Abstract
Objective
Although a time of increased independence and autonomy, adolescence is also a time of vulnerabilities, through increased risk-taking and the emergence of psychopathology. Neurodevelopmental changes during this period may provide a neurobiological basis for this normative rise in deleterious behaviors. Thus, the objective of this review was to identify neurodevelopmental processes underlying the emergence of risk-taking and psychopathology in adolescence, and discuss implications of these findings for prevention.
Method
This article reviews literature examining developmental and contextual factors influencing neural functioning in systems mediating threat, reward, and cognitive control. This literature is discussed from the perspective of the Triadic Neural Systems Model of motivated behavior.
Results
Neuroimaging research suggests that neurodevelopmental and contextual factors both contribute to a shift in the functional equilibrium among the Triadic nodes. This equilibrium shift may contribute to negative outcomes of adolescent behavior. Most importantly, the balance of this equilibrium and its sensitivity to social and appetitive contexts may be exploited to facilitate prevention of deleterious outcomes.
Conclusion
Understanding developmental and contextual factors that influence functioning in motivational neural circuits can inform research on adolescent risk-taking, and may provide targets for novel preventions, for example through the use of incentives to reduce deleterious outcomes.
Introduction
Adolescence is a transitional period between childhood and adulthood, marked by distinct changes in physical, psychological, and social functioning (Ernst et al., 2006). The specific time course of adolescence can vary widely across individuals depending on many factors, including nutrition, gender, cultural values, and socioeconomic status (Spear, 2007). Although a time of promise and opportunity, this developmental period is also characterized by emerging vulnerability factors, including affective lability, dominant peer-oriented social influence, and increased risk-taking (Dahl, 2004; Ernst and Hardin, 2009; Ernst and Paulus, 2005).
Developmental shifts during adolescence can be understood from an evolutionary perspective as they aid progression toward autonomy (e.g., Steinberg and Belsky, 1996); however, they may come with great costs. Indeed, adolescence is the only period of human development during which the primary causes of morbidity and mortality are directly attributable to overt actions and behaviors (e.g., driving recklessly, suicide) rather than disease (Patton et al., 2009). Additionally, adolescence is a prime period for the onset of mental illness, which often persists into adulthood (Kessler et al., 2005). Thus, understanding the mechanisms underlying emotional, social, and behavioral changes during adolescence may inform the development of novel prevention efforts to reduce the costly consequences of these changes.
The following review offers an overview of core neurobiological mechanisms including functional anatomy and ontogeny of the neural systems underlying motivated behavior. We will draw from the Triadic Neural Systems Model of motivated behavior (Ernst et al., 2006), which emphasizes contributions of three neural systems in the unique cognitive and affective architecture of adolescent development; namely, systems mediating threat, reward, and cognitive control (Figure 1). Notably, the Triadic Model was not specifically developed to inform the prevention of noxious behaviors among adolescents, but was spawned to explain the developmental changes in neural functioning that have been observed across different contexts (e.g., threatening, rewarding, and social) over the course of human development. Thus, the primary goals of this review are to (1) provide knowledge of normative adolescent neural functioning using the Triadic Model as a theoretical framework, and (2) spark interest in the field of prevention research to translate this knowledge into incentive-based preventions of deleterious outcomes among adolescents.
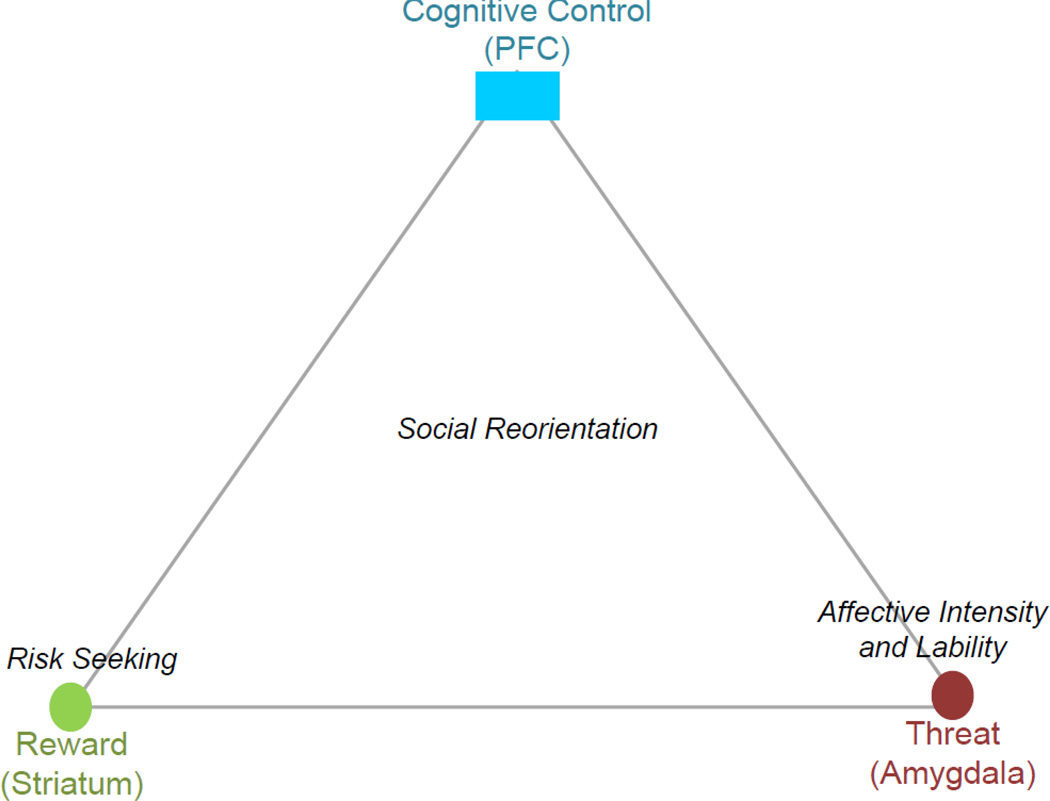
Figure 1.
