Abstract
Rationale: Prevalence of pulmonary nontuberculous mycobacterial (PNTM) disease varies by geographic region, yet the factors driving these differences remain largely unknown.
Objectives: To identify spatial clusters of PNTM disease at the county level and to describe environmental and sociodemographic factors predictive of disease.
Methods: PNTM cases identified from a nationally representative sample of Medicare Part B beneficiaries from 1997 to 2007 were geocoded by county and state of residence. County-level PNTM case counts and Medicare population data were then uploaded into SaTScan to identify significant spatial clusters and low-risk areas of disease. High-risk and low-risk counties were then compared to identify significant sociodemographic and environmental differences.
Measurements and Main Results: We identified seven significant (P < 0.05) clusters of PNTM cases. These high-risk areas encompassed 55 counties in 8 states, including parts of California, Florida, Hawaii, Louisiana, New York, Oklahoma, Pennsylvania, and Wisconsin. Five low-risk areas were also identified, which encompassed 746 counties in 23 states, mostly in the Midwest. Counties in high-risk areas were significantly larger, had greater population densities, and higher education and income levels than low-risk counties. High-risk counties also had higher mean daily potential evapotranspiration levels and percentages covered by surface water, and were more likely to have greater copper and sodium levels in the soil, although lower manganese levels.
Conclusions: Specific environmental factors related to soil and water exposure appear to increase the risk of PNTM infection. Still, given that environmental sources of NTM are ubiquitous and PNTM disease is rare, both host susceptibility and environmental factors must be considered in explaining disease development.
Keywords: nontuberculous mycobacteria, pulmonary, spatial, cluster, epidemiology
At A Glance Commentary
Scientific Knowledge on the Subject
Prevalence of pulmonary nontuberculous mycobacterial (PNTM) disease varies greatly by geographic region, yet the factors driving these differences remain largely unknown.
What This Study Adds to the Field
We identified several distinct geographic areas in the United States where PNTM disease is clustered among persons 65 years of age and older, and detected socioeconomic, demographic, and environmental factors predictive of an increased county-level risk for PNTM disease.
Pulmonary nontuberculous mycobacterial (PNTM) disease is increasingly recognized as an important cause of morbidity in the United States, especially among older adults (1). Infection with PNTM is typically thought to result from environmental exposures, including soil and water sources (2). Studies suggest that the prevalence of PNTM disease is increasing throughout the United States, especially among older adults (1, 3–5), although disease prevalence estimates vary widely by geographic region (1, 3, 5, 6). Although these geographic differences in prevalence are likely due to a combination of environmental, genetic, and socioeconomic influences, the precise geospatial factors driving these dynamics remain largely unknown.
Previous studies have identified U.S. geographic areas that appear to have increased rates of PNTM disease. In one study on U.S. Medicare beneficiaries, Hawaii was found to have the highest prevalence of PNTM in the United States, with an 11-year period prevalence of 396 cases per 100,000 persons—nearly four times that observed overall in the total U.S. population (5). Environmental conditions unique to Hawaii such as soil-related characteristics may contribute to greater levels of NTM exposure, although the specific factors driving the disease dynamics in Hawaii remain unknown (5). A higher prevalence of PNTM disease as well as NTM exposure as measured by purified protein derivative-Battey (PPD-B; for Mycobacterium intracellulare) sensitivity has been described among older adults living in southeastern and western states (3, 5, 7, 8), and greater numbers of mycobacteria have been isolated from environmental samples in southeastern states than from other locations surveyed (9, 10). Regional factors, individual behaviors, and local exposures may all contribute to the true risk of infection.
Characterizing the spatial epidemiology of PNTM disease is essential for understanding the environmental and sociodemographic determinants of NTM exposure and disease risks. Using national Medicare claims and geospatial data, we sought to describe the epidemiology of spatial clusters of PNTM disease among older adults in the United States. Some preliminary results from this study were previously reported in the form of an abstract (11).
Methods
Data were obtained from the Centers for Medicare and Medicaid’s Carrier Standard Analytic File, using a 5% sample of all Medicare Part B beneficiaries from 1997 to 2007. All individuals with one or more medical claims submitted from noninstitutional outpatient care providers that included the International Classification of Diseases, 9th revision (ICD-9) code associated with PNTM disease (031.0) were identified and termed “PNTM cases.” Individuals were excluded if they were less than 65 years old, enrolled in a health maintenance organization, enrolled in Medicare Part B for less than 1 month, or resided outside of the United States. The county and state of residence for each PNTM case were geocoded into latitude and longitude coordinates by county centroid. For PNTM cases who moved during the study period, the county and state of residence where PNTM was first diagnosed were used. Relocation patterns were also evaluated.
