Abstract
Strategies combatting cognitive decline among the growing aging population are vital. We tested whether environmental enrichment could reverse age-impaired rapid spatial search strategy acquisition concomitantly with hippocampal neurogenesis in rats. Young (5–8 mo) and aged (20–22 mo) male Fischer 344 rats were pair-housed and exposed to environmental enrichment (n=7 young, 9 aged) or housed individually (n=7 young, 7 aged) for ten weeks. After five weeks, hidden platform trials (5 blocks of 3 trials; 15m IBI), a probe trial, and then visible platform trials (5 blocks of 3 trials; 15m IBI) commenced in the water maze. One week after testing, rats were given 5 daily bromodeoxyuridine (BrdU, 50mg/kg; i.p.) injections and perfused 4 weeks later to quantify neurogenesis. Although young rats outperformed aged rats, aged enriched rats outperformed aged individually-housed rats on all behavioral measures. Neurogenesis decreased with age but enrichment enhanced new cell survival, regardless of age. The novel correlation between new neuron number and behavioral measures obtained in a rapid water maze task among aged rats, suggests that environmental enrichment increases their ability to rapidly acquire and flexibly use spatial information along with neurogenesis.
Keywords: adult neurogenesis, learning, memory, enrichment, water maze, age-related, Fisher 344, neural progenitor cell, hippocampus
1. Introduction
Altered hippocampal function likely contributes to age-related changes in cognitive ability because hippocampus-dependent tasks are sensitive to age-related cognitive decline (Foster, 1999). Decades ago, the standard Morris water maze task revealed impaired performances among some senescent rats (Gage et al., 1984, Rapp et al., 1987). More recent behavioral assessments have sought to increase task sensitivity to age-related cognitive decline (Kennard and Woodruff-Pak, 2011), so that the deficits and their underlying mechanisms can be better characterized and potentially manipulated. Here we employ a rapid water maze task sensitive to age-related cognitive decline to test whether daily exposure to an enriched environment can reverse the effects of age on hippocampal function concomitantly with hippocampal neurogenesis.
Neurogenesis is a striking form of neural plasticity that persists throughout life in the hippocampus and olfactory bulbs of all mammals investigated, including humans (Altman and Das, 1965, Cameron et al., 1993, Eriksson et al., 1998). Although the precise role that new neurons play in hippocampal integrity is debated, new neuron number in young animals generally correlates with their performance measures in hippocampus-dependent tasks (Deng et al., 2010 but see Epp and Galea, 2009). Manipulations that attenuate neurogenesis chronically associate with impaired performances (Shors et al., 2002, Madsen et al., 2003, Raber et al., 2004, Snyder et al., 2005, Saxe et al., 2006, Winocur et al., 2006) while those that potentiate neurogenesis associate with better performances (Ormerod et al., 2004, van Praag et al., 2005). Post-mortem signs of hippocampal neurogenesis in human patients who exhibited profound memory impairments are scarce (Crossen et al., 1994, Roman and Sperduto, 1995, Siffert and Allen, 2000, Correa et al., 2004, Monje et al., 2007, Coras et al., 2010).
Hippocampal neurogenesis declines with age in rodents primarily because neural progenitor cells (NPCs) become increasingly quiescent, although NPCs that do divide may be less likely to produce surviving neuronal progeny (Kuhn et al., 1996, Kempermann et al., 1997, Cameron and McKay, 1999, Lichtenwalner et al., 2001, Nacher et al., 2003, Hattiangady and Shetty, 2008). While several studies have related new neuron number and cognitive measures in aged rats (Lemaire et al., 2000, Drapeau et al., 2003, Driscoll et al., 2006, Drapeau et al., 2007), dogs (Siwak-Tapp et al., 2007), and non-human primates (Aizawa et al., 2009), the strength of this relationship among aged rats tested in the water maze varies. For example, new neuron number appears unrelated to the performances of aged rats in water maze tasks that distribute training across 8–10 days (Bizon and Gallagher, 2003, Merrill et al., 2003, Bizon et al., 2004) but related in protocols that mass training across 2–3 days (Drapeau et al., 2003, Driscoll et al., 2006). Moreover, new neuron survival in the hippocampi of aged rats is enhanced by their participation in early but not later trials of the distributed water maze protocol (Drapeau et al., 2007). These results suggest that the strength of the relationship between neurogenesis and water maze performance in aged rats may depend upon the speed of learning demanded by the task.
In aged rodents, daily exposure to environmental enrichment primarily stimulates neurogenesis by increasing the probability that new neurons survive to maturity (Kempermann et al., 1997, 1998, Kempermann et al., 2002, Segovia et al., 2006, Leal-Galicia et al., 2008) and improves the rapid acquisition of spatial information in a condensed water maze task (Kumar et al., 2012). Here we tested the hypothesis that daily exposure to environmental enrichment would reverse age-related impairments in rats’ abilities to rapidly acquire a spatial search strategy concomitantly with ongoing rates of neurogenesis.
2. Materials and Methods
2.1. Subjects
Young (5–8 mo) and aged (20–22 mo) sexually naïve male F344 rats obtained from the National Institute of Aging colony at Harlan Sprague Dawley (Indianapolis, IA) were treated in accordance with University of Florida and federal policies regarding the ethical use of animals for experimentation. Rats exhibiting signs of aggression (bites and scratches) or age-related health problems (poor grooming, hunching, excessive porphyrin secretion, weight loss and tumors) were euthanized humanely.
2.2. Differential experience: Environmental enrichment and individual housing
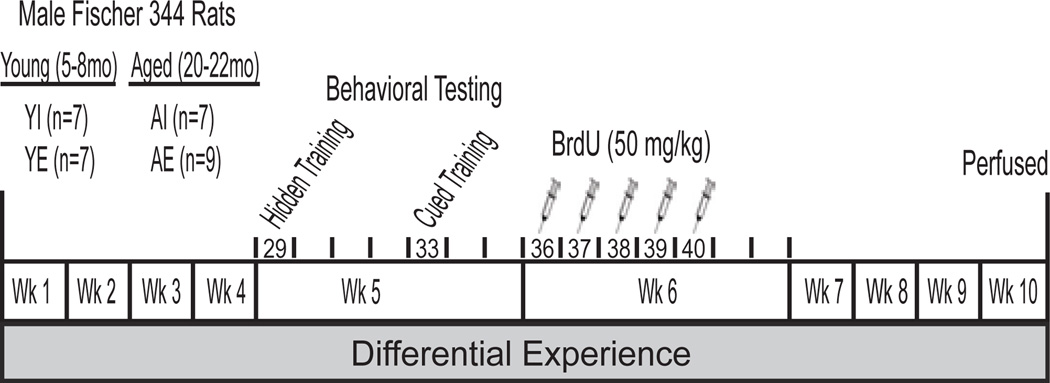
For the 10 week experiment, the rats were housed in a 12:12h light cycle with access to food and water ad libitum either individually (n=7 young [YI] and n=7 aged [AI]) or pair housed with 2–3h of access daily to an enriched environment (n=7 young [YE] and n=9 aged [AE]). The goal of the differential experience protocol was to provide opportunities for the enriched group to engage in a variety of hippocampus-dependent behaviors while limiting them for the individually housed group. The enriched environment consisted of a large wooden box, empty water maze tank or large wire cage containing assorted three-dimensional toys (e.g. plastic tubes, balls and various objects), food and water. The environment and toys were randomly rotated daily to maintain novelty. Daily exposure to this environment modifies hippocampal electrophysiology and facilitates the rapid acquisition of a spatial search strategy in aged rats (Foster and Dumas, 2001, Kumar et al., 2007, Kumar et al., 2011). Behavioral testing commenced in the 4th week of differential experience and BrdU injections commenced one week after behavioral testing was completed. The rats were perfused 4 weeks after the final BrdU injection to quantify neurogenesis (see Fig. 1).
Figure 1. Experiment timeline.
Rats were housed individually (n=7 young, n=7 aged) or in pairs and exposed to an enriched environment daily (n=7 young, n=9 aged) for ten weeks. In the 5th week, rats were trained and tested on hidden platform trials and then visible platform trials 3d later. Beginning one week after testing, rats were injected daily with BrdU (50mg/kg) over 5 days and then perfused 4 weeks later to quantify neurogenesis.
2.3. Water maze training and testing
A black water maze tank (1.7m d) filled with water (27±2°C) to a depth of 8cm below its rim was housed in a well-lit room. A Columbus Instruments tracking system recorded escape latencies (sec) and pathlengths (cm). Hidden and cued platform training consisted of 5 blocks (15min inter-block-interval) of 3–60s trials (20s inter-trial-interval) administered in a single session. This massed protocol is sensitive to both age-related cognitive decline (Foster et al., 2003, Foster and Kumar, 2007, Carter et al., 2009) and the effects of differential experience on cognition in aged rats (Kumar et al., 2012). Rats were dried between blocks.
2.3.1. Hidden platform trials
After 4 weeks of differential experience, rats were trained over a single session to locate a platform (29 cm d) hidden ~1cm below the water surface in the NE quadrant of the pool in the presence of highly visible extra-maze cues. Rats were first habituated to the pool by being given 3 opportunities to climb onto the platform from different directions. On the subsequent hidden platform trials, the rats were released randomly from N, S, W or E start locations and given 60s to locate the hidden platform before being guided.
2.3.2. Probe trial
A 60s free swim probe trial during which the platform was removed from the pool was conducted 15min after the last hidden platform training block. The rats were released from the quadrant opposite the goal quadrant and discrimination scores (t(G)−t(O)/t(G)+t(O), where t(O) is time spent in the opposite quadrant and t(G) is time spent in the goal quadrant), served as our strength of learning measure.
2.3.2. Cued trials
Three days after the hidden platform training session, rats were trained to locate the now flagged platform that protruded ~1.5 cm above the water in water maze tank now surrounded by a black curtain to mask distal cues. The rats were guided to the flagged platform if they failed to escape the maze within 60s. The N, S, E and W release points and the location of the flagged platform were changed on each trial.
2.4. Bromodeoxyuridine injections and histology
Bromodeoxyuridine (BrdU) was dissolved in fresh 0.9% sterile saline (20 mg/ml (w/v)) and injected intraperitoneally (2.5ml/kg or 50mg/kg) once per day over five days, starting one week after behavioral testing to minimize the well-known effects of learning on neurogenesis (Gould et al., 1999, Epp et al., 2010). This BrdU dose safely and effectively labels dividing NPCs in the hippocampus of young and aged adult rodents (Kolb et al., 1999, Cameron and McKay, 2001, Drapeau et al., 2003).
Four weeks after the final BrdU injection, the rats were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine (Webster Veterinary, Sterling, MA) and perfused transcardially with ice-cold isotonic saline and 4% paraformaldehyde (Electron Microscopy Sciences). By 4 weeks, many new cells express mature neuronal proteins and are relatively permanent (Cameron and McKay, 2001, van Praag et al., 2002). Extracted brains were stored overnight in perfusate, equilibrated in 30% sucrose (~ 4 days) at 4°C and then sectioned coronally through the rostral caudal extent of the hippocampal dentate gyrus at 40 µm intervals using a freezing stage sledge microtome (American Optical Corp). Sections were stored at −20°C in 30% ethylene glycol, 25% glycerin and 45% 0.1 M sodium phosphate buffer (v/v/v) until immunostained.
2.5. Immunohistochemistry
Free floating sections were stained immunohistochemically to quantify 28–32 day-old BrdU+ cells and confirm their neuronal or glial phenotypes as described previously (Palmer et al., 2000, Ormerod et al., 2004). Sections were washed repeatedly between steps in tris-buffered saline (TBS; pH 7.4).
2.5.1. Enzyme substrate immunostaining
BrdU+ cells were revealed enzymatically on every 12th section through the dentate gyrus of each rat and counted under light microscopy to estimate total new cell numbers (Fig. 3). Sections were incubated in 0.3% H2O2 for 10 min to quench endogenous peroxidase, rinsed in 0.9% NaCl and then incubated in 2M HCl for 20min at 37°C to denature DNA. The sections were then blocked in a solution of 3% normal donkey serum (NDS) and 0.1% triton-x in TBS and incubated overnight in blocking solution containing rat anti-BrdU (1:500; AbD Serotec, Raleigh, NC) at 4°C and then for 4h in biotinylated secondary anti-rat IgG (Jackson ImmunoResearch, West Grove, PA; 1:500) at RT. Next, the sections were incubated in avidin-biotin horseradish peroxidase (Vector Laboratories, Burlingame, CA) and then reacted in a solution of 0.02% 3,3’-diaminobenzidine tetrahydrochloride (DAB; Sigma Aldrich, St. Louis, MO) and 0.5% H2O2. Sections were mounted on glass slides, dried overnight, dehydrated in an alcohol series and then coverslipped under permount (Fisher Scientific).
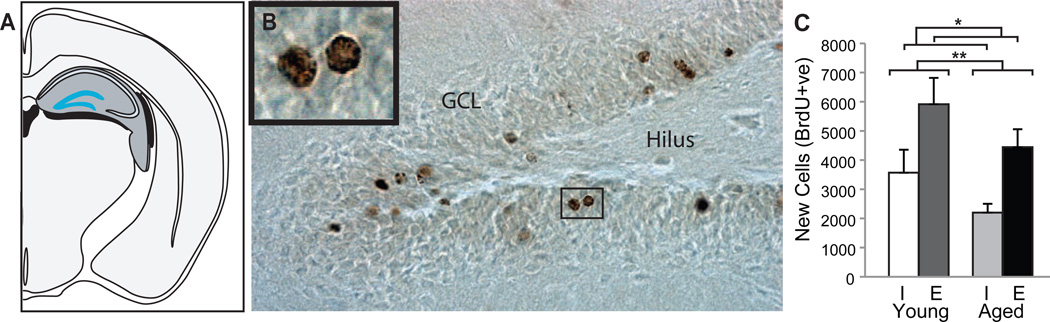
Figure 3. Exposure to an enriched environment reversed the effects of age on neurogenesis.
Rats were given five daily injections of BrdU (beginning one week after behavioral testing) and perfused 4 weeks later. The total number of new cells surviving 4 was estimated stereologically using BrdU+ cell counts obtained under light microscopy from every 12th section through the DG. The bar graph depicts group means (± S.E.M.) of total new cell number in the dentate gyri of YI (white bars), YE (dark gray bars), AI (light gray bars) and AE (black bars) rats. (A) Coronal view of the rat brain. The dentate GCL is highlighted in turquoise. (B) Photomicrograph of new (BrdU+) cells in the dentate gyrus of an aged rat. Representative examples of ~4 week-old cells labeled with BrdU+ (in brown) revealed enzymatically with DAB. (C) Total new cells numbers declined with age but were potentiated by enrichment, regardless of age. More new cells survived ~4 weeks in the dentate gyri of young versus aged and enriched versus individually-housed rats. *p≤0.05 and **p≤0.01.
2.5.2. Fluorescent immunostaining
The % of BrdU+ cells expressing neuronal or glial protein was quantified on sections that were immunostained using fluorescent secondary antibodies under confocal microscopy (Fig. 4). The sections were blocked in a solution of 3% NDS and 0.1% Triton-X in TBS and then incubated overnight at 4°C in blocking solution containing primary antibodies raised against the mature neuronal protein neuronal nuclei (mouse anti-NeuN, 1:500; Chemicon, Temecula, CA) and the immature neuronal protein doublecortin (goat anti-DCX, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) or the oligodendrocyte precursor marker chondroitan sulfate proteoglycan (rabbit anti-NG2, 1:500; Chemicon) and the astrocyte protein glial fibrillary acidic protein (chicken anti-GFAP, 1:750; EnCor Biotech, Alachua, FL). The next day, sections were incubated in maximally cross-adsorbed FITC-conjugated anti-mouse and Cy5-conjugated anti-goat secondary antibodies to reveal neurons or FITC-conjugated anti-rabbit and Cy5-conjugated anti-chicken secondary antibodies to reveal glia for 4h at RT (Jackson ImmunoResearch; 1:500). Sections were then fixed in 4% paraformaldehyde, rinsed in 0.9% NaCl, incubated in 2M HCl and then incubated overnight at 4°C in rat anti-BrdU (AbD Serotec; 1:500) and then Cy3-conjugated anti-rat secondary for 4h at RT the next day before being incubated in DAPI (Calbiochem, San Diego, CA; 1:10,000) for 10min and then mounted on glass slides under 2.5% diazobicyclooctane (in TBS with 10% polyvinyl alcohol and 20% glycerol).
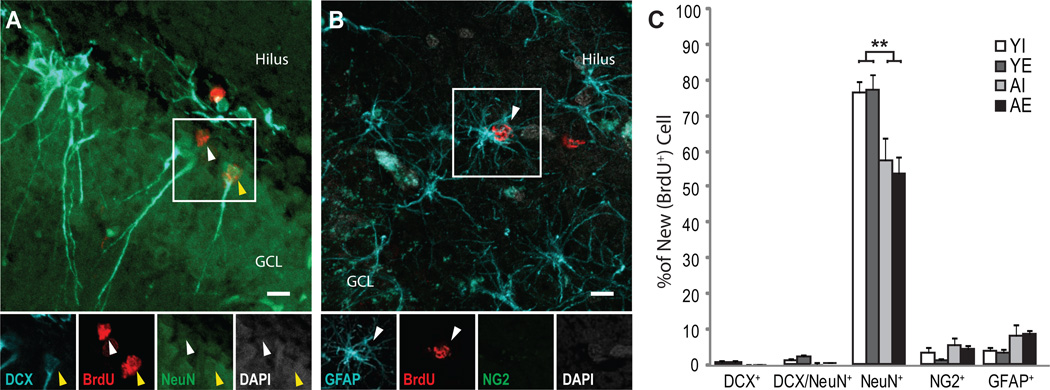
Figure 4. Fewer new cells expressed mature neuronal phenotypes in the dentate gyri of aged rats.
At least 100 BrdU+ cells per rat were examined under confocal microscopy (40 × objective with 2.3× digital zoom) to calculate proportions expressing markers of immature (DCX+), transitioning (DCX/NeuN+) or mature (NeuN+) neurons, as well as GFAP+ astrocytes or NG2+ oligodendrocyte precursors, which were revealed using fluorescent immunohistochemistry. (A,B) Confocal images of new neurons and astrocytes in the dentate gyrus of an adult rat. Representative images of ~4 week old BrdU+ cells (in red) that express the neuronal markers DCX (in blue) and/or NeuN (in green; A) or the glial markers GFAP (in blue) or NG2 (in green; B). (C) The proportion of new cells expressing neuronal phenotypes decreased with age and was unaffected by enrichment regardless of age. Mean (± S.E.M.) % of new cells in the dentate gyri of YI (white bars), YE (dark gray bars), AI (light gray bars) and AE (black bars) rats expressing neuronal and glial phenotypes are shown. In all rats, the majority of ~4 week-old cells expressed mature neuronal phenotypes. However, a lower % of new cells expressing mature neuronal phenotypes and a higher % of new cells expressing glial phenotypes was detected in aged versus young rats. No effect of differential experience on the % of new cells expressing either phenotype was detected in young or aged rats. *p≤0.05 and **p≤0.01.
2.6. Cell quantification
2.6.1. Total New Cell Number
BrdU+ cells distributed through the subgranular zones (SGZ) and granule cell layers (GCL; Fig. 3A) were counted on sets of every 12th section (9–10 sections per rat) through the rostral-caudal extent of the hippocampal dentate gyrus under a 40X objective on a Zeiss Axio Observer Z1 inverted microscope and optical fractionator principles (West et al., 1991, Kempermann et al., 2002). The first section of each rat’s set was randomly selected from the 1st–11th section of dentate gyrus. Because BrdU+ cells are typically distributed irregularly, we counted all new cells (mean [±S.E.M.] total BrdU+ cells: YI =297±66; YE=493±75; AI=183±25; AE=370±51), excluding obvious cell ‘caps’ that could represent cells in adjacent cell sections and multiplied that number by 12 (the section interval) to generate a stereological estimate of total cell number (see Fig. 3) without fractionating section thickness (Kempermann et al., 2002). Because age- or enrichment-related changes in BrdU+ cell nucleus diameter could affect cell estimates estimated this way, we confirmed that nuclear diameters of ~10–20 BrdU+ cells completely contained within one of these sections in 3–4 rats per group were consistent (YI=8.48±0.40µm, YE=8.19±0.13µm, AI=8.79±0.33µm and AE=8.79±0.22µm; age effect [F(1,9)=2.82], enrichment effect [F(1,9)=0.29], interaction effect [F(1,9)=0.28], all p values=n.s.). Because exposure to enriched environments can increase hippocampal volumes, we measured GCL and SGZ areas (in mm2) under a 20× objective using Axiovision software and then calculated volumes using a truncated cone formula that accurately predicts the volume of many biological regions (Uylings et al., 1986, Galea et al., 2000, Seifert et al., 2010): Volume = ⅓I (h1 + √h1 * √h2 + h2), where I is the distance between sections (480µm) and the two section areas for which volumes between are calculated are h1 and h2. Although neither age nor differential experience affected BrdU+ cell nuclei diameters, BrdU+ cells per mm2 as well as total cell estimates are reported because of expected effects of enrichment on dentate volumes.
2.6.2. New Cell Phenotypes
To determine whether new cells differentiated into neurons or glia, we examined at least 100 BrdU+ cells on quadruple fluorescent-stained sections (2–4 in young rats and 4–6 in aged rats) randomly selected from a set of every 12th section through the dentate gyrus for the co-expression of neuronal and glial proteins using a Zeiss meta LSM 710 fully spectral laser scanning confocal microscope (with 405, 488, 510, 543, and 633 laser lines) under a 40× objective (and 2.3× digital zoom). BrdU+ cells were considered co-labeled when a full “z-dimension” scan revealed its BrdU/DAPI+ nucleus was unambiguously associated with DCX and/or NeuN, NG2 or GFAP. The % of BrdU+ cells expressing each protein calculated (Fig. 4).
2.7. Statistical analyses
Statistical analyses were performed using STATISTICA software (StatSoft; Tulsa, OK). Analyses of variance (ANOVA) explored the effects age (young, aged) and experience (individually housed, enriched group-housed), on cognitive (latencies, pathlengths and probe trial discrimination index scores), health (body mass, swim speeds) and neurogenesis (new cell numbers, % and total new neurons and glia) measures and Newman Keuls post hoc tests revealed group differences. Chi square tests revealed the number of animals that performed at or above chance on probe trials and Pearson product moment correlations (r) tested relationships between neurogenesis and behavioral measures. The alpha level for all statistical tests was set at α=0.05.
3. Results
3.1. Daily enrichment partially reverses the effect of age on spatial ability
3.1.1. Enrichment enhances spatial learning in aged rats
Because measures of pathlength and latency over trials were correlated positively (r(29)=0.82; p<0.0001), we report only pathlengths to avoid redundancy. An ANOVA exploring the effects of age (young versus aged), training block (blocks 1–5) and differential experience (individually housed versus enriched) on pathlength (Fig. 2A) revealed significant effects of age (F(1,26)=15.65; p<0.001) and training block (F(4,104)=5.85; p<0.001) and significant age × environment (F(1,26)=5.57; p<0.05), age × training block (F(4,104)=4.94; p<0.001) and age × environment × training block (F(4,104)=3.24; p<0.05) interaction effects. All rats improved their performances across blocks (block 1>3–5 and 3>5; p values <0.005) but, as expected, young rats outperformed aged rats. Across all blocks, AI rats performed more poorly than AE rats (p<0.05) and young rats in either group (p’s<0.001) while YI and YE rats’ performances improved equally rapidly across training blocks (p’s>0.1). Specifically, AI rats performed significantly more poorly on training blocks 3, 4 and 5 (p’s<0.01) and AE rats performed significantly more poorly only on training block 5 relative to YI and YE rats (p’s<0.01).
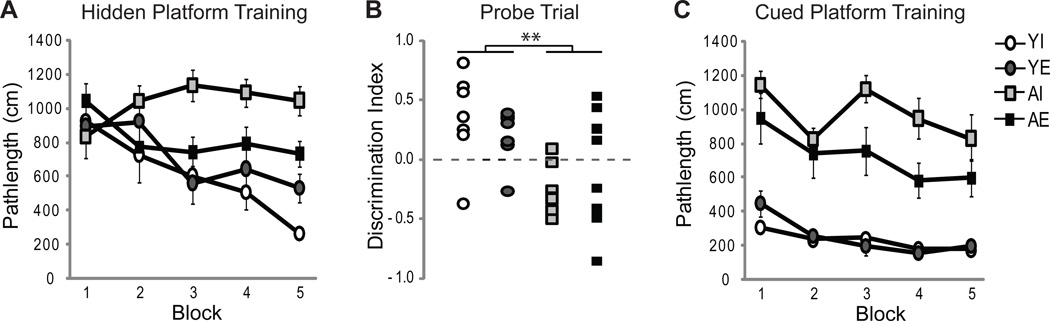
Figure 2. Exposure to an enriched environment subdues age-dependent impairments on hidden and visible platform trials.
The rats were trained on hidden platform trials (A), tested on a probe trial administered 15 min after the last hidden platform trial (B) and cued platform trials 3 days later (C). Line graphs depict group means (± S.E.M.) of measures obtained from the YI (white circles), YE (dark gray circles), AI (light gray squares) and AE (black squares) groups. (A) Enrichment enhanced the ability of aged rats to rapidly acquire a spatial search strategy. On all training blocks combined, young rats swam more directly to the hidden platform than aged rats. AI rats swam more circuitous routes to the hidden platform than either AE rats or young rats in either group. B) Probe trial DI scores varied by age and experience. Chi square tests confirmed that the % of rats that performed above or below chance (DI score = 0, dashed line) decreased with age but increased with exposure to enrichment. Specifically, 69% of enriched rats performed above chance whereas only 50% of individually housed rats performed above chance. (C) Previous experience influences performance on visible platform trials. Young rats outperformed aged rats on all training blocks, including the initial training block, likely because they retained procedural information from the spatial task and AE rats outperformed AI rats.
An ANOVA on swim speeds (mean ± [S.E.M.] cm/s = 26.06±1.07 [YI], 29.92±2.07 [YE], 19.45±0.94 [AI] and 18.68±1.64 [AE]) revealed a significant effect of age (F(1,26)=33.49; p<0.001) and a significant environment × training block interaction (F(4,104)=2.68; p<0.05). Young rats swam significantly faster than aged rats across all training blocks and enriched rats swam significantly faster during the first training block relative to all other training blocks (p’s<0.05). The effects of age on swim speed are unsurprising because aged rats were heavier than young rats (mean [± S.E.M.] g= 336.83±6.38 [YI], 347.37±4.44 [YE], 419.21±10.78 [AI] and 406.74±8.21 [AE]; F(1,26) = 78.87; p<0.001). The effect of enrichment on the performance of aged rats on hidden trials is likely related to cognition because enrichment neither affected swim speeds nor body mass in aged rats.
An ANOVA exploring the effects of age, training block, and environment on the percent of time spent in the outer annulus during hidden platform trials (mean [± S.E.M.] % = 56.07±4.47 [YI], 43.00±4.15 [YE], 81.98±0.92 [AI] and 64.32±3.13 [AE]) revealed significant effects of age (F(1,26)=46.63; p<0.0001), environment (F(1,26)=19.76; p<0.0001), training block (F(4,104)=3.32; p<0.05) and a significant age × training block interaction effect (F(4,104)=3.95; p<0.01). Overall, the % of time spent in the outer annulus significantly decreased in young versus aged rats, in enriched versus individually housed rats and on later versus earlier training blocks (block 1–2>5). While all young rats decreased their time spent in the outer annulus across blocks (block 1 and 2>3–5; p’s<0.01), aged rats maintained their time across blocks (p’s>0.78).
An ANOVA revealed a significant effect of age on probe trial discrimination index scores (F(1,26)=8.40, p<0.01) but no effect of environment (Fig. 2B). Because one YI and one YE rat performed at chance (i.e. discrimination index = 0) and only one AI rat performed above chance, Chi square tests on the % rats performing above or below chance were employed to confirm effects of age (χ2 = 78.55, p < 0.0001) and differential experience (χ2 = 14.44, p < 0.0005), with 69% of enriched rats performing above chance and only 50% of individually housed rats performing above chance. The effect of differential experience was mainly due to an effect of environmental enrichment observed in aged rats (χ2 = 71.59, p<0.0005). Taken together, these data confirm that although young rats outperformed aged rats, enrichment enhanced the ability of aged rats to rapidly acquire a spatial search strategy.
3.1.2. Enrichment enhances cue discrimination learning in aged rats
An ANOVA exploring the effects of age, training block, and environment on pathlength for the cued discrimination task revealed significant effects of age (F(1,26)=62.37; p<0.001) and training block (F(4,104)=9.92; p<0.001) but no effect of enrichment. Post-hoc tests confirmed that young rats swam more directly to the visible platform than aged rats but that all groups exhibited improved performances across training blocks (block 1>2>3, 4 and 5; p’s≤0.05). An ANOVA across blocks within each age and treatment group indicated a significant effect of training in three groups (YE: F(4,24)=5.68; p<0.005; AE: F(4,32)=3.66; p<0.05; AI: F(4,24)=3.28; p<0.05), with a tendency (p=0.07) for a training effect in YI animals. This tendency was due, in part, to near asymptotic performance on the first training block. Indeed, young rats exhibited shorter pathlengths on the first block of cue training relative to the first block of spatial training (Figs. 2A and C) indicating a carry-over effect of prior training on the spatial task. Finally, age tended to interact with environment (p<0.10) and post hoc tests indicated that AE rats swam shorter pathlengths than AI rats (p=0.05; Fig. 2C).
An ANOVA exploring the effects of age, training block, and environment on average swim speed across visible platform trials (YI=27.88±1.04cm/s, YE=26.28±1.33cm/s, AI=20.69±1.10cm/s, and AE=22.74±1.31cm/s) confirmed that young rats swam significantly faster than aged rats (F(1,26)=18.82; p<0.001), but no there was no effect of differential experience. Age tended to interact with training block (F(4,104)=2.32; p=0.062) such that young rats increased their swim speeds (block 1<4–5; p’s<0.05, while aged rats maintained their slower swim speeds (p’s>0.74) across all blocks.
An ANOVA exploring the effects of age, training block, and environment on the % time spent in the outer annulus during cued platform training (YI=36.00±2.94%, YE=28.90±2.94%, AI=75.14±4.08% and AE=64.15±4.69%) revealed significant effects of age (F(1,26)=88.64; p<0.0001) and differential experience (F(1,26)=5.24; p<0.05). Less time was spent in the outer annulus by young versus aged rats and by enriched versus individually housed rats. While all rats decreased the time they spent in the outer annulus across blocks (F(4,104)=12.57; p<0.0001; training blocks 1and 2>3–5; p’s<0.05), young rats ventured from the maze wall in early trials (block 1>2>3–5; p’s<0.001) whereas aged rats ventured from the wall only in later training blocks (block 3>4,5; p’s<0.05; age × block interaction effect: F(4,104)=8.18; p<0.001).
3.2. The effect of enrichment overcomes the effect of age on neurogenesis
3.2.1 Enriched environment reverses the effect of age on total new cell number
An ANOVA revealed significant effects of age (F(1,26)=4.26; p<0.05) and environment (F(1,26)=11.14; p<0.01) on the total number of new (BrdU+) cells produced and/or surviving 4 weeks in young and aged rats (Fig. 3). More new cells were found in the dentate gyri of young versus aged rats and in enriched versus individually housed rats (Fig. 3C). Enrichment similarly increased the number of new cells in the dentate gyri of both young and aged rats by ~2,300. This effect of enrichment appears robust because we found similarly increased new cell densities (mean ±S.E.M. = enriched: 1345.13±204.12 cells/mm3 versus individually housed: 881.10±156.82 cells/mm3, F(1,26)=5.83; p<0.05) despite increased GCL volumes (enriched=3.97±0.31mm3 vs. individually housed=3.25±0.17mm3; F(1,26)=7.81; p<0.01). Neither cell density nor GCL volume was affected by age or the interaction between age and environment.
3.2.2. Enriched environment does not reverse the effect of age on neuronal differentiation
We calculated the proportion of BrdU+ cells that co-expressed markers for immature (DCX+), transitioning (DCX/NeuN+), or mature (NeuN+) neurons, or GFAP+ astrocytes, or NG2+ oligodendrocyte precursors (Fig. 4A–C). An ANOVA exploring the effects of age and environment on the % of new cells expressing each phenotype revealed significant effects of age (F(1,26)=7.99; p<0.01) and phenotype (F(1,104)=532.30; p<0.001) and a significant age × phenotype interaction effect (F(1,104)=17.18; p<0.001; Fig. 4D). Consistent with the extended survival period of the study, the majority of new cells expressed mature neuronal phenotypes (p’s<0.0001 vs. all other phenotypes). Of the < 10% of BrdU+ cells expressing glial or immature neuronal phenotypes, astrocyte phenotypes were expressed most frequently (p’s<0.01 vs. immature and transitioning neurons). Significantly fewer BrdU+ cells expressed mature neuronal phenotypes in aged versus young rats (p<0.0001) and this effect was not reversed by enrichment. In fact, a higher proportion of BrdU+ cells in aged versus young rats did not co-express the markers of differentiation employed in this study (YI=13.80±3.79, YE=15.94±5.07, AI=27.85±8.10, AE=33.05±4.39 % BrdU+ cells; F(1,26)=8.00; p<0.01).
3.2.3 Enriched environment increases net neurogenesis
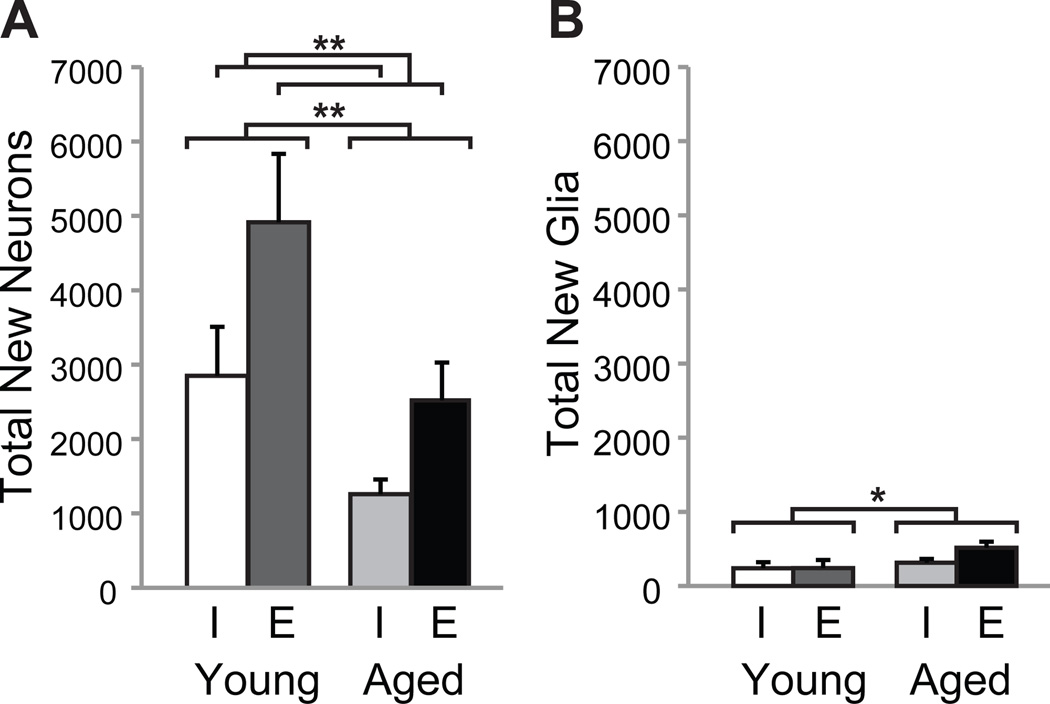
We next determined the total number of new neurons (immature, transitioning and mature neurons combined) and new glia (oligodendrocytes and astrocytes) by multiplying the estimated total number of BrdU+ cells by the proportion of BrdU+ cells co-expressing each phenotype (Fig. 5). An ANOVA exploring the effects of age and environment on total new neuron number revealed statistically significant effects of age (F(1,26)=10.32; p<0.01) and environment (F(1,26)=7.18; p<0.05). More new neurons were found in the dentate gyri of young versus aged rats and in enriched versus individually-housed rats (Fig. 5A). Importantly, no age × environment interaction was observed indicating that enrichment increased net neurogenesis similarly in young and aged rats. An ANOVA exploring the effects of age and environment on total new glia revealed a statistically significant effect only for age (F(1,26)=4.26; p=0.05), such that more new glia (primarily astrocytes) were found in the dentate gyri of aged versus young rats (Fig. 5B). However, the reliability of this effect requires replication in future work because of the low frequency with which new glia were observed.
Figure 5. Net neurogenesis declines with age but is increased by exposure to enrichment whereas age-dependent increases in gliogenesis are unaffected by enrichment.
Net neurogenesis and gliogenesis was calculated by multiplying total new cell numbers (Fig. 3) by % of new cells expressing neuronal and glial phenotypes (Fig. 4), respectively. The bar graphs depict group mean (± S.E.M.) numbers of neurons (A) or glia (B) in the dentate gyri of YI (white bars), YE (dark gray bars), AI (light gray bars) and AE (black bars) rats. (A) Net neurogenesis declines with age but increases with enrichment, independent of age. Neurogenesis declined with age and was potentiated by exposure to an enriched environment regardless of age. A few weeks of exposure to an enriched environment, therefore, returned levels of hippocampal neurogenesis in aged rats to those observed in young individually-housed rats. (B) Age-dependent increases in gliogenesis are unaffected by exposure to enrichment. We detected a small but significant increase in gliogenesis in aged versus young rats that was unaffected by differential experience. *p≤0.05 and **p≤0.01.
3.3. Higher rates of neurogenesis relate to better water maze performances in aged rats
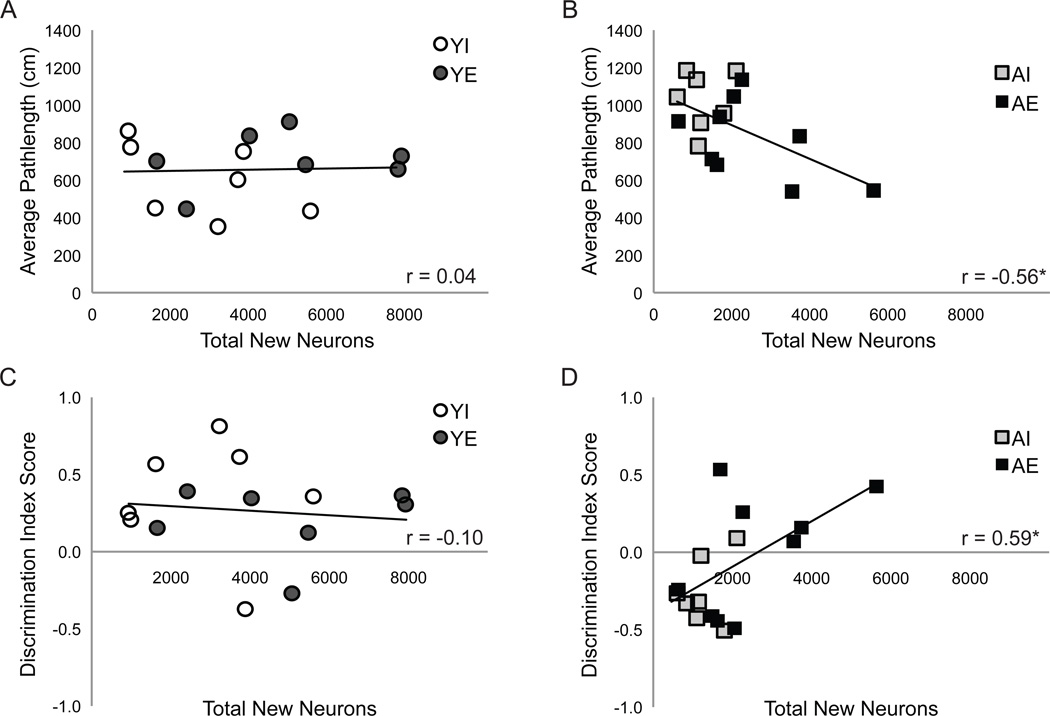
Pearson product-moment correlations were employed to measure the relationships between ongoing neurogenesis and measures of water maze performance (mean pathlength and discrimination index) in each age group. Note that longer pathlengths on hidden platform trials are indicative of more circuitous routes and therefore poorer performance whereas higher discrimination index scores indicate better discrimination between the target and opposite quadrants on probe trials and therefore better performance. New neuron number correlated significantly with average pathlength across hidden platform trials (r=−0.56, p<0.05 Fig. 6B) and probe trial discrimination index scores (r=0.59, p<0.05; Fig. 6D), in aged but not young rats.
Figure 6. New neuron number correlates with measures of spatial ability.
Graphs depict total neuron number plotted against mean pathlengths across hidden platform training blocks (A and C) or against probe trial discrimination index scores (B and D) for YI (white circles), YE (dark gray circles), AI (light gray squares) and AE (black squares) rats. Mean pathlengths correlated negatively with total new neuron number in aged rats (r=−0.56; A). Note that shorter pathlengths indicate better performances. (D) The number of new neurons and discrimination index score are correlated positively in aged rats (r=0.59). * p ≤ 0.05.
4. Discussion
In the current study, we confirmed that hippocampal neurogenesis and spatial learning are compromised by age and that exposure to environmental enrichment potentiates neurogenesis, regardless of age. We found that environmental enrichment improves the performances of aged but not young rats on a water maze task in which the hidden platform location is learned in a single day. We propose that this task requires the ability to rapidly acquire and flexibly use spatial information that appears intact and therefore unaffected by enrichment in young rats but compromised and improved by enrichment in aged rats. We also reveal a novel age-specific relationship between total new neuron number and indices of ability in a rapid water maze task.
Decreased hippocampal neurogenesis is characteristic of aging (Kuhn et al., 1996, Cameron and McKay, 1999, Nacher et al., 2003). Although our single experiment endpoint cannot disentangle the effects of age on NPC proliferation versus new cell survival, our effect is consistent with the well-known effects of age on NPC proliferation. Fewer BrdU+ cells expressed neuronal markers and more BrdU+ cells were devoid of differentiation markers in the hippocampi of aged versus young rats, which is consistent with some reports that neuronal differentiation is compromised by age (Kempermann et al., 1998) Our finding that gliogenesis increased with age has been noted by others (Bizon et al., 2004) but because so few new glia were detected in the dentate gyri of either young or aged rats, the reliability of this effect should be tested in future work. Overall, our findings support published work showing age-related decreases in neurogenesis are mediated by increasing NPC quiescence across life and because fewer NPC progeny adopt neuronal fates.
Environmental enrichment increases neurogenesis in aged rodents by potentiating neuronal differentiation and new cell survival (Kempermann et al., 1998, Kempermann et al., 2002, Segovia et al., 2006, Leal-Galicia et al., 2008). Indeed, we found similar enrichment-induced increases in the number of new cells surviving 4–5 weeks in the dentate gyri of young and aged rats (Fig. 3). However, enrichment neither reversed the effects of age on the proportion of BrdU+ cells that expressed neuronal phenotypes nor increased the proportion in young rats (Fig. 4). Other studies showing that exposure to enriched environments potentiates neuronal differentiation in young and aged have employed running wheels, larger social groups and earlier more extended exposures to enriched environments, which could each potentiate different components of neurogenesis and probably each require more detailed investigation (Lazarov et al., 2010, Lugert et al., 2010). Overall, we show that just a few weeks of exposure to environmental enrichment can increase net neurogenesis (Fig. 5) in the hippocampus of aged rats by robustly enhancing new cell survival to the extent that it overcomes the effects of age on NPC proliferation (Fig. 3) and neuronal fate choice (Fig. 4).
We expanded upon work showing that exposure to environmental enrichment enhances the ability of aged rats to discriminate the spatial location of a platform hidden in water maze tasks that distribute training across days (Frick and Fernandez, 2003, Fernandez et al., 2004, Lores-Arnaiz et al., 2006) by confirming that it also enhances their ability to discriminate a platform spatially (Fig. 1A and B; Kumar et al., 2012) and visually (Fig. 1C) in a water maze task that masses training sessions into a single day. We did not observe the anticipated beneficial effect of enrichment on water performances in our young rats (Schrijver et al., 2002, Leggio et al., 2005). However, the effects of weeks’ rather than longer exposures to enrichment on spatial ability may be gender-dependent and only observable in water maze protocols that distribute training across days (for example (Frick et al., 2003, Harburger et al., 2007). The rapidly asymptotic performances of YE and YI rats across hidden platform training blocks is consistent with the notion that the rapid water maze task may be sensitive to performance impairments but not enhancements in young rodents and precludes a meaningful evaluation of the relationship between their measures of neurogenesis and spatial ability.
Our data showing that AE and AI rats exhibited similar anxiety levels (% time spent in the outer annulus), fitness (swim speeds and body mass) and perhaps visual acuity (similar performances were exhibited on early visible platform blocks), suggests that enrichment reverses age-related changes in systems mediating spatial and visual discrimination, independent of overt effects on sensorimotor ability. Exposure to enrichment improves cerebellar, in addition to hippocampal function (Greenough and Volkmar, 1973, Camel et al., 1986, Kumar et al., 2012), which could improve both spatial and visual discrimination. In addition, our unpublished data and previous research (Gerlai, 2001, Ormerod and Beninger, 2002) suggests that training on sequential tasks (including spatial versus visual discrimination) may beneficially or detrimentally affect performance on the second task. Indeed, young rats appeared to readily employ procedural information they acquired on spatial discrimination trials about escaping the water maze on early visual discrimination blocks (Fig. 2A versus 2C).
Exposure to an enriched environment produces many effects in the hippocampus that could relate to improved spatial discrimination in aged rats. For example, exposure to an enriched environment increases hippocampal and vascular volumes as well as morphological and electrophysiological measures of plasticity in aged rats (Leventhal et al., 1999, Palmer et al., 2000, Hattiangady and Shetty, 2008, Kumar et al., 2012). In support of other work employing water maze protocols with massed training schedules (Drapeau et al., 2003, Driscoll et al., 2006), measures of neurogenesis and spatial ability correlated strongly (Fig. 6). This rapid task may be more sensitive to the relationship than distributed training water maze protocols (Bizon and Gallagher, 2003, Merrill et al., 2003, Bizon et al., 2004) because it taxes the hippocampus by requiring faster acquisition and more flexible use of spatial information (Foster, 2012). We also may have simply increased the variability within our measures enough to detect the relationship by exposing aged rats to differential experience.
We cannot conclude that neurogenesis mediates spatial ability from our correlational data. However, our data do suggest that neurogenesis may be a marker of spatial ability and hippocampal integrity in aged rats because aged rats with higher ongoing rates of neurogenesis exhibited better spatial ability than those with lower rates. Indeed, environmental enrichment increases the expression of factors associated with enhanced spatial ability and neurogenesis, such as brain-derived neurotrophic factor (Lee et al., 2002, Obiang et al., 2011) and stimulates the production of factors that are down-regulated with age and are known to be neurogenic, such as fibroblast growth factor-2, vascular endothelial growth factor and insulin growth factor-1 (Shetty et al., 2005). Our data do suggest that future work investigating the relationship between neurogenesis and hippocampal function across age may provide insight into the etiology and potential interventions for age-related cognitive decline.
In summary, we found that several weeks of daily exposure to an enriched environment partially reverses the effects of age on the rapid acquisition of a spatial search strategy in the water maze, potentially through its effects on neurogenesis because we found higher ongoing rates of neurogenesis in aged rats that exhibited better performances in the task. Our data suggest that engaging in mentally and physically stimulating activity could reverse some aspects of age-related cognitive decline perhaps by potentiating neurogenesis.
Acknowledgements
The authors thank Melissa Ferguson, Prasanna Durairaj, Jose Herrera, and Vijay Parekh for technical assistance and Dr. Gerry Shaw for his gift of chicken anti-GFAP. The study was supported by grants from the National Institutes of Health (AG014979, AG036800, and AG037984) to TCF, the McKnight Brain Research Foundation to TCF and BKO, and the Broad Foundation for Biomedical Research to BKO. RBS was a National Science Foundation Graduate Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that all research reported in this manuscript was conducted in the absence of commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58:403–407. doi: 10.1538/expanim.58.403. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Camel JE, Withers GS, Greenough WT. Persistence of visual cortex dendritic alterations induced by postweaning exposure to a "superenriched" environment in rats. Behav Neurosci. 1986;100:810–813. doi: 10.1037//0735-7044.100.6.810. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. NatNeurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. The journals of gerontology. 2009;64:850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, Buchfelder M, Stefan H, Beck H, Steindler DA, Blümcke I. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133:3359–3372. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proc Natl Acad Sci U S A. 2009;106:2927–2932. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Epp JR, Galea LA. Hippocampus-dependent strategy choice predicts low levels of cell proliferation in the dentate gyrus. Neurobiol Learn Mem. 2009;91:437–446. doi: 10.1016/j.nlm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Epp JR, Haack AK, Galea LA. Task difficulty in the Morris water task influences the survival of new neurons in the dentate gyrus. Hippocampus. 2010;20:866–876. doi: 10.1002/hipo.20692. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fernandez CI, Collazo J, Bauza Y, Castellanos MR, Lopez O. Environmental enrichment-behavior-oxidative stress interactions in the aged rat: issues for a therapeutic approach in human aging. Ann N Y Acad Sci. 2004;1019:53–57. doi: 10.1196/annals.1297.012. [DOI] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca(2+) channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Dumas TC. Mechanism for increased hippocampal synaptic strength following differential experience. J Neurophysiol. 2001;85:1377–1383. doi: 10.1152/jn.2001.85.4.1377. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. Spatial working memory and hippocampal size across pregnancy in rats. Horm Behav. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Lambert TJ, Frick KM. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behav Brain Res. 2007;185:43–48. doi: 10.1016/j.bbr.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. JNeurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard JA, Woodruff-Pak DS. Age sensitivity of behavioral tests and brain substrates of normal aging in mice. Front Aging Neurosci. 2011;3:9. doi: 10.3389/fnagi.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Pedersen B, Ballermann M, Gibb R, Whishaw IQ. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J Neurosci. 1999;19:2337–2346. doi: 10.1523/JNEUROSCI.19-06-02337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. JNeurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee W, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2012;33:828.e821–828.e817. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thinschmidt JS, Foster TC, King MA. Aging effects on the limits and stability of long-term synaptic potentiation and depression in rat hippocampal area CA1. J Neurophysiol. 2007;98:594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Galicia P, Castaneda-Bueno M, Quiroz-Baez R, Arias C. Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem. 2008;90:511–518. doi: 10.1016/j.nlm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lores-Arnaiz S, Bustamante J, Arismendi M, Vilas S, Paglia N, Basso N, Capani F, Coirini H, Costa JJ, Arnaiz MR. Extensive enriched environments protect old rats from the aging dependent impairment of spatial cognition, synaptic plasticity and nitric oxide production. Behav Brain Res. 2006;169:294–302. doi: 10.1016/j.bbr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Obiang P, Maubert E, Bardou I, Nicole O, Launay S, Bezin L, Vivien D, Agin V. Enriched housing reverses age-associated impairment of cognitive functions and tPA-dependent maturation of BDNF. Neurobiol Learn Mem. 2011;96:121–129. doi: 10.1016/j.nlm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Beninger RJ. Water maze versus radial maze: differential performance of rats in a spatial delayed match-to-position task and response to scopolamine. Behav Brain Res. 2002;128:139–152. doi: 10.1016/s0166-4328(01)00316-3. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73:209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- Segovia G, Yague AG, Garcia-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res Bull. 2006;70:8–14. doi: 10.1016/j.brainresbull.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Zheng Z, Ormerod BK, Cohn MJ. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat Commun. 2010;1:23. doi: 10.1038/ncomms1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffert J, Allen JC. Late effects of therapy of thalamic and hypothalamic tumors in childhood: vascular, neurobehavioral and neoplastic. Pediatr Neurosurg. 2000;33:105–111. doi: 10.1159/000028985. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG, Hofman MA. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods. 1986;18:19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]