The Triadic Neural Systems Model of motivated behavior emphasizes the contributions of three neural systems in the unique cognitive and affective architecture of adolescent development; namely, a threat system, a reward system, and a cognitive control system. Within the Triadic Model, the striatum represents the reward system, and is associated with approach; the amygdala represents the threat system, and plays a significant role in avoiding aversive (e.g., fearful) stimuli; and the prefrontal cortex is the center of the cognitive control system, which serves to regulate threat and reward functions. Of the developmental changes typically observed in adolescence, hyperactivity in the striatum is chiefly responsible for increased risk-seeking, while hyperactivity in the amygdala is implicated in affective lability. Social reorientation involves interactions among all three systems.
To set the stage for the Triadic Model, we first provide a brief review of typical adolescent neurodevelopmental processes that may help to account for the emergence of specific vulnerabilities, including affective lability, peer primacy, and risk-taking during adolescence. Second, we introduce the Triadic Neural Systems Model. Third, we present selected functional neuroimaging studies that inform the dynamic function of the triadic nodes in different contexts across adolescence into adulthood. We then address the implications of this work for prevention, with a focus on how existing prevention models can be applied to take adolescent neural development into consideration by applying incentives to encourage behavior change, and conclude with future directions.
The Adolescent Brain
Adolescence is characterized by significant changes in brain structure and function which continue into early adulthood (for recent reviews, see Casey et al., 2008; Ernst and Mueller, 2008; Steinberg, 2008). Structurally, the proportion of white to grey matter changes across adolescence: white matter increases and grey matter decreases, partly reflecting myelination and synaptic pruning respectively (Gogtay et al., 2006; Mabbott et al., 2006; Paus et al., 1999). These processes provide faster communication, and more efficient neural coding, with structural and functional maturation of specific brain regions occurring asynchronously (Ernst and Mueller, 2008; Gogtay et al., 2004). Specifically, structural changes progress from posterior to anterior dorsal regions, with parietal grey matter loss occurring most prominently from childhood to adolescence, while frontal grey matter decreases more dramatically from adolescence to adulthood (Gogtay et al., 2004; Sowell et al., 2004). Much less is known about developmental changes in the structure of subcortical regions. Evidence suggests that striatal volumes decrease with age (Giedd et al., 1996); however, hippocampal volume increases with age among males (Suzuki et al., 2005).
Functional neuroimaging research has recently begun to elucidate the functional significance of these structural changes, as well as the role of adolescent neurodevelopment on behavior. Researchers have theorized that imbalanced maturation of limbic regions relative to prefrontal regions may be one mechanism by which potentially damaging behaviors emerge during adolescence (e.g., Casey et al., 2008; Steinberg, 2008). Refining this theoretical approach, we identify the specific systems that are engaged in this equilibrium between limbic and prefrontal regions. We also believe that the proposed “imbalance” does not necessarily reflect different degrees of maturation, but results from functional biases in these systems emerging during adolescence to facilitate behaviors essential for species survival. That is, we propose that adolescent-specific patterns of neural development involve context-specific shifts in the functional equilibrium among the three neural systems that support reward, threat, and cognitive control (Ernst et al., 2006). In the context of affective (i.e., appetitive, aversive, or social) stimuli, adolescents display preferential recruitment of limbic regions charged with processing emotionally laden stimuli (e.g., threat, reward, peers), relative to cognitive control structures in the prefrontal cortex (PFC). This preferential recruitment of limbic structures relative to cognitive control structures when processing evocative stimuli may explain adolescent tendencies toward emotional intensity, risk-taking, and reduced cognitive control over emotions and behavior when affectively aroused. However, if adolescents are provided with incentives specifically for engaging cognitive control functions, the functional equilibrium among these nodes can shift to appear more similar to adult patterns of functioning. Therefore, adolescents may be particularly amenable to prevention strategies that involve the use of incentives to bring about healthy behavioral changes.
Overall, developmental changes in behavioral, emotional, and social functioning are normative in that they facilitate progression toward fulfillment of adult roles and expectations. However, perturbations in the neurodevelopment of the systems underlying any of these functional domains could potentially result in deadly consequences. Thus, understanding the mechanisms underlying normative development to identify targets for preventing negative consequences of problematic adolescent behaviors is imperative. The Triadic Neural Systems Model of Motivated Behavior (Triadic Model; Ernst et al., 2006) provides a heuristic framework for understanding the neural basis of adolescent behavior, and is described below.
The Triadic Neural Systems Model of Motivated Behavior
The Triadic Model assumes that behavior reflects the output of the functional integration of three distinct, yet overlapping neural systems. Although these specialized circuits will be initially discussed in isolation, they are functionally interconnected through substantial direct and indirect projections, and the circuits themselves are highly overlapping (e.g., Carmichael and Price, 1995; Ernst and Fudge, 2009; Fuster, 2001; McDonald et al., 1999). Moreover, the particular functions that these neural circuits play in the Triadic Model are specific to the context of goal-directed action, and should not be viewed as exclusive of other functions supported by these structures (Ernst et al., 2006).
The threat system refers to the emotion-related neural system. Although this system is involved in positive and negative emotions, it is uniquely implicated in threat-related processes and serves as a key mediator of avoidant behavior (e.g., LeDoux, 2000). This system is comprised principally of the amygdala, hippocampus, and insula, which are consistently associated with response to aversive stimuli (Rauch et al., 2003). Behaviorally, emotion-related processes seem to follow a curvilinear developmental trajectory such that affective intensity and reactivity peak during adolescence (Arnett, 1999; Larson et al., 2002; Silk et al., 2003; Weinstein et al., 2007).
The reward system refers to the neural system primarily implicated in the processing of appetitive stimuli and functions to facilitate approach behavior. This neural system comprises subcortical and cortical structures that are major sites of dopaminergic neurotransmission, including the striatum (caudate nucleus, putamen and nucleus accumbens) and medial and orbital prefrontal cortices (Jensen et al., 2003; Kringelbach, 2005). Behaviorally, reward-related processes also seem to follow a curvilinear developmental trajectory, whereby reward sensitivity peaks in adolescence (Ernst and Spear, 2009).
Finally, the control system refers to cortical regions involved in the modulation of subcortical function through “top-down” cognitive control. This node relies on prefrontal cortical structures, including medial prefrontal structures that encode specialized functions, such as inhibition, as well as conflict detection, monitoring, and resolution (Amodio and Frith, 2006; Bush et al., 2000; Carter and van Veen, 2007). Behaviorally, control processes mature linearly with age, in conjunction with an increase in cortical size during childhood (Durston et al., 2001; Giedd et al., 1996), and changes in dendritic density, axonal size, and increased myelination (Paus et al., 1999; Yakovlev, 1967).
These systems provide a foundation for understanding the neural basis of the characteristics that emerge during adolescence (Figure 1). Risk-taking reflects a hyperactive reward system (serving to approach stimuli or situations) combined with hypoactivation of the threat system (serving to reduce avoidance of potentially threatening stimuli or situations, respectively) in the context of appetitive stimuli. Affective lability and reactivity, indicates hyperresponsivity of the threat system, resulting in poor regulation of emotional responses in the context of potential threat. Hyperactivation in both the reward and threat systems in the context of appetitive or aversive stimuli respectively, indicates poor capacity of the control system to modulate functioning in these two systems within their particular contexts. Finally, social peer primacy represents a shift in social value, in terms of magnitude (reinforcement from peer affiliation and acceptance) and quality (family to peer), and may reflect re-attribution of positive and negative values to distinct social stimuli (Ernst and Hardin, 2009). Thus, the shift in social orientation during adolescence directly interacts with the dynamics of the three maturing nodes of the Triadic Model.
The next section reviews functional neuroimaging studies that illustrate the differential recruitment of the Triadic nodes across adolescence and within different contexts (see Table 2 for a summary of all studies reviewed below). We will restrict the review to studies that have directly compared neural functioning of systems involved in the Triadic Model between youths and adults. In doing so, we will argue that imbalanced functioning of the threat and reward systems relative to the control system in certain contexts can result in an increased risk of engaging in potentially harmful behaviors and developing psychopathology.
Table 2.
Summary of results from the fMRI studies discussed in the current review. The table is organized according to the three neural systems that were studied: the threat system, the reward system, and the control system. The information provided for each study includes author names and year of publication, age and size of each sample recruited, task used, stage(s) analyzed, and neuroimaging results from group comparisons (i.e., youths versus adults).
| Authors | Age Group |
Task | Stage analyzed |
Threat Node Results |
Reward Node Results |
Cognitive Control Node Results |
Other |
|---|---|---|---|---|---|---|---|
| Threat Context | |||||||
| Guyer et al., 2008 | 31 adolescents (9–17 yo); 30 adