Abstract
Evidence suggests the muscle mechanoreflex, a circulatory reflex that raises blood pressure and heart rate (HR) upon activation of mechanically sensitive afferent fibers in skeletal muscle, is overactive in hypertension. However, the mechanisms underlying this abnormal reflex function have yet to be identified. Sensory input from the mechanoreflex is processed within the nucleus tractus solitarius (NTS) in the medulla oblongata. Within the NTS, the enzymatic activity of nitric oxide synthase (NOS) produces nitric oxide (NO). This centrally-derived NO has been shown to modulate muscle reflex activity and serves as a viable candidate for mediating the mechanoreflex dysfunction that develops in hypertension. We hypothesized that mechanoreflex dysfunction in hypertension is mediated by abnormal alterations in NO production in the NTS. Mechanically sensitive afferent fibers were stimulated by passively stretching hindlimb muscle before and after blocking the endogenous production of NO within the NTS via microdialysis of the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 1 and 5 mM) in normotensive Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Changes in HR and mean arterial pressure (MAP) in response to stretch were significantly larger in SHR compared to WKY prior to L-NAME dialysis. Attenuating NO production via L-NAME in normotensive rats recapitulated the exaggerated cardiovascular response to stretch observed in SHR. Dialyzing L-NAME in SHR further accentuated the increases in HR and MAP elicited by stretch. These findings support the contention that reductions in NO production within the NTS contribute to the generation of abnormal cardiovascular control by the skeletal muscle mechanoreflex in hypertension.
Keywords: blood pressure, heart rate, exercise
INTRODUCTION
In many cases, hypertensive individuals experience exaggerated increases in mean arterial pressure (MAP), heart rate (HR), and systemic vascular resistance during both static and dynamic exercise (Aoki et al., 1983; Pickering, 1987; Seguro et al., 1991; Delaney et al., 2010). This augmented circulatory response to physical activity is potentially dangerous as it may increase the risk for stroke, arrhythmia, and/or myocardial infarction during or immediately after exercise (Pickering, 1987; Hoberg et al., 1990; Mittleman et al., 1993; Mittleman & Siscovick, 1996; Kokkinos et al., 2002). As a result, understanding the mechanism(s) responsible for generating this cardiovascular hyperexcitability during exercise in hypertension is important and clinically relevant.
To this end, recent evidence from a rat model of human hypertension suggests that the exercise pressor reflex (EPR) contributes significantly to the exaggerated cardiovascular response to exercise over a wide range of work intensities (Smith et al., 2006; Mizuno et al., 2011). The EPR is a neural drive originating in skeletal muscle that elicits an increase in MAP and HR when skeletal muscle contracts primarily by increasing sympathetic nerve activity and, to a lesser extent, by withdrawing parasympathetic nerve activity (McCloskey & Mitchell, 1972; Mitchell et al., 1983; Mark et al., 1985). EPR activity is determined by two distinct components: the muscle metaboreflex and muscle mechanoreflex (Kaufman et al., 1983). The metaboreflex signals a mismatch between oxygen supply and demand to working muscle. It is stimulated when by-products of skeletal muscle metabolism activate predominantly unmyelinated group IV afferent fibers, which are primarily chemically sensitive (McCloskey & Mitchell, 1972; Kaufman et al., 1982). This component of the EPR has been recently implicated as a significant contributor to the abnormally accentuated cardiovascular response to exercise in hypertensive humans (Sausen et al., 2008; Delaney et al., 2010). The second component of the EPR, the muscle mechanoreflex, is activated during muscle contraction by mechanical stimuli such as pressure and stretch (Stebbins et al., 1988; Nobrega et al., 1994). Stimulation of the mechanoreflex is mediated primarily by activation of mechanoreceptors predominantly associated with thinly-myelinated group III afferent neurons terminating in collagen tissue between skeletal fibrocytes (McCloskey & Mitchell, 1972; Kaufman et al., 1983; Andres et al., 1985). Recent evidence from our laboratory derived from studies in rats suggests that the abnormally exaggerated EPR activity manifest in hypertension is likewise mediated by the muscle mechanoreflex (Leal et al., 2008; Mizuno et al., 2011). The current study focuses on this component of the EPR as the mechanisms underlying mechanoreflex overactivity in hypertension remain undetermined.
Second order dorsal horn neurons, presumptively receiving input from mechanically sensitive afferent fibers associated with the muscle mechanoreflex, project to the nucleus tractus solitarius (NTS) in the medulla oblongata (Potts et al., 2002). Functional, electrophysiological and neuroanatomical evidence suggests that the NTS is a major sensory nucleus and a central site of cardiovascular regulation during adaptive behaviors such as exercise (Kalia et al., 1981; Person, 1989; Toney & Mifflin, 1995). While many neurotransmitters and neuromodulators within the NTS may be involved in processing muscle reflex input, current research has established a modulatory role for nitric oxide (NO) (Li & Potts, 2001). Within the NTS, L-arginine is oxidized by nitric oxide synthase (NOS) to produce NO and L-citrulline (Moncada & Higgs, 1993). This centrally-produced NO has been shown to affect muscle reflex function. For example, it has been demonstrated that increases in MAP caused by activation of muscle reflexes are attenuated when NO production is experimentally increased in the NTS of normotensive cats (Smith et al., 2005a). These experiments suggest that NO contributes to the regulation of muscle reflex activity in the central nervous system.
Given the role of NO within the NTS in processing sensory input from skeletal muscle afferent fibers, impairment in the L-arginine-NO pathway in the NTS is a plausible candidate for the development of muscle mechanoreflex dysfunction in hypertension. As NO has been shown to buffer muscle reflex induced increases in MAP in healthy animals (Smith et al., 2005a), it is logical to suggest that a decrease in its production would reduce this buffering capacity resulting in the exaggerated cardiovascular response observed in hypertension. Therefore, we hypothesized that mechanoreflex dysfunction in hypertension is mediated by abnormal alterations in NO production in the NTS. To test this hypothesis, we performed microdialysis in the NTS of normotensive Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats to block endogenous NO production while stimulating mechanically sensitive afferent fibers by passively stretching hindlimb skeletal muscle.
METHODS
Ethical Approval
Experiments were performed in 20 SHR and 25 WKY age-matched (14–20 weeks old) male rats (Harlan, Indianapolis, IN). Animals were housed in standard rodent cages on 12-h light-dark cycles and were given food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas at Southwestern Medical Center at Dallas. In addition, all studies were conducted in accordance with the United States Department of Health and Human Services National Institutes of Health Guide for the Care and Use of Laboratory Animals and are in accordance with the policies of Experimental Physiology.
General Surgical Procedures
Rats were anesthetized with isoflurane gas (2–3%) in pure oxygen, intubated, and mechanically ventilated (Harvard Apparatus) for the duration of the experiment. To minimize edema, 0.15 mg dexamethasone was given intramuscularly in the left hindlimb. Both carotid arteries and a jugular vein were catheterized (PE-50, polyethylene tubing) to record blood pressure as well as to administer fluids. To maintain fluid balance and stabilize the animals, 1 M NaHCO3, 5% dextrose Ringers solution was infused intravenously at a rate of 2 mL/hr when necessary. In addition, arterial blood gases and pH were measured throughout experimentation using an automated blood gas analyzer (50 µL blood samples; Model ABL 5, Radiometer) to ensure variables were maintained within physiological ranges (arterial PO2 of >80 Torr; arterial PCO2 of 35–45 Torr; pH, 7.3–7.4). Body temperature was maintained between 36.5 and 38.0°C by an isothermal pad (Deltaphase). Animals were held in a stereotaxic head unit (Kopf Instruments) and a pre-collicular decerebration was performed. Briefly, holes were drilled into the parietal skull and the bone superior to the central sagittal sinus removed. The dura mater was cut and the cerebrum aspirated. Once the superior and inferior colliculi were within view, a pre-collicular section was made and the forebrain aspirated rendering the animal insentient. To minimize bleeding, small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the internal skull surface and the cranial cavity was packed with cotton. Immediately after decerebration, gas anesthesia was discontinued and the animal was allowed to stabilize for one hour.