For each U.S. county, the number of PNTM cases and total county population of Medicare enrollees for the year 2000 were recorded and uploaded into SaTScan (version 7.0; Kulldorff M. and Information Management Services, Inc.). Because of the rare nature of this disease, PNTM county-level case counts were assumed to carry a Poisson distribution in models used to detect areas with significantly (P ≤ 0.05) greater than expected numbers of PNTM cases, termed clusters or high-risk areas, based on the overall U.S. prevalence of PNTM observed. Areas with significantly fewer than expected PNTM cases, termed low-risk areas, were also detected. Specifically, a spatial scan test was conducted to calculate the likelihood of being a case both within and outside of a defined circular window within the grid covered by the U.S. study population, with the null hypothesis stating that both areas were of equal risk. Through 999 iterations, this window scanned the total geographic area, varying the diameter from zero (a single county centroid) to a maximal radius set to never include more than 25% of the total population, facilitating detection of both smaller and larger clusters. All counties included within the high-risk and low-risk areas were identified and maps of all findings were generated with ArcView GIS 9.2 (Environmental Systems Research Institute, Inc., Redlands, CA).
County-level environmental and sociodemographic data available were analyzed for all counties identified within high-risk and low-risk areas. Data evaluated included population and socioeconomic factors from the U.S. Census Bureau (i.e., population density, urban/rural composition, racial/ethnic, sex, age distributions, employment status, income/poverty levels, education levels, and insurance status); and environmental and climatic factors from the U.S. Census Bureau, U.S. Forest Service, and the U.S. Geological Survey (i.e., elevation, geochemical soil properties, agricultural use, percentage of surface water, temperature, precipitation, and potential evapotranspiration—a measure of the atmosphere’s ability to remove water from the surface through the processes of evaporation and transpiration and convert it to atmospheric water vapor) (12–14).
High-risk and low-risk counties were described and compared, using a two-tail Student t test. Logistic regression was used to evaluate factors associated with high-risk counties. Variables with significant (P < 0.05) odds ratios in univariate models were included in multivariate models built by backward stepwise selection. Effect modification and confounding among variables were also evaluated. All statistical analyses were performed with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Nearly 2.3 million individuals from all 50 states and the District of Columbia were included in the sample of Medicare Part B beneficiaries evaluated for 1997–2007, from which 16,508 PNTM claims representing 2,548 unique cases were identified. Seventy-five percent of cases had PNTM submitted as the primary medical claim at least once and an average of 6.5 PNTM claims were submitted per case. A total of 4,328 treating physicians were identified on the claims, for an average of 1.7 physicians per case.
Over the 11-year time period evaluated, 54 cases (2.1%) had claims reported from at least one county/state location. Of these, 27 represented changes in state and 17 changes in U.S. region (i.e., northeast to southeast, and so on). For five cases with claims reported from one or more county/state location, the first recorded location change was subsequently followed by another claim from the original location of residence, suggesting that this move was temporary, such as a seasonal change in residence.
Cluster Analysis
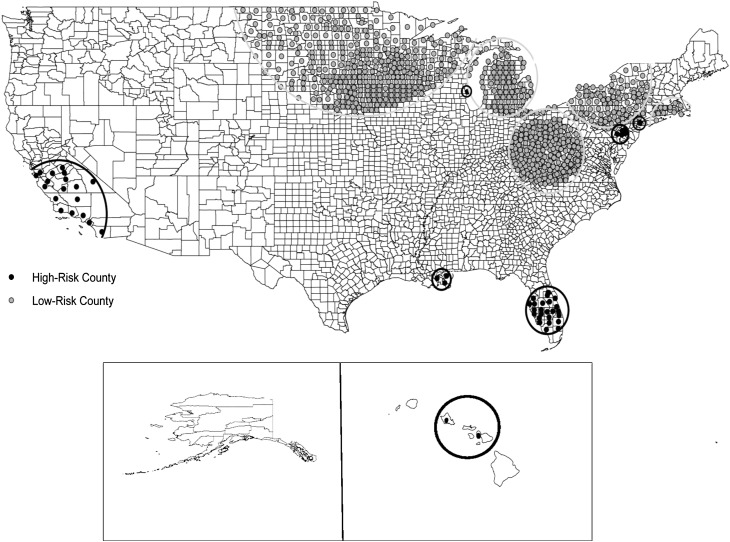
SaTScan identified seven significant (P < 0.05) clusters of PNTM cases. These high-risk areas encompassed 55 counties in 8 states, including parts of California, Florida, Hawaii, Louisiana, New York, Oklahoma, Pennsylvania, and Wisconsin. Five low-risk areas were also identified that encompassed 746 counties in 23 states. The majority of the low-risk areas were located in the Midwest (Table 1 and Figure 1). High-risk areas averaged 8 counties (range, 1–24) and had a mean radius of 104 km (range, 0–344); low-risk areas averaged 132 counties (range, 16–247) and had a mean radius of 341 km (range, 107–689). The relative risk (RR) of having a PNTM diagnosis when residing in a high-risk area compared with all other areas in the United States ranged from 1.9 for a cluster centered in Highlands County, Florida (radius, 159 km; P < 0.0001) to 6.5 for a cluster centered in Plaquemines Parish, Louisiana (radius, 70 km; P < 0.0001) (Table 1).
TABLE 1.
SUMMARY OF ALL SIGNIFICANT CLUSTERS IDENTIFIED BY SaTScan OF PULMONARY NONTUBERCULOUS MYCOBACTERIAL DISEASE AMONG U.S. MEDICARE BENEFICIARIES 65 YEARS OF AGE AND OLDER
| Cluster Type | Centroid County and State | No. of Counties (Radius, km) | Relative Risk | P Value |
| High risk | Highlands, FL | 24 (159.4) | 1.9 | <0.0001 |
| Santa Barbara, CA | 18 (344.5) | 2.0 | <0.0001 | |
| Montgomery, PA | 5 (42.2) | 2.2 | 0.0001 | |
| New York, NY | 1 (0) | 2.7 | 0.002 | |
| Milwaukee, WI | 1 (0) | 3.6 | <0.0001 | |
| Kalawao, HI | 3 (114.8) | 3.7 | <0.0001 | |
| Plaquemines, LA | 3 (70.2) | 6.5 | <0.0001 | |
| Low risk | Washington, RI | 16 (106.7) | 0.5 | 0.02 |
| Iosco, MI | 93 (351.4) | 0.4 | <0.0001 | |
| Roane, WV | 208 (268.5) | 0.4 | <0.0001 | |
| Polk, MN | 247 (689.7) | 0.4 | <0.0001 | |
| Cayuga, NY | 95 (289.0) | 0.3 | <0.0001 |
Figure 1.
Significant clusters of counties identified by SaTScan as being at either high or low risk for pulmonary nontuberculous mycobacterial (PNTM) disease among U.S. Medicare beneficiaries 65 years of age and older.
Compared with counties in low-risk areas, counties in high-risk areas were significantly larger, had a greater population density, and had higher education and income levels (Table 2). The total land area in high-risk counties included a greater proportion of area as surface water than low-risk counties (19 vs. 6%, respectively). High-risk counties also had greater levels of precipitation, mean minimal and maximal temperatures, mean daily potential evapotranspiration, and lower elevation than low-risk counties (Table 2). The mean doctor-to-patient ratio for PNTM cases was 1.6 in high-risk counties and 2.0 in low-risk counties (P = 0.1).
TABLE 2.
COMPARISON OF SELECTED CHARACTERISTICS OF HIGH-RISK AND LOW-RISK COUNTIES FOR PULMONARY NONTUBERCULOUS MYCOBACTERIAL DISEASE AMONG U.S. MEDICARE BENEFICIARIES 65 YEARS OF AGE AND OLDER
| Variable* | High-Risk County | Low-Risk County | P Value |
| Demographic | |||
| Median age | 37.8 | 38.3 | 0.37 |
| ≥65 yr old, % | 16.1 | 16.0 | 0.85 |
| Female, % | 50.2 | 50.5 | 0.07 |
| Race/ethnicity | |||
| White | 63.3 | 92.0 | <0.0001 |
| Black | 8.6 | 2.2 | <0.0001 |
| Asian | 4.9 | 0.6 | <0.0001 |
| Hispanic | 19.1 | 1.8 | <0.0001 |
| Socioeconomic | |||
| Population density, per square mile | 391 | 85 | <0.0001 |
| Bachelor’s degree or higher, % | 22 | 16 | <0.0001 |
| Median household income, $ | 40,941 | 36,331 | 0.0002 |
| Deaths per 1,000 persons | 9.3 | 10.7 | 0.0001 |
| Environmental | |||
| Total area, square miles | 1,861 | 827 | <0.0001 |
| Proportion of area as surface water, % | 19.0 | 5.9 | <0.0001 |
| Mean precipitation, inches | 85.9 | 74.2 | 0.0006 |
| Mean daily potential evapotranspiration, mm | 6.5 | 4.8 | <0.0001 |
| Mean minimal temperature, °C | 12.9 | 3.0 | <0.0001 |
| Mean maximal temperature, °C | 24.8 | 14.9 | <0.0001 |
| Elevation, m | 227.7 | 398.3 | <0.0001 |
| Geochemical soil levels, ppm | |||
| Selenium | 0.3 | 0.4 | 0.02 |
| Mercury | 0.09 | 0.06 | 0.08 |
| Lead | 22.8 | 21.3 | 0.41 |
| Zinc | 52.4 | 69.1 | 0.0005 |
| Copper | 17.4 | 15.6 | 0.14 |
| Aluminum | 3.7 | 4.7 | <0.0001 |
| Sodium | 0.8 | 0.7 | 0.14 |
| Manganese | 401.7 | 743.1 | <0.0001 |
| Iron | 2.0 | 2.6 | 0.0005 |
All population-level demographic factors are based on year 2000 U.S. Census Bureau data.
When evaluated in multivariate models, several socioeconomic and environmental factors remained highly predictive of high-risk counties (Table 3). For each 0.1-mm increase in the mean daily potential evapotranspiration, a county was 4.0 (1.6–10.1) times more likely to be high-risk. In addition, each 10% increase in the percentage of a county area covered by surface water increased a county’s risk by 4.6 (1.5–14.6) times. High-risk counties were also significantly more likely to have greater copper and sodium levels in the soil, and lower manganese levels. High-risk counties also had significantly greater population densities and median income levels, with each additional 100 persons per square mile and $1,000 in median income level increasing the likelihood of being high-risk by 40% (P < 0.01).
TABLE 3.
MULTIVARIATE LOGISTIC REGRESSION MODEL OF ENVIRONMENTAL AND SOCIOECONOMIC FACTORS SIGNIFICANTLY ASSOCIATED WITH HIGH-RISK COUNTIES FOR PULMONARY NONTUBERCULOUS MYCOBACTERIAL DISEASE AMONG U.S. MEDICARE BENEFICIARIES 65 YEARS OF AGE AND GREATER
| Variable | Adjusted Odds Ratio (95% Confidence Interval) | P Value |
| Population per square mile, per 100-person increase | 1.4 (1.1–1.7) | 0.003 |
| Median household income, per $1,000 increase | 1.4 (1.1–1.8) | 0.004 |
| Proportion of area as surface water, per 10% increase | 4.6 (1.5–14.6) | 0.009 |
| Mean daily potential evapotranspiration, per 0.1-mm increase | 4.0 (1.6–10.1) | 0.004 |
| Copper soil levels, per 1-ppm increase | 1.2 (1.0–1.4) | 0.008 |
| Sodium soil levels, per 0.1-ppm increase | 1.9 (1.2–2.9) | 0.004 |
| Manganese levels, per 100-ppm increase | 0.7 (0.4–1.0) | 0.03 |
Discussion
By applying spatial epidemiologic tools to a nationally representative population-based sample of Medicare beneficiaries with PNTM, we identified several distinct geographic areas in the United States where PNTM disease diagnosis is clustered among persons at least 65 years of age. In addition, by comparing counties located in clusters with those found in low-risk areas, we detected socioeconomic, demographic, and environmental factors predictive of an increased county-level risk for PNTM disease. Although other studies have found geographic variation in the prevalence of PNTM in the United States (1, 3, 5, 15, 16), this is the first study to describe environmental and socioeconomic determinants of PNTM disease clustering by county in the United States.
The majority of the high-risk counties identified here are located in southeastern and western states, which is consistent with previously identified high-risk areas for both exposure and disease, particularly in Florida, California, Hawaii, and Louisiana (1, 3, 5, 8). Greater levels of human exposure to NTM (as measured by PPD-B) (7), as well as higher levels of NTM in the environment (13) and greater rates of NTM isolation from humans (14), have been previously reported in these states, especially in the southeast, where in a study of Navy recruits in the 1960s who had resided in only a single county, more than 60% from Florida and Louisiana demonstrated evidence of prior exposure to NTM (7). However, in this same study, less than one-quarter of California residents tested positive for evidence of prior exposure (7), perhaps indicating a change in NTM ecology and disease dynamics over time (5). Although these states are recognized as being high-risk for PNTM disease, our results identify more precise locations within these states that are at greatest risk. In Louisiana, three southern, coastal parishes were identified within the cluster, including Plaquemines, Jefferson, and St. Bernard Parishes. These parishes, along with Orleans Parish, which borders this cluster, all lie within the delta plain of the Mississippi River, representing an estuarine habitat with an extensive network of wetlands and thick, underlying layers of peat (17). In previous studies, high numbers of mycobacteria have been retrieved from peat lands and East Coast estuaries (9, 18). In addition, in a nationwide study on NTM among patients at cystic fibrosis centers, Orleans Parish was observed to have the highest NTM isolation prevalence of any of the 21 sites studied (8).
In both Florida and California, the southern and central regions appear to carry the highest risk of PNTM disease, as opposed to northern counties, which were not included in the clusters. Although these clusters overlap with the most populated areas in these states, likely reflecting socioeconomic, lifestyle, and demographic differences, they also represent areas where the climate differs quite dramatically from areas north of the clusters. In Florida, the climate transitions from tropical to subtropical just above the northern boundary of the cluster (19), and in California, the mean temperatures of counties in the clusters versus those to the north differ by 2–10°C, depending on the location (coast vs. the central valley).
In Hawaii, three counties were included in the cluster identified; however, actual cases were reported from only two of these counties, Maui and Honolulu, with the third county, Kalawao, just serving as the central point of the cluster between the other two high-risk areas. Similarly, although PNTM cases in our data set were reported from Hawaii County, this area was not included in the cluster identified. In a previous study, Hawaii was found to have the highest prevalence of PNTM in the country (5). This increased risk was attributed to having both a large Asian/Pacific Islander population, which has a twofold increased risk for PNTM disease, as well as a greater environmental potential for exposure, possibly due to unique conditions present on the island (5, 20, 21). For instance, many of the zonal soil groups in the Hawaiian Islands are classified as being humic (22), containing humic acid as a principal component that has been associated with sources of high numbers of NTM (18). Therefore, it is likely that the islands of Molokai and Hawaii, on which Kalawao and Hawaii Counties are, respectively, located, may also represent high-risk environments for NTM; however, it is impossible to speculate further on the environmental potential for exposure and disease risk given these data and findings.
In addition to the historically documented high-risk areas and states, clusters were also detected in several northeastern and north–central areas, including in New York, Pennsylvania, and Wisconsin. Between 2000 and 2003, Bodle and colleagues noted an increase in NTM among patients without human immunodeficiency virus in New York, New York, where our New York cluster was located, within a population not identified by NTM specialty centers, thus removing the concern of referral center bias (15). Clusters were also identified in the greater Philadelphia metropolitan area and in Milwaukee, Wisconsin, the latter of which was also the location of a center with a high isolation prevalence of NTM among patients with cystic fibrosis (8). These clusters may reflect increased efforts in diagnosis and detection of NTM by specialists in these areas. However, the analysis conducted of physicians diagnosing PNTM in these clusters does not appear to support the hypothesis that a few specialists are responsible for the greater than expected numbers of cases in these areas. Still, without further local investigation it is difficult to determine whether other factors may also be contributing to the observed elevated risk for PNTM disease.
When comparing high-risk counties with low-risk counties, certain socioeconomic and demographic factors had a strong and significant association with an increased risk for PNTM disease. Counties located in clusters had greater population densities, median household income levels, more racially/ethnically diverse populations, and proportions of college graduates than low-risk counties. These factors are related to the tendency for clusters to cover more urban areas, which has been linked previously with NTM disease (8, 23). Our findings are also similar in magnitude to those of Olivier and colleagues, who also found that NTM culture–positive study subjects with cystic fibrosis were approximately 40% more likely to live in nonrural areas with greater college education and median income levels (8). In our multivariate analysis, population density and median household income level were the socioeconomic factors most predictive of high-risk counties. These variables likely serve as indicators of better access to more specialized medical resources that are necessary for NTM to be properly diagnosed.
Even after controlling for population density and median household income level, several environmental variables related to water/moisture availability and soil properties were independently associated with clustering of PNTM disease. High rates of the mean daily potential evapotranspiration, a measure of the ability of the atmosphere to remove water from the surface through the processes of evaporation and transpiration and convert it to atmospheric water vapor, as well as the proportion of the county composed of surface waters, the likely sources of drinking water, were both strongly associated with high-risk counties. Surface waters serve as one of the main environmental sources of mycobacteria, with the highest numbers isolated from the acid brown-water swamps of the southeastern coast (18, 24); this same area had clusters detected in this analysis. Further, drinking water systems using surface water were more likely to have NTM than systems using a groundwater source (25). In fact, groundwater has been shown to rarely contain NTM (26). These results suggest that surface and atmospheric water sources and levels contribute to an increased risk for NTM exposure and therefore to the higher prevalence of disease in these areas.
Soils also serve as a large natural source of mycobacteria, and certain soil properties likely promote the growth and persistence of NTM in the environment. In our analysis, soils in counties located in clusters were more likely to have higher copper and sodium levels, but lower manganese levels, than in low-risk counties. NTM are more frequent in brackish water (9) and have been shown to grow well in the presence of high salt concentrations, such as in brackish water environments (16). In a pilot drinking water distribution system, it was shown that disinfection led to an increase in the proportion of cells of Mycobacterium avium in biofilms, particularly copper pipe biofilms (27). Also, in a study by Falkinham, Mycobacterium immunogenum cells were shown to grow rapidly on copper surfaces, forming biofilms within 2 weeks (28). Therefore, NTM may flourish in soils with elevated sodium and copper levels, and even outcompete other bacterial species for nutrients and resources. Although studies evaluating the significance of manganese levels on NTM growth in the environment are lacking, one study showed that antioxidants containing manganese appear to inhibit growth of Mycobacterium abscessus in human macrophages (29). However, whether this effect is due to manganese specifically or whether NTM growth would be inhibited in a natural environment is unknown.
Although environmental exposure to NTM occurs through both water and soil sources, attributing their relative roles in the development of PNTM disease is difficult because of ubiquity of exposure to both these sources, and the need to consider both frequency and duration to these common exposures. One study conducted in Palm Beach County, Florida, which was identified here as a high-risk county, obtained detailed measurements on the intensity of soil-related exposures and found that participation in occupations with greater amounts of soil exposure was significantly associated with a positive Mycobacterium avium sensitin skin test reaction, with a dose–response relationship (30). Other studies have linked NTM exposure and infection to various water-related sources, including natural water sources, public drinking water, and even household showerheads and hot tubs (24, 31–34). In addition, other household-related factors such as humidifier and air-conditioning system use, the latter of which likely occurs with greater frequency in hot, humid climates, may possibly concentrate NTM and also serve as sources of exposure. All of these potential sources of exposure may lead to the development of NTM infections; however, because of the ubiquity of the exposure and rarity of this disease, host-related factors such as behavioral practices and genetic susceptibility must also be key factors in determining PNTM disease development.
Spatial epidemiologic analyses are limited to the quality and precision of the data being evaluated. The use of county-level data limits the ability to identify clusters of disease at finer spatial scales, and may mask important associations between PNTM disease and environmental, socioeconomic, and demographic factors that exist at a more local level, such as in specific niches, especially within larger, more diverse counties. Similarly, by performing a cluster analysis and evaluating only high-risk and low-risk counties, we are unable to evaluate other counties not selected by SaTScan, including neighboring counties, which may also share an increased or decreased disease burden. In addition, the use of PNTM cases identified on the basis of ICD-9 codes in Medicare claims underestimates the true disease burden (1, 35), and the population of PNTM cases captured in this study may differ in epidemiologically relevant ways from individuals with PNTM disease who were not included in our study population, which could in turn affect the geographic risk factors identified. Differential use of Medicare or coding of PNTM by region or population subgroup could be one of these factors, although we have not found this to have a strong influence in other analyses (5, 36). Further, although our analysis focused on associations between cases and their reported county and state of residence, the interval between exposure and disease onset is unknown, and therefore some disease could be due to organisms acquired elsewhere. However, given the consistency of these findings with prior studies related to geographic risk factors, and the low proportion of the study population that moved during the study period, the disease cluster patterns are not likely to be explained by human movement patterns.
Specific environmental factors related to soil and water exposure appear to increase the risk of PNTM infection. Still, even within high-risk areas with increased levels of exposure to ubiquitous environmental sources of NTM, the development of PNTM disease is rare, suggesting that both host susceptibility and environmental factors must be considered in identifying risk factors for PNTM disease. Additional studies at a finer spatial scale are needed to further elucidate the complex interactions driving PNTM disease dynamics.
Supplementary Material
Acknowledgments
The authors thank Elizabeth Hilborn for assistance with identifying national geospatial data sources, and Michael Adjemian for consultations regarding spatial analytic methods.
Footnotes
Supported by the Division of Intramural Research, NIAID.
Author Contributions: Study concept and design—J.A., R.P.; acquisition of data—J.A., A.E.S., D.R.P., S.M.H., J.O.F.; analysis and interpretation of data—J.A., K.N.O., A.E.S., D.R.P., J.O.F.; drafting of the manuscript—J.A., D.R.P.; critical revision of the manuscript for important intellectual content—J.A., K.N.O., A.E.S., J.O.F., S.M.H., D.R.P.; statistical analysis—J.A., D.R.P.; study supervision—D.R.P.
Originally Published in Press as DOI: 10.1164/rccm.201205-0913OC on July 05, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med 2010;182:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangione EJ, Huitt G, Lenaway D, Beebe J, Bailey A, Figoski M, Rau MP, Albrecht KD, Yakrus MA. Nontuberculous mycobacterial disease following hot tub exposure. Emerg Infect Dis 2001;7:1039–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, Prevots DR. Nontuberculous mycobacteria–associated lung disease in hospitalized persons, United States, 1998–2005. Emerg Infect Dis 2009;15:1562–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002;23:553–567 [DOI] [PubMed] [Google Scholar]
- 5.Adjemian J, Olivier KN, Seitz A, Holland S, Prevots R. Prevalence of pulmonary nontuberculous mycobacterial infections among U.S. Medicare beneficiaries, 1997–2007. Am J Respir Crit Care Med 2012;185:881–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 2010;182:977–982 [DOI] [PubMed] [Google Scholar]
- 7.Edwards LB, Acquaviva FA, Livesay VT, Cross FW, Palmer CE. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis 1969;99:1–132 [PubMed] [Google Scholar]
- 8.Olivier KN, Weber DJ, Wallace RJ, Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, et al. Nontuberculous mycobacteria. I. Multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 2003;167:828–834 [DOI] [PubMed] [Google Scholar]
- 9.Falkinham JO, III, Parker BC, Gruft H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am Rev Respir Dis 1980;121:931–937 [DOI] [PubMed] [Google Scholar]
- 10.Brooks RW, Parker BC, Gruft H, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. V. Numbers in eastern United States soils and correlation with soil characteristics. Am Rev Respir Dis 1984;130:630–633 [DOI] [PubMed] [Google Scholar]
- 11.Adjemian J, Olivier K, Seitz A, Holland S, Prevots R. Spatial clusters of nontuberculous mycobacterial lung disease in the United States [abstract]. Am J Respir Crit Care Med 2012;185:A2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Census Bureau. Index of U.S. Census Bureau data, 2000 [accessed 2012 July 12]. Available from: http://www2.census.gov/census_2000/datasets/
- 13.United States Forest Service. Publications and data [accessed 2012 July 12]. Available from: http://nrs.fs.fed.us/data/urban/
- 14.United States Geological Survey. Mineral resources on-line spatial data [accessed 2012 July 12]. Available from: http://tin.er.usgs.gov/geochem/doc/averages/countydata.htm.
- 15.Bodle EE, Cunningham JA, Della-Latta P, Schluger NW, Saiman L. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis 2008;14:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George KL, Parker BC, Gruft H, Falkinham JO. Epidemiology of infection by nontuberculous mycobacteria. II. Growth and survival in natural waters. Am Rev Respir Dis 1980;122:89–94 [DOI] [PubMed] [Google Scholar]
- 17.McCulloh RP, Heinrich PV, Good B. Geology and hurricane-protection strategies in the greater New Orleans area. Public Information Series No. 11. Louisiana Geological Survey: Public Information Series 2006;11:1–31 Available from: http://www.lgs.lsu.edu/deploy/uploads/11strategies.pdf [Google Scholar]
- 18.Kirschner RA, Jr, Parker BC, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria: Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. Am Rev Respir Dis 1992;145:271–275 [DOI] [PubMed] [Google Scholar]
- 19.Florida Climate Center. 1981–2000 normals [2012 Jul 12]. Office of the State Climatologist. Available from: http://climatecenter.fsu.edu/products-services/data#1981-2000Normals.
- 20.Good RC. From the Centers for Disease Control and Prevention: isolation of nontuberculous mycobacteria in the United States, 1979. J Infect Dis 1980;142:779–783 [DOI] [PubMed] [Google Scholar]
- 21.Hennessee CT, Seo JS, Alvarez AM, Li QX. Polycyclic aromatic hydrocarbon–degrading species isolated from Hawaiian soils: Mycobacterium crocinum sp. nov., Mycobacterium pallens sp. nov., Mycobacterium rutilum sp. nov., Mycobacterium rufum sp. nov. and Mycobacterium aromaticivorans sp. nov. Int J Syst Evol Microbiol 2009;59:378–387 [DOI] [PubMed] [Google Scholar]
- 22.United States Department of Agriculture. Soil survey of the territory of Hawaii: islands of Hawaii, Kauaii, Lanai, Maui, Molokai, and Oahu. Soil Survey Series 1955;1939:1–64.
- 23.O'Brien RJ, Geiter LJ, Snider DE., Jr The epidemiology of nontuberculous mycobacterial diseases in the United States: results from a national survey. Am Rev Respir Dis 1987;135:1007–1014 [DOI] [PubMed] [Google Scholar]
- 24.Falkinham JO., III Nontuberculous mycobacteria in the environment. Clin Chest Med 2002;23:529–551 [DOI] [PubMed] [Google Scholar]
- 25.Falkinham JO, III, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl Environ Microbiol 2001;67:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin EC, Parker BC, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. VII. Absence of mycobacteria in southeastern groundwaters. Am Rev Respir Dis 1987;136:344–348 [DOI] [PubMed] [Google Scholar]
- 27.Norton CD, LeChevallier MW, Falkinham JO., III Survival of Mycobacterium avium in a model distribution system. Water Res 2004;38:1457–1466 [DOI] [PubMed] [Google Scholar]
- 28.Falkinham JO., III Effects of biocides and other metal removal fluid constituents on Mycobacterium immunogenum. Appl Environ Microbiol 2009;75:2057–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberley-Deegan RE, Lee YM, Morey GE, Cook DM, Chan ED, Crapo JD. The antioxidant mimetic, MnTE-2-PyP, reduces intracellular growth of Mycobacterium abscessus. Am J Respir Cell Mol Biol 2009;41:170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed C. Environmental risk factors for infection with Mycobacterium avium complex. Am J Epidemiol 2006;164:32–40 [DOI] [PubMed] [Google Scholar]
- 31.Falkinham JO., III Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 2011;17:419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA 2009;106:16393–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.du Moulin GC, Stottmeier KD, Pelletier PA, Tsang AY, Hedley-Whyte J. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 1988;260:1599–1601 [DOI] [PubMed] [Google Scholar]
- 34.Glazer CS, Martyny JW, Lee B, Sanchez TL, Sells TM, Newman LS, Murphy J, Heifets L, Rose CS. Nontuberculous mycobacteria in aerosol droplets and bulk water samples from therapy pools and hot tubs. J Occup Environ Hyg 2007;4:831–840 [DOI] [PubMed] [Google Scholar]
- 35.Winthrop KL, Baxter R, Liu L, McFarland B, Austin D, Varley C, Radcliffe L, Suhler E, Choi D, Herrinton LJ. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf 2011;20:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seitz A, Olivier K, Adjemian J, Holland S, Prevots R. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000–2007. Chest (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.