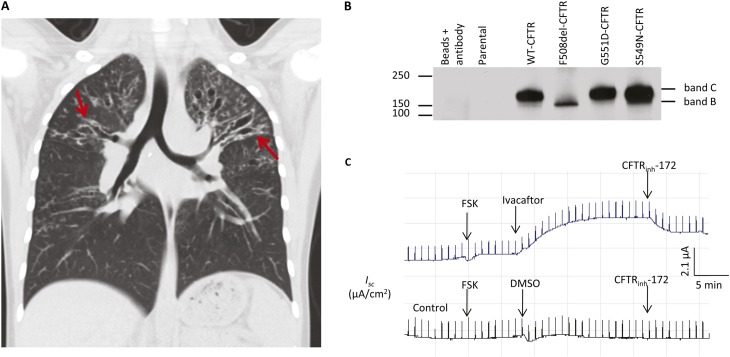
We presented a clinical case of a young Hispanic patient with CF heterozygous for c.1521_1523delCTT (p.Phe508del or F508del)/c.1646G>A (p.Ser549Asn or S549N) mutations (please note that the legacy names for the mutations will be used in this letter). This individual was diagnosed with CF at 5 months of age after multiple hospitalizations due to dehydration, failure to thrive, and emesis. She also had a prolonged and persistent cough thought secondary to a diagnosis of pertussis at 4 months of age. At diagnosis, she was also noted to have a hypochloremic, hypokalemic metabolic alkalosis. She was below the fifth percentile on all aspects of her growth curve. Sweat chloride levels were abnormal with values of 87.1 mmol/L (right arm) and 78.2 mmol/L (left arm). She was empirically given pancreatic replacement enzymes and fat soluble vitamin replacement. By the age of 3 years, she was found to be colonized by two strains of nonmucoid Pseudomonas aeruginosa as well as a mucoid Pseudomonas that was her predominant isolate by 4 years of age. Over the past 2 years, she has had three additional hospitalizations for pulmonary exacerbations (April 2010, April 2011, and October 2011). Pulmonary function tests (PFTs) have continued to worsen with FEV1 values of 50 to 60% predicted (the most recent FEV1 prior to hospitalization in October 2011 was 51% predicted) and FEF25–75 values in the low end of 20 to 40% predicted (the most recent value was 20% predicted). She has had increasing pulmonary symptoms and has developed worsening sinusitis. Chest computed tomography scan completed in October 2011 showed areas of extensive bronchiectasis and fibrotic change, predominantly affecting the upper lobes. Areas of disease were observed in the lower lobes as well (Figure 1A and Figure E1 in the online supplement). There are numerous areas of “tree-in-bud” opacities present essentially involving all lobes of both lungs (Figure 1A).
Figure 1.
S549-CFTR is a regulation mutant and associates with a severe cystic fibrosis (CF) lung disease in a young Hispanic patient with CF. (A) Chest computed tomography (coronal view) shows extensive bilateral upper lobe bronchiectasis (arrows, left > right) with relatively less involvement of the lower lung fields. (B) S549N-CFTR has normal protein expression levels. HEK-293 cells were transiently transfected with pcDNA3 containing WT, S549N, G551D, or F508del cDNA, lysed 48 hours after transfection, immunoprecipitated with α-24-1 monoclonal antibody and Western blotted for CFTR by using α-NBD-1-R polyclonal antibody. (C) Ivacaftor (100 μM) restored Cl− channel function of S549N-CFTR expressed in CFBEo− cells. The cells were grown on Costar Transwell permeable supports until they reached resistance of more than 1,400 Ω and then mounted in an Ussing chamber. FSK (10 μM) was used to activate CFTR channel function and CFTRinh-172 was used to verify the observed signal were CFTR mediated. DMSO (the solvent for Ivacaftor) was used as a negative control. See Reference 12 for technical details. DMSO = dimethyl sulfoxide; WT = wild-type; FSK = forskolin.
F508del is the most common CFTR mutation, with more than 90% of patients with CF carrying it on at least one allele. F508del-CFTR protein is insufficiently folded and is trapped in the endoplasmic reticulum and targeted for degradation (1). Like c.1652G>A (p.Gly551Asp or G551D), S549N mutation occurs in the signature sequence (LSGGQ) of the first nucleotide-binding domain on the CFTR protein (Figure E2A). S549N mutation was first reported by Curtis and colleagues in a Pakistani individual who was homozygous for S549N and demonstrated severe malnutrition, growth retardation, and advanced pulmonary disease (6). S549N mutation was found to be prevalent among Hispanic, African American, and Asian populations (4).
We characterized S549N-CFTR at the protein level to understand its molecular characteristics and the associated severe disease phenotype and, more importantly, to explore using mutation-specific therapy for medical interventions. S549N-CFTR was expressed as a mature form of CFTR (band C) with levels comparable to wild-type CFTR (Figure 1B). Surface labeling assay and fluorescence microscopy data showed that S549N-CFTR was expressed at the plasma membrane of cells (Figures E3A and E3B). However, S549N-CFTR lacks Cl− channel function (Figures E2B and E2C). These molecular characteristics correlate well with the severe CF phenotype we observed and suggest that, like G551D, S549N-CFTR is probably a regulation mutant.
Ivacaftor (VX-770) is a CFTR potentiator that can restore the defective gating of G551D and several other regulation mutants (7–9) and has been approved by U.S. Food and Drug Administration for treating patients with CF aged 6 and older with G551D mutation (10). We expressed S549N-CFTR in polarized human cystic fibrosis bronchial epithelial cells (CFBEo−) and tested the effect of ivacaftor on CFTR channel function. Our results showed that ivacaftor restored the defective channel function of S549N-CFTR (Figure 1C), suggesting that ivacaftor has potential clinical benefit for patients with CF with S549N mutation. Our biochemical findings are consistent with data from a recent study in which ivacaftor was found to potentiate the channel open probability and total Cl− transport of S549N-CFTR expressed in Fischer rat thyroid cells (9). To further confirm S549N is a regulation mutant and can be rescued by CFTR potentiators, we also tested the effect of another known CFTR potentiator, P1, on the channel function of S549N-CFTR expressed in CFBEo− cells or human embryonic kidney (HEK-293) cells and found that P1 exerted a potentiating effect similar to that of ivacaftor (Figures E4 and E5).
Because this subject in our study is heterozygous for F508del/S549N mutations, she would be an ideal candidate for the ongoing combinational trials using VX-809 (11) and ivacaftor and, most likely, will benefit from these trials.
Supplementary Material
Footnotes
Author Contributions: The manuscript was written by S.Y. and W.Z. supervised by A.P.N. and D.C.S. The project was designed and supervised by A.P.N. S.Y. conducted site-directed mutagenesis and surface labeling, F.A.I.K. performed iodide efflux assays, and H.P. performed immunoprecipitation and Western blotting experiments. A.R. conducted Isc measurements. K.A. performed immunofluorescence microscopy studies. C.A.D., S.S., and D.C.S. did the clinical studies. J.C.K. assisted in site-directed mutagenesis. All authors discussed the results and commented on the manuscript.
Supported in part by U.S. National Institutes of Health funding (DK074996 and DK080834 to A.P.N.) and U.S. Cystic Fibrosis Foundation funding to D.C.S.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990;63:827–834 [DOI] [PubMed] [Google Scholar]
- 2.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993;73:1251–1254 [DOI] [PubMed] [Google Scholar]
- 3.Penmatsa H, Frederick CA, Nekkalapu S, Conoley VG, Zhang W, Li C, Kappes J, Stokes DC, Naren AP. Clinical and molecular characterization of S1118F-CFTR. Pediatr Pulmonol 2009;44:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohlfs EM, Zhou Z, Heim RA, Nagan N, Rosenblum LS, Flynn K, Scholl T, Akmaev VR, Sirko-Osadsa DA, Allitto BA, et al. Cystic fibrosis carrier testing in an ethnically diverse US population. Clin Chem 2011;57:841–848 [DOI] [PubMed] [Google Scholar]
- 5.Sugarman EA, Rohlfs EM, Silverman LM, Allitto BA. CFTR mutation distribution among Hispanic US and African American individuals: evaluation in cystic fibrosis patient and carrier screening populations. Genet Med 2004;6:392–399 [DOI] [PubMed] [Google Scholar]
- 6.Curtis A, Richardson RJ, Boohene J, Jackson A, Nelson R, Bhattacharya SS. Absence of cystic fibrosis mutations in a large Asian population sample and occurrence of a homozygous S549N mutation in an inbred Pakistani family. J Med Genet 1993;30:164–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 2009;106:18825–18830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. ; VX08–770–102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, Urrutia A, Joubran J, Seepersaud S, Sussky K, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros 2012;11:237–245 [DOI] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Foundation. FDA Approves Kalydeco (VX-770)—first drug that targets the underlying cause of cystic fibrosis [accessed 2012 January 31]. Available from: http://www.cff.org/aboutCFFoundation/NewsEvents/1-31-FDA-Approves-Kalydeco.cfm.
- 11.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011;108:18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, Naren AP. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 2007;13:940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.