Activation of Mechanically Sensitive Afferent Fibers in Skeletal Muscle
The pelvis of the rat was stabilized with steel posts within a customized stereotaxic frame and the right hindlimb was fixed in one position using clamps attached to the tibial bone. The gastrocnemius and soleus muscles were isolated, and the calcaneal bone cut. The Achilles’ tendon was connected to a force transducer (Grass Instruments, FT10) for the measurement of muscle tension. Afferent fibers associated with the muscle mechanoreflex were then selectively activated by passively stretching the gastrocnemius and soleus muscles of the hindlimb. This technique has been shown to be a valid procedure to preferentially stimulate mechanically sensitive afferent fibers (Stebbins et al., 1988).
Microdialysis Procedures
Animals were held in a stereotaxic head unit (Kopf Instruments). A limited occipital craniotomy was performed to expose the dorsal surface of the brainstem and a microdialysis probe (Bioanalytical Systems, model CMA 11, 0.24 mm outer diameter, 1 mm membrane tip) was stereotaxically positioned unilaterally within the NTS in the area known to receive projections from mechanically sensitive muscle afferent fibers in rats (coordinates: 0.0 mm rostro-caudal to the calamus scriptorius, 0.5 mm lateral to the calamus scriptorius and 0.5 mm below the dorsal medullary surface) (Paxinos & Watson, 1986; Potts et al., 2002). Probes were continuously perfused at a rate of 2.5 µl/min with artificial cerebral spinal fluid (aCSF) buffered to a pH of 7.4. The aCSF contained 0.2 % bovine serum albumin, 0.1 % bacitracin, and the following ions (in mM): 6.2 K+, 134 Cl−, 2.4 Ca2+, 150 Na+, 1.3 P−, 13 HCO3− and 1.3 Mg2+. After the probe was inserted, the preparation was allowed to stabilize for a minimum of one hour.
Experimental Protocols
The cardiovascular response to activation of mechanically sensitive afferent fibers was obtained during the individual administration of aCSF (pre/control), during either 1mM L-NAME (WKY, n=10; SHR, n=5) or 5 mM L-NAME (WKY, n=10; SHR, n=10), and during repeat administration of aCSF (post/recovery). To assess whether blockade of NO production exhibited a graded effect, two doses of L-NAME were utilized. In these experiments, the microdialysis probe was placed ipsilateral to the stretched muscle. The aCSF and L-NAME were dialyzed a minimum of 45 minutes before stretching the muscle. Using a calibrated 9.5 mm rack and pinion system (Harvard Apparatus), preferential activation of mechanically sensitive afferent fibers was induced by passively stretching the gastrocnemius and soleus muscles of the hindlimb for 30 seconds. The amount of developed hindlimb muscle tension during passive stretch was matched to that known to be produced during maximal hindlimb muscle contraction in WKY and SHR rats (approximately 1200 g) (Smith et al., 2001; Smith et al., 2010). During dialysis of each substance, two reproducible responses were obtained with a minimum recovery period of 15 minutes between each stretch. Before all stretch maneuvers, hindlimb muscles were preloaded by stretching to 70–100 g of tension. In additional control experiments in SHR (n=5) and WKY (n=5) animals, mechanically sensitive afferent fibers were activated before and after the microdialysis of D-NAME (5 mM), the inactive isomer of L-NAME.
Validation of Probe Placement
To verify probe placement, Evans blue dye was dialyzed into the NTS at the conclusion of experimentation. Subsequently, the brainstem was excised and fixed in 10% phosphate buffered formalin and stored at 4°C. Medullary tissue was blocked, and 40 µm sections were cut serially using a cryostat (Cambridge Instruments). Sections were placed on coated slides and examined to establish the neuroanatomical location of probe placement. The perfusion area of the probe was verified by the distribution of the dye.
Corollary Experiments
Stimulation of mechanically sensitive afferent fibers induces increases in MAP and HR predominantly via activation of the sympathetic nervous system (Mitchell et al., 1983). To ascertain whether the hemodynamic responses to stretch were indeed mediated by the sympathetic nervous system, the stretch protocol was additionally performed before and after ganglionic blockade (hexamethonium, 30 mg kg−1) in a subset of WKY (n=4) and SHR animals (n=4). Validation of this dose of hexamethonium as an effective ganglionic blocker has been previously established in our laboratory (Smith et al., 2006).
Morphological Measurements
At the conclusion of testing procedures, insentient decerebrated animals were killed by intravenous injection of saturated potassium chloride (4M, 2 ml/kg). Use of this procedure adheres to the guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. Subsequently, the heart and lungs were excised and wet weights obtained. Tibial length was also measured to assess heart mass / tibial length ratios.
Data Acquisition
In all physiologic experiments, baseline and reflex changes in MAP, HR, and developed tension were recorded. Baseline values were established by monitoring each variable for a period of 15 min. After a steady state had been reached for a minimum of 5 min, baseline values for each measured variable were determined over a 30 sec period prior to reflex activation. The greatest change in each variable from this baseline in response to reflex activation was taken as the peak value.
Statistical Analyses
All cardiovascular and contractile force data were acquired, recorded, and analyzed using data acquisition software (Spike 2, version 3, Cambridge Electronic Design, Ltd) for the CED micro 1401 system (Cambridge Electronic Design Ltd). Statistical analyses were performed using Student t tests (e.g. comparison of morphometric and baseline hemodynamics), one-way repeated measures analysis of variance (ANOVA; e.g. comparison of cardiovascular responses during D-NAME microdialysis) and two-way repeated measures ANOVA (e.g. comparison of cardiovascular responses during L-NAME microdialysis). When ANOVA was found to be significant, a Student-Newman-Keuls multiple comparison test was utilized to identify differences between specific group means. Results are presented as means ± S.E.M. The significance level was set at P<0.05. All statistical analyses were performed using Sigma Stat for Windows (SPSS Inc.)
RESULTS
Characterization of Hypertensive Model
Morphometric and hemodynamic baseline data for WKY and SHR animals are presented in Table 1. Ratios of heart weight to both body weight and tibial length were significantly greater in SHR than WKY. However, the lung weight to body weight ratio was not different between groups. Baseline MAP was significantly higher in SHR compared to WKY but baseline HR was not different between groups.
Table 1.
Morphometric characteristics and baseline hemodynamics.
| WKY | SHR | |
|---|---|---|
| n | 25 | 20 |
| Body weight, g | 351±7 | 402±8* |
| Heart weight/body weight, mg/g | 2.9±0.1 | 3.4±0.1* |
| Lung weight/body weight, mg/g | 5.8±0.5 | 6.9±0.4 |
| Heart weight/tibial length, mg/mm | 26.1±0.7 | 34.5±0.6* |
| MAP, mmHg | 103±5 | 146±7* |
| HR, beats/min | 413±14 | 419±9 |
Data are means ± S.E.M. MAP, mean arterial pressure; HR, heart rate.
Significantly different from WKY, P<0.05.
Verification of Probe Placement
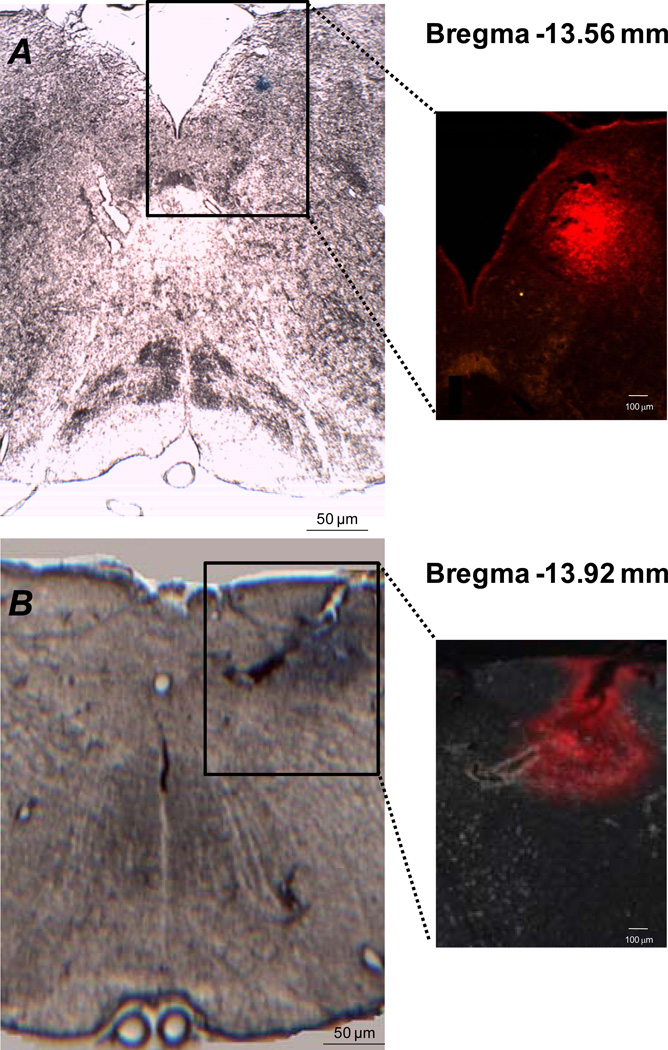
To verify probe placement, Evans blue dye was dialyzed at the end of experimentation for 40 minutes. Representative examples of slices of brainstem medullary tissue identifying the distribution of dye marking the probe perfusion area are shown in Figure 1. At bregma −13.56 mm, dye was found to be located in the solitary tract as well as the central, dorsolateral, intermediate and medial subnuclei of the NTS. At bregma −13.92, the dye spread was found to be more diffuse being partially located in the solitary tract as well as the dorsolateral, interstitial, ventral and ventrolateral divisions of the NTS continuing laterally into portions of the reticular formation (Paxinos & Watson, 1986). This analysis confirmed that the probe perfusion area was within the NTS region although not exclusively limited to the NTS.
Figure 1. Microdialysis probe placement and dye distribution within the NTS.
Photomicrographs of brain slices in which the probe perfusion area was identified by the dialysis of Evans blue dye. Panel A, brain slice located −13.56 mm from bregma (interaural −4.56 mm). Panel B, brain slice located −13.92 mm from bregma (interaural −4.92 mm). The magnified area depicted with each slice identifies the spread of Evans blue dye using red fluorescence. The microdialysis probe was placed 0.0 mm rostro-caudal to the calamus scriptorius, 0.5 mm lateral to the calamus scriptorius and 0.5 mm below the dorsal medullary surface. Minimal structural damage was caused by placement of the dialysis probe.
Dialysis of L-NAME within the NTS Alters the Cardiovascular Response to Muscle Stretch
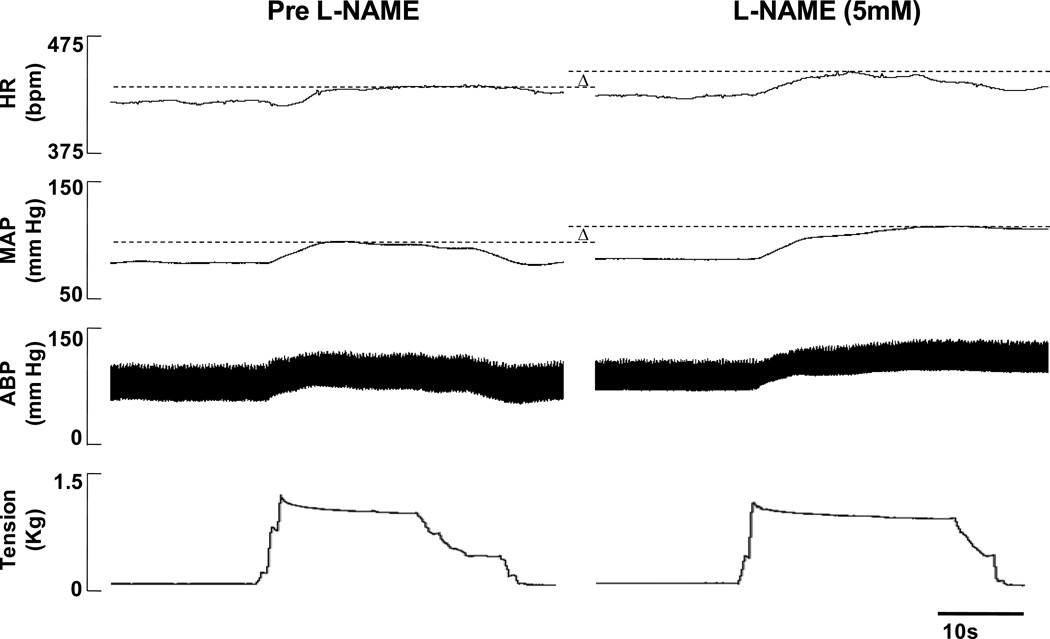
In both WKY and SHR, the HR and MAP responses to passive muscle stretch were enhanced by the dialysis of L-NAME within the NTS as compared to control aCSF trials. An example of this finding is presented in representative tracings from a WKY animal in Figure 2. In agreement with previous reports (Leal et al., 2008; Mizuno et al., 2011), the peak HR and MAP responses characteristically occurred within the first 10–20s from stretch onset remaining relatively sustained until the muscle was relaxed. In addition, the temporal profiles for the HR and MAP responses to stretch were similar.
Figure 2. Characteristic cardiovascular responses to passive muscle stretch before (Pre) and during L-NAME dialysis within the NTS.
In this representative tracing from a normotensive WKY rat, both the HR and MAP responses to activation of mechanically sensitive afferent fibers via hindlimb passive stretch were augmented as a result of dialyzing L-NAME within the NTS. The peak responses elicited during dialysis of the control substance aCSF (Pre L-NAME) and during L-NAME (5mM) are marked with dashed lines and the differences indicated by Δ. Responses during dialysis of aCSF post L-NAME (i.e. recovery) were not different from the pre-trial and are not shown.
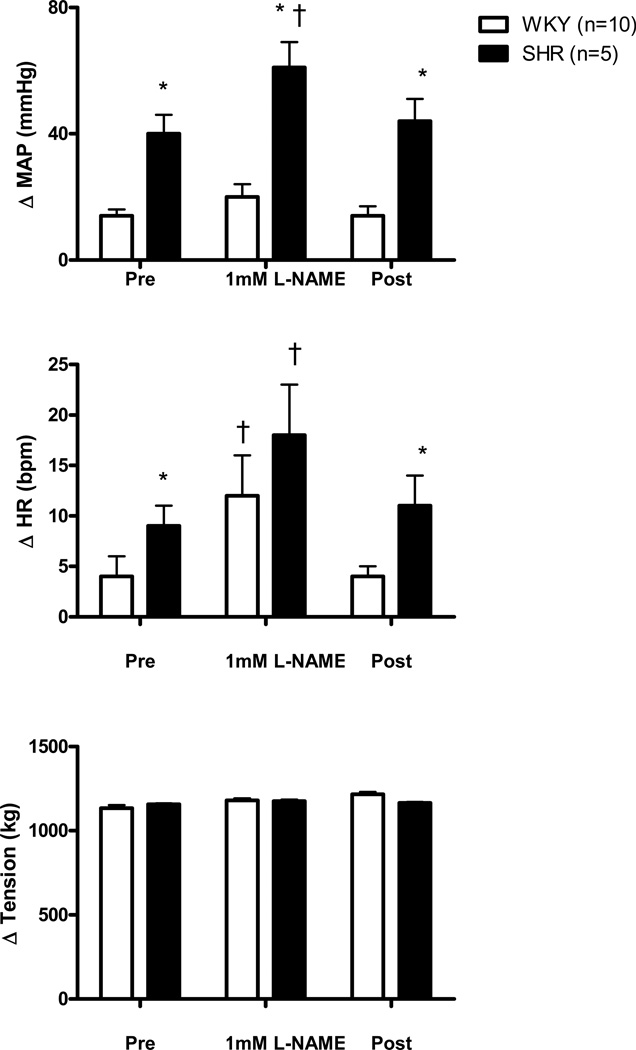
The effect of microdialyzing 1 mM L-NAME into the NTS on the MAP and HR responses to activation of mechanically sensitive afferent fibers in WKY and SHR animals is presented in Figure 3. In agreement with previous reports (Leal et al., 2008; Mizuno et al., 2011), the HR and MAP responses to stretch were significantly greater in SHR compared to WKY during the control aCSF trial. In WKY, inhibiting NOS activity within the NTS with L-NAME significantly enhanced the HR response to passive muscle stretch compared to control aCSF conditions. Interestingly, this L-NAME induced HR response in WKY (12 ± 4 bpm) was similar to that obtained in SHR (9 ± 2 bpm) prior to L-NAME administration. In WKY, the MAP response to passive stretch was 14 ± 2 mmHg prior to the dialysis of L-NAME and 20 ± 4 mmHg during L-NAME dialysis (no statistical difference). In SHR, microdialysis of 1 mM L-NAME significantly increased the HR and MAP responses to stretch compared to control aCSF conditions. When the passive stretch procedure was repeated during the dialysis of aCSF at the end of the experimental protocol, the circulatory responses returned to pre-L-NAME levels in both groups of animals.
Figure 3. Cardiovascular responses to activation of mechanically sensitive afferent fibers in skeletal muscle before (Pre), during, and after (Post) 1 mM L-NAME dialysis within the NTS.
Passive stretch of hindlimb skeletal muscle induced increases in MAP and HR from baseline that were significantly greater in SHR as compared to WKY. In addition, dialysis of 1mM L-NAME into the NTS significantly enhanced the tachycardic response to hindlimb muscle stretch in both WKY and SHR as well as the pressor response to stretch in hypertensive animals as compared to pre and post control trials. * Significantly different from WKY rats. † Significantly different from Pre (control) and Post (recovery) responses in which aCSF was dialyzed. P<0.05.
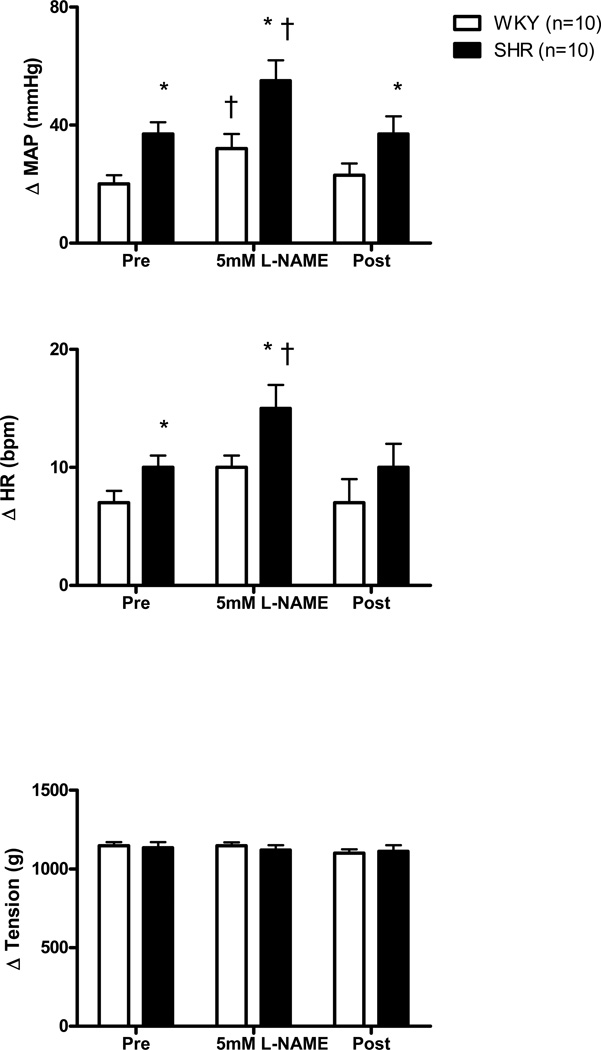
The effect of dialyzing 5 mM L-NAME into the NTS on the MAP and HR responses to stretch in WKY and SHR animals is presented in Figure 4. As previously demonstrated (Leal et al., 2008; Mizuno et al., 2011), both the HR and MAP responses to activation of mechanically sensitive afferent fibers were significantly greater in SHR compared to WKY during the control aCSF trial. In WKY, the HR response during the dialysis of L-NAME as compared to before was not statistically larger. However, the L-NAME induced HR response in WKY (10 ± 1 bpm) was comparable to that obtained in SHR (12 ± 2 bpm) prior to L-NAME administration. In WKY, NOS blockade in the NTS with 5 mM L-NAME significantly increased the MAP response to stretch compared to control aCSF conditions. This resultant change in MAP (32 ± 5 mmHg) was similar to that produced in SHR (37 ± 3 mmHg) prior to the dialysis of L-NAME. In SHR, microdialysis of 5 mM L-NAME significantly increased the HR and MAP responses to stretch compared to control aCSF conditions. In both groups of animals, the cardiovascular responses returned to pre L-NAME levels after the administration of aCSF in the recovery period. Within groups, It should be noted that the magnitude of the changes induced by 5 mM L-NAME were similar to those induced by 1 mM L-NAME.
Figure 4. Cardiovascular responses to activation of mechanically sensitive afferent fibers before (Pre), during, and after (Post) 5 mM L-NAME dialysis within the NTS.
Passive stretch of hindlimb skeletal muscle during dialysis of 5 mM L-NAME caused significantly larger increases in MAP from baseline in SHR and WKY compared to pre and post control trials. The HR response, from baseline, to hindlimb muscle stretch in SHR animals was also significantly enhanced during dialysis of L-NAME as compared to pre and post control trials. * Significantly different from WKY rats. † Significantly different from Pre (control) and Post (recovery) responses in which aCSF was dialyzed. P<0.05.
Dialysis of D-NAME within the NTS Has No Effect on the Cardiovascular Response to Muscle Stretch
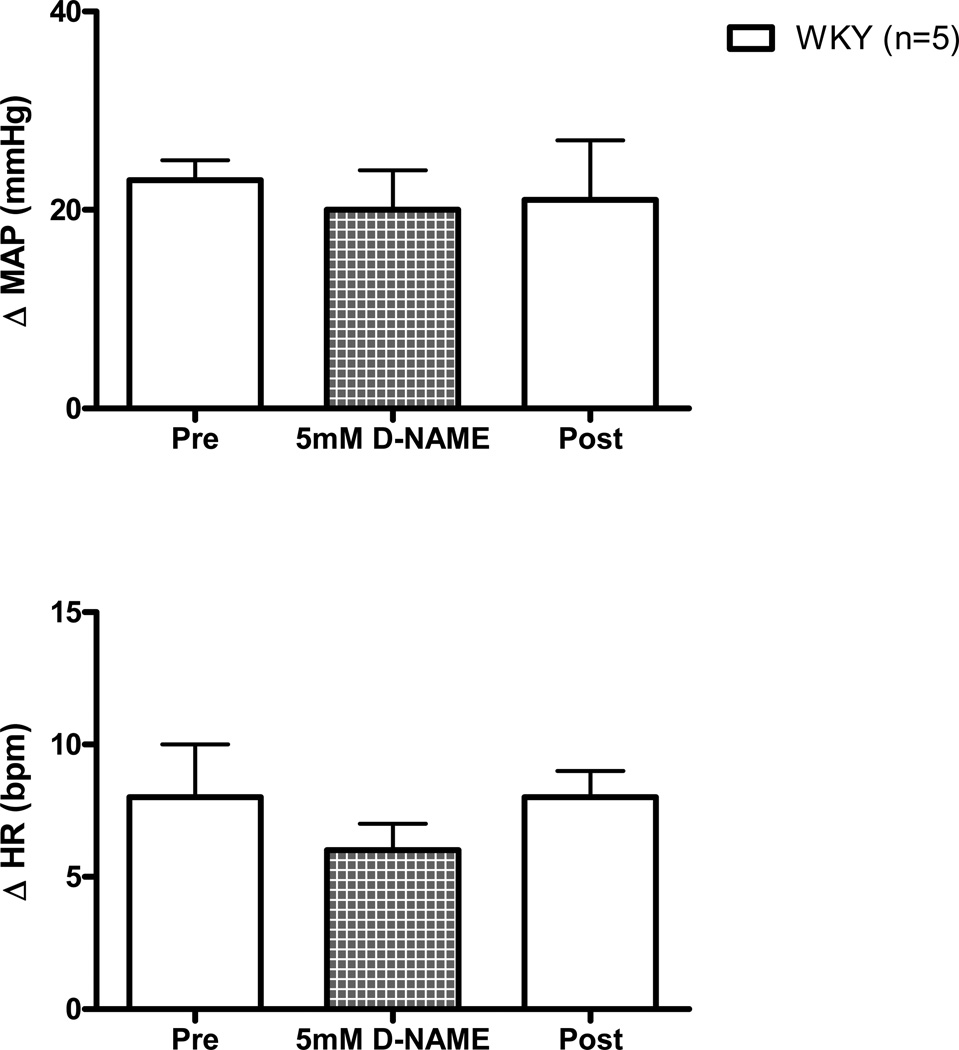
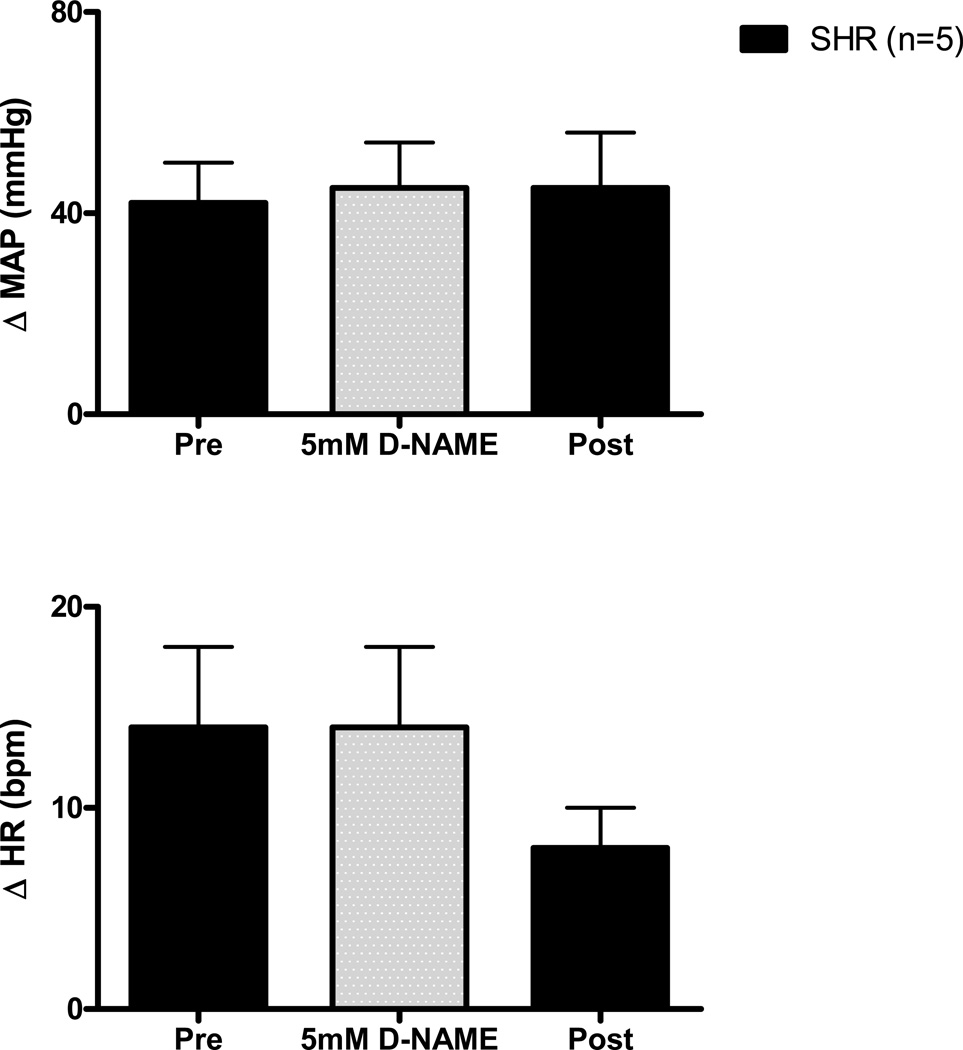
As a control experiment, activation of mechanically sensitive afferent fibers was repeated in WKY and SHR animals during the dialysis of D-NAME (5 mM) within the NTS, the inactive enantiomer of L-NAME. D-NAME had no significant effect on the hemodynamic response to passive stretch in either WKY (Figure 5) or SHR (Figure 6).
Figure 5. Cardiovascular responses to activation of mechanically sensitive afferent fibers before (Pre), during, and after (Post) the dialysis of D-NAME (5 mM) within the NTS of WKY rats.
Dialysis of the inactive enantiomer D-NAME had no effect on the cardiovascular response (from baseline) to passive stretch of hindlimb skeletal muscle in WKY. aCSF was dialyzed during Pre (control) and Post (recovery) trials.
Figure 6. Cardiovascular responses to activation of mechanically sensitive afferent fibers before (Pre), during, and after (Post) the dialysis of D-NAME (5 mM) within the NTS of SHR rats.
Dialysis of the inactive enantiomer D-NAME had no effect on the cardiovascular response (from baseline) to passive stretch of hindlimb skeletal muscle in SHR. aCSF was dialyzed during Pre (control) and Post (recovery) trials.
Effects of Ganglionic Blockade on the Cardiovascular Response to Stretch
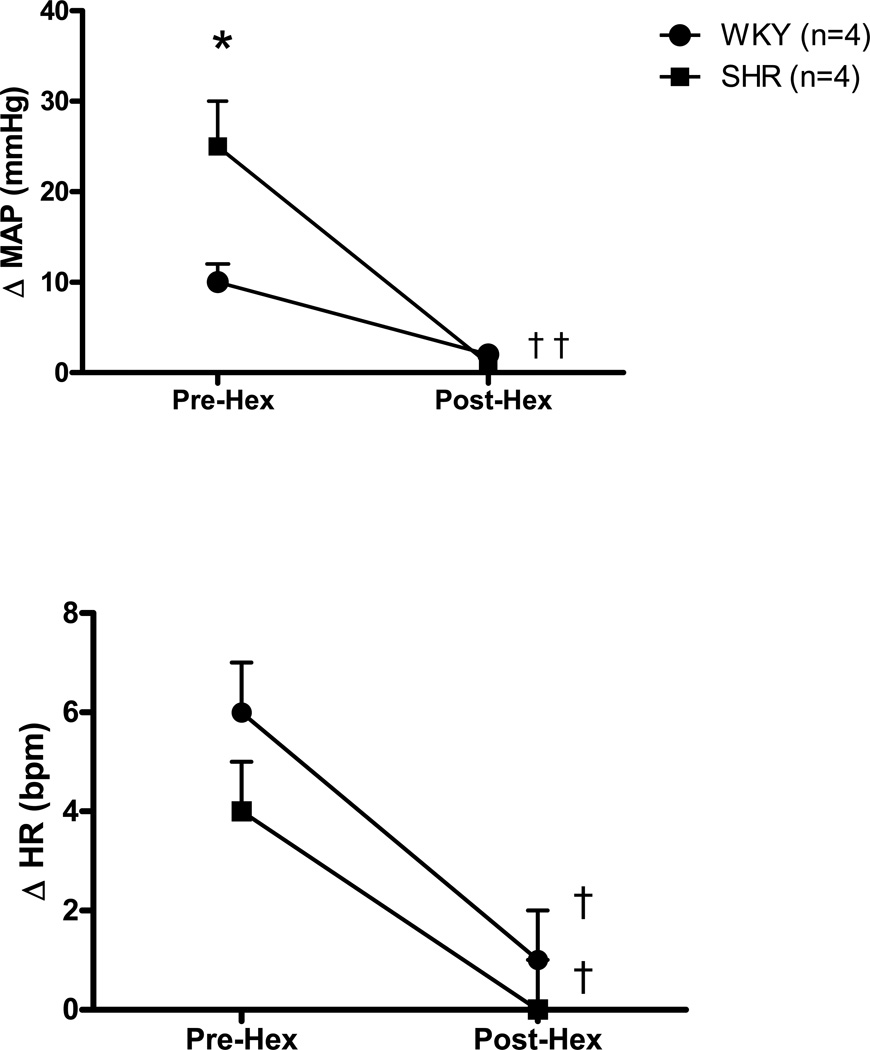
Administration of hexamethonium, a ganglionic blocker, was used to inhibit sympathetic nerve activity before and after activation of mechanically sensitive afferent fibers. Ganglionic blockade virtually abolished the MAP and HR response to passive stretch in both WKY and SHR (Figure 7).
Figure 7. The effects of ganglionic blockade on the cardiovascular response to activation of mechanically sensitive afferent fibers in skeletal muscle.
Ganglionic blockade with hexamethonium significantly reduced the HR and MAP responses to passive muscle stretch in WKY and SHR animals. *Significantly different from WKY rats. † Significantly different from Pre-Hex response.
DISCUSSION
Utilizing the same rat model as the current study, we recently provided evidence consistent with the concept that overactivity of the skeletal muscle mechanoreflex contributes importantly to the exaggerated cardiovascular response to exercise manifest in hypertension (Leal et al., 2008; Mizuno et al., 2011). With the intent of providing a foundation for our understanding of the central neurochemical mechanisms responsible for mechanoreflex dysfunction in hypertension, this study was designed to examine the role of NO in regulating the cardiovascular response to activation of mechanically sensitive afferent fibers in skeletal muscle. The results of the study produced the following major findings: (i) blocking NO production within the NTS of normotensive rats recapitulates the augmented cardiovascular response to passive muscle stretch observed in hypertensive rats, (ii) blocking NO production within the NTS of hypertensive rats further enhances the exaggerated cardiovascular response to stretch, and (iii) normal and heightened circulatory responses to activation of mechanically sensitive afferent neurons in normotensive and hypertensive rats are mediated by the sympathetic nervous system. Collectively, these findings suggest that reductions in NO production within the NTS may contribute significantly to the generation of exaggerated, sympathetically-mediated cardiovascular responses to mechanoreflex activation in hypertension.
Perhaps the most interesting finding from this study was that, in more than one instance, blocking NO production within the NTS of normotensive rats reproduced the exaggerated increases in MAP and HR to passive muscle stretch observed in hypertensive animals. This suggests that, in rats, NO within the NTS is involved in the processing of sensory afferent input from skeletal muscle and, subsequently, the modulation of the cardiovascular response elicited by activation of mechanically sensitive afferent fibers. These findings are corroborated by experiments in larger mammals (e.g. cats) demonstrating that increasing NO production within the NTS attenuates the cardiovascular response to EPR sensory input to which mechanically sensitive afferent fibers are known to contribute (Li & Potts, 2001; Smith et al., 2005a). More importantly, the current findings provide initial evidence that a decrease in NO availability within the NTS maintains the potential to generate an abnormally elevated cardiovascular response to passive muscle stretch in otherwise healthy animals. It is postulated that similar mechanisms may lead to the development of mechanoreflex overactivity in hypertension. It should be noted that dialyzing L-NAME within the NTS of hypertensive rats likewise accentuated the already exaggerated pressor and tachycardic responses manifest in these animals. This finding suggests that, although NO production may be reduced in SHR, it is not completely absent. As an alternative explanation, the results of such experiments could also indicate that the effects of NO on mechanoreflex function are blunted in hypertension.
Interestingly, the effects of L-NAME within each group of animals was largely independent of the dose administered. In other words, no graded effect of L-NAME was apparent. This was an unexpected finding as the dialysis of larger doses of L-NAME (20 mM) within the NTS than utilized in the current investigation have been shown to have little to no effect on muscle reflex function in cats (Smith et al., 2005a). The discrepancy between studies cannot be readily explained but likely represents a species difference between rats and cats and/or technical differences between each study. Regardless of the underlying cause, the differences between investigations highlight the need for further research in this area. It is also noteworthy that the effects of L-NAME were statistically variable within the normotensive group (i.e. WKY). For example, at the 1 mM dose the tachycardic response to muscle stretch was significantly augmented whereas at the 5 mM dose the pressor response was significantly enhanced. Likely, the discrepancies are due to the variability in the measurements. Regardless, in most instances, L-NAME increased the HR and/or MAP response to stretch in WKY to the same level obtained in untreated SHR.
The finding that NO within the NTS may play a role in the development of muscle reflex overactivity in hypertension is not surprising. Emerging evidence suggests that centrally-derived NO plays an important role in the regulation of the cardiovascular system in humans as well as animals. For example, systemic NOS inhibition in humans has been shown to elicit hypertension being mediated, at least in part, by elevations in sympathetic nerve activity (Owlya et al., 1997; Sander et al., 1999). Moreover, systemic L-NAME infusion of sufficient duration to allow its crossing the blood-brain barrier has been shown to increase central sympathetic outflow (Young et al., 2009). Collectively, these findings in humans suggest that, within the central nervous system, NO operates to restrain sympathetic nerve activity. Loss of this ability could contribute to the exaggerated sympathetically mediated cardiovascular response to exercise in humans. Whether this mechanism alters mechanoreflex function in hypertensive patients, as implicated by the current study in rats, remains to be elucidated.
Currently, it is not clear what factors could lead to a decrease in NO availability/production within the hypertensive brainstem nor is it clear the sequalae of events that would trigger these factors. It is possible that the availability of NO is reduced due to an increased production of reactive oxygen species that scavenge the molecule and/or the uncoupling of NOS, which occurs when reactive oxygen species oxidize tetrahydrobiopterin; a co-factor of NOS. Both of these physiological processes have been documented in hypertensive animals (Kietadisorn et al., 2012). Another cause of an L-arginine-NO pathway impairment could be a decrease in the expression/activity of the NOS isoforms present within the NTS; namely neuronal NOS, endothelial NOS and inducible NOS. Unfortunately, studies to date describing NOS expression/activity within the brainstem of hypertensive rats have been conflicting. Some studies have shown NOS expression and activity within the medulla to be decreased during infancy, but significantly increased in adulthood compared to normotensive controls (Plochocka-Zulinska & Krukoff, 1997; Qadri et al., 2003; Waki et al., 2006). However, other studies show basal levels of NOS expression and activity are decreased in the NTS of adult hypertensive rats compared to their normotensive counterparts (Pontieri et al., 1998; Ferrari & Fior-Chadi, 2005). Given that blocking NOS activity in normotensive rats mimicked the exaggerated cardiovascular response to passive stretch observed in hypertensive animals, data from the experiments presented support the idea that basal NOS levels or activity are decreased in the NTS of SHR. Using the same rationale, the data further suggest that, although NOS expression/activity may be decreased in SHR it is not completely absent as NOS blockade further augments the enhanced cardiovascular response to stretch in hypertensive animals. The contradictory findings in the literature regarding NOS within the NTS of hypertensive animals and its effect on hemodynamic regulation illustrate the need for additional comprehensive experiments in which both activity and expression are accurately and reliably quantified.
The finding that ganglionic blockade with hexamethonium almost completely abolished the circulatory response to activation of mechanically sensitive afferent fibers in this study confirmed that the responses elicited were predominately sympathetically mediated. It should be noted, however, that the magnitude of the sympathetic response cannot be discerned from the use of this technique. As such, it remains unclear whether the enhanced MAP and HR responses observed in this study were mediated via exaggerated increases in sympathetic nerve activity or from an augmented end organ response (e.g. vascular smooth muscle; adrenal medulla) to a normal level of reflex-induced sympathetic input. Regardless of which is the case, blockade of sympathetic activity has been shown to mitigate aberrant mechanoreflex function in hypertension identifying the sympathetic nervous system as a potential target for the treatment of reflex overactivity in this disease.
Limitations
Several limitations could affect the interpretation of results from the current study. First, the use of the microdialysis technique presents a few issues for consideration. We performed unilateral microdialysis due to spatial constraints and to reduce surgical trauma preserving the functional and structural integrity of the medullary brainstem. However, it has been established, in the rat, that second order, presumptively mechanosensitive, dorsal horn neurons project bilaterally to the NTS (Gamboa-Esteves et al., 2001; Potts et al., 2002). Given that the use of unilateral microinjection and microdialysis techniques to assess the circulatory responses to activation of cardiovascular reflexes have been validated on several occasions (Lewis et al., 1991; Pontieri et al., 1998; Smith et al., 2005a), we contend that the results of the current study were not significantly limited by using this approach. It is also acknowledged that, in addition to perfusing several NTS subnuclei known to receive afferent input from skeletal muscle afferents, the dialysis procedure partially perfused regions of the medulla just outside of the NTS (e.g. dorsal and intermediate reticular nucleus). As such, the findings reported may have partly resulted from alterations in the activity of neurons in these regions and should be considered when interpreting results. Finally, it is possible that perfusion of the control substance aCSF within the NTS could itself effect the cardiovascular response to passive stretch by altering NO availability. However, the stretch-induced changes in HR and MAP during aCSF dialysis were similar, if not the same, as those previously reported in WKY and SHR animals in which microdialysis was not performed (Leal et al., 2008; Mizuno et al., 2011). These findings suggest that aCSF alone does not influence the cardiovascular response to passive stretch.
A second limitation of the present study concerns the technique used for activation of mechanically sensitive afferent fibers in skeletal muscle. Passive muscle stretch, the method used to stimulate these afferent fibers, may not completely mimic the manner by which the afferent neurons are activated during physiological exercise. For example, it has been shown in cats that only a portion (25–75 %) of mechanically sensitive group III afferent fibers with receptive fields in the gastrocnemius and soleus muscles respond to both muscle contraction and passive stretch (Hayes et al., 2005). However, both contraction and stretch produce similar effects on afferent discharge rates, as well as increases in MAP and HR in these animals (Hayes & Kaufman, 2001; Hayes et al., 2005). In addition, hindlimb stretch has been shown to increase renal sympathetic efferent discharge rates and the same efferents responsive to stretch are also activated by hindlimb contraction (Hayes & Kaufman, 2001). Further, gadolinium, a known blocker of stretch sensitive receptors, effectively diminishes group III afferent discharge and the concomitant increases in MAP and HR that occur in response to passive muscle stretch (Hayes & Kaufman, 2001; Smith et al., 2005b; Mizuno et al., 2011). As such, this stretching technique is commonly used in animals to activate mechanically sensitive group III afferent fibers in skeletal muscle. Although it is tempting to interpret findings elicited during passive stretch as being directly representative of mechanoreflex activity, conclusions must be tempered with this limitation in mind. It should be noted that ideologically similar passive stretching techniques have recently been utilized in humans elucidating the muscle mechanoreflex contribution to cardiovascular control (Fisher et al., 2005; Cui et al., 2006; Cui et al., 2008a; Cui et al., 2008b; Drew et al., 2008a; Drew et al., 2008b; Momen et al., 2008; Cui et al., 2010). Results from these studies have proven invaluable in extending the findings from animal investigation to humans.
Lastly, it must be acknowledged that the arterial baroreflex is known to modulate input from skeletal muscle reflexes and vice versa. For example, it has been demonstrated that the cardiovascular response to activation of muscle reflexes is enhanced in normotensive animals after barodenervation (Waldrop & Mitchell, 1985). This finding suggests that the baroreflex maintains the potential to buffer muscle reflex activity under normal conditions. Baroreflex afferent fibers are known to project to the NTS and the activity of the reflex is likewise modulated by NO within this nuclei (Paton et al., 2001; Smith et al., 2005a). As such, it is possible that the dialysis of L-NAME within the NTS may affect baroreflex function which could, in turn, modulate the cardiovascular response to activation of mechanically sensitive muscle afferent fibers. That being stated, blockade of NO production with L-NAME has been shown to increase the sensitivity of the arterial baroreflex (Smith et al., 2005a). Such an increase in baroreflex sensitivity would be expected to enhance its buffering capacity of muscle reflex input reducing the magnitude of the cardiovascular response to stretch. Since the circulatory response to stretch after L-NAME administration was found to be accentuated despite the possibility that baroreflex buffering capacity may have been enhanced, it is our contention that the interpretation of results would be minimally affected by such an alteration in baroreflex sensitivity. An alternative scenario is also important to acknowledge. Recent evidence in humans suggests that activation of mechanically sensitive afferent fibers decreases arterial baroreflex sensitivity (Drew et al., 2008a). This could reduce the buffering capacity of the baroreflex contributing, in part, to the cardiovascular responses observed during control trials. Again, however, it is possible that the dialysis of L-NAME could have affected baroreflex function, possibly restoring its sensitivity and hence buffering capacity. For the same reasons previously stated, this would not be expected to significantly alter interpretation of results.
Future Directions
As stated, the current study provides a foundation for our understanding of the central neurochemical mechanisms underlying mechanoreflex dysfunction in hypertension. This has been achieved by stimulating mechanically-sensitive afferent fibers in a static fashion presumably without the production of metabolites during experimental manipulation of NO within the brainstem. Given that static exercise is only one form of physical activity it will be important to determine whether the findings reported in this investigation are valid for more dynamic manipulations of the muscle mechanoreflex. In addition, several studies in animals (Rotto et al., 1990; Adreani & Kaufman, 1998) and humans (Bell & White, 2005; Cui et al., 2008a; Cui et al., 2008b; Momen et al., 2008) have demonstrated that muscle metabolites sensitize mechanoreceptors. Whether this relationship is altered in hypertension remains to be determined as does the role of centrally-produced NO in processing this afferent information. Additional studies in both animals and humans are needed to address these important questions.
Relevance
Determining the central neurochemical mechanisms underlying muscle reflex dysfunction may provide invaluable insight into the development of treatments designed to reduce the hemodynamic risks associated with exercise in hypertension. To this end, this investigation has provided evidence supporting the concept that a reduction in NO production within the NTS contributes to the muscle mechanoreflex dysfunction manifest in this disease.
ACKNOWLEDGEMENTS
This research was supported by grants from the National Institutes of Health (HL-094075 to A.K.L. and HL-088422 to S.A.S.) and the Lawson & Rogers Lacy Research Fund in Cardiovascular Diseases (to J.H.M.). The authors thank Martha Romero and Julius Lamar, Jr. for their expert technical assistance.
REFERENCES
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Andres KH, During Mv, Schmidt RF. Sensory innervation of the Achilles tendon by group III and IV afferent fibers. Anat Embryol. 1985;172:145–156. doi: 10.1007/BF00319597. [DOI] [PubMed] [Google Scholar]
- Aoki K, Sato K, Kondo S, Pyon C, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J. 1983;47:802–809. doi: 10.1253/jcj.47.802. [DOI] [PubMed] [Google Scholar]
- Bell MP, White MJ. Cardiovascular responses to external compression of human calf muscle vary during graded metaboreflex stimulation. Exp Physiol. 2005;90:383–391. doi: 10.1113/expphysiol.2004.029140. [DOI] [PubMed] [Google Scholar]
- Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol. 2006;576:625–634. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Leuenberger UA, Blaha C, Yoder J, Gao Z, Sinoway LI. Local adenosine receptor blockade accentuates the sympathetic responses to fatiguing exercise. Am J Physiol. 2010;298:H2130–H2137. doi: 10.1152/ajpheart.00083.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Mascarenhas V, Moradkhan R, Blaha C, Sinoway LI. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptoRs) stimulation in healthy humans. Am J Physiol. 2008a;294:R458–R466. doi: 10.1152/ajpregu.00475.2007. [DOI] [PubMed] [Google Scholar]
- Cui J, Moradkhan R, Mascarenhas V, Momen A, Sinoway LI. Cyclooxygenase inhibition attenuates sympathetic responses to muscle stretch in humans. Am J Physiol. 2008b;294:H2693–H2700. doi: 10.1152/ajpheart.91505.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney E, Greaney J, Edwards D, Rose W, Fadel P, Farquhar W. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol. 2010;299:H1318–H1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew RC, Bell MPD, White MJ. Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol. 2008a;104:716–723. doi: 10.1152/japplphysiol.00956.2007. [DOI] [PubMed] [Google Scholar]
- Drew RC, McIntyre DB, Ring C, White MJ. Local metabolite accumulation augments passive muscle stretch-induced modulation of carotid-cardia but not carotid-vasomotor baroreflex sensitivity in man. Exp Physiol. 2008b;93:1044–1057. doi: 10.1113/expphysiol.2008.042234. [DOI] [PubMed] [Google Scholar]
- Ferrari MFR, Fior-Chadi DR. Differential expression of nNOS mRNA and protein in the nucleus tractus solitarii of young and aged Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens. 2005;23:1683–1690. doi: 10.1097/01.hjh.0000179163.68634.c3. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Bell MPD, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol. 2005;90:773–781. doi: 10.1113/expphysiol.2005.030577. [DOI] [PubMed] [Google Scholar]
- Gamboa-Esteves FO, Tavares I, Almeida A, Batten TFC, McWilliam PN, Lima D. Projection sites of superficial and deep spinal dorsal horn cells in the nucleus tractus solitarii of the rat. Brain Res. 2001;921:195–205. doi: 10.1016/s0006-8993(01)03118-3. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol. 2001;280:2153–2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–1896. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mauther HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65:583–589. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mei SS, Kao FF. Central projections from ergoreceptors (C fibers) in muscle involved in cardiopulmonary responses to static exercise. Circ Res. 1981;48:I48–I62. [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol. 2012;302:E481–E495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehab. 2002;22:178–183. doi: 10.1097/00008483-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol. 2008;295:H1429–H1438. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Ohta H, Machado B, Bates JN, Talman WT. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii decreases arterial pressure and heart rate via activation of soluble guanylate cyclase. Eur J Pharmacol. 1991;202:135–136. doi: 10.1016/0014-2999(91)90269-v. [DOI] [PubMed] [Google Scholar]
- Li J, Potts JT. NO formation in nucleus tractus solitarii attenuates pressor response evoked by skeletal muscle afferents. Am J Physiol. 2001;280:H2371–H2379. doi: 10.1152/ajpheart.2001.280.5.H2371. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mittleman M, Siscovick D. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996;14:263–270. doi: 10.1016/s0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol. 2011;300:H968–H977. doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen A, Cui J, McQuillan P, Sinoway LI. Local prostaglandin blockade attenuates muscle mechanoreflex-mediated renal vasoconstriction during muscle stretch in humans. Am J Physiol. 2008;294:H2184–H2190. doi: 10.1152/ajpheart.00948.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. New Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Williamson JW, Friedman DB, Araujo CG, Mitchell JH. Cardiovascular responses to active and passive cycling movements. Med Sci Sports Exerc. 1994;26:709–714. doi: 10.1249/00005768-199406000-00009. [DOI] [PubMed] [Google Scholar]
- Owlya R, Vollenweider L, Trueb L, Sartori C, Lepori M, Nicod P, Scherrer U. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation. 1997;96:3897–3903. doi: 10.1161/01.cir.96.11.3897. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando, FL: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Person RJ. Somatic and vagal afferent convergence on solitary tract neurons in cat: electrophysiological characteristics. Neurosci. 1989;30:283–295. doi: 10.1016/0306-4522(89)90254-6. [DOI] [PubMed] [Google Scholar]
- Pickering TG. Pathophysiology of exercise hypertension. Herz. 1987;12:119–124. [PubMed] [Google Scholar]
- Plochocka-Zulinska D, Krukoff TL. Increased gene expression of neuronal nitric oxide synthase in brain of adult spontaneously hypertensive rats. Brain Res Mol Brain Res. 1997;48:291–297. doi: 10.1016/s0169-328x(97)00101-0. [DOI] [PubMed] [Google Scholar]
- Pontieri V, Venezuela MK, Scavone C, Michelini LC. Role of endongenous nitric oxide in the nucleus tractus solitarii on baroreflex control of heart rate in spontaneously hypertensive rats. J Hypertens. 1998;16:1993–1999. doi: 10.1097/00004872-199816121-00021. [DOI] [PubMed] [Google Scholar]
- Potts JT, Lee SM, Anguelov PI. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Auto Neurosci. 2002;98:64–69. doi: 10.1016/s1566-0702(02)00034-6. [DOI] [PubMed] [Google Scholar]
- Qadri F, Arens T, Schwarz EC, Hauser W, Dendorfer A, Dominiak P. Brain nitric oxide synthase activity in spontaneously hypertensive rats during the development of hypertension. J Hypertens. 2003;21:1623–1624. doi: 10.1097/00004872-200309000-00018. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol. 1990;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol. 2008 doi: 10.1007/s00421-008-0910-8. In Press. [DOI] [PubMed] [Google Scholar]
- Seguro C, Sau F, Zedda N, Scano G, Cherchi A. Arterial blood pressure behavior during progressive muscular exercise in subjects with stable arterial hypertension. Cardiologia. 1991;36:867–877. [PubMed] [Google Scholar]
- Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol. 2010;588.7:1179–1189. doi: 10.1113/jphysiol.2009.184952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Li J. Independent modification of baroreceptor and exercise pressor reflex function by nitric oxide in nucleus tractus solitarius. Am J Physiol. 2005a;288:2068–2076. doi: 10.1152/ajpheart.00919.2003. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005b;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol. 2006;577:1009–1020. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effects of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol. 1988;65:1539–1547. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- Toney GM, Mifflin SW. Time-dependent inhibition of hindlimb somatic afferent transmission within nucleus tractus solitarius: an in vivo intracellular recording study. Neurosci. 1995;68:445–453. doi: 10.1016/0306-4522(95)00156-d. [DOI] [PubMed] [Google Scholar]
- Waki H, Murphy D, Yao ST, Kasparov S, Paton JFR. Endothelial NO synthase activity in nucleus tractus solitarii contributes to hypertension in spontaneously hypertensive rats. Hypertension. 2006;48:644–650. doi: 10.1161/01.HYP.0000238200.46085.c6. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Mitchell JH. Effects of barodenervation on cardiovascular responses to muscle contraction. Am J Physiol. 1985;249:H710–H714. doi: 10.1152/ajpheart.1985.249.4.H710. [DOI] [PubMed] [Google Scholar]
- Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD, Fadel PJ. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J Physiol. 2009;587:4977–4986. doi: 10.1113/jphysiol.2009.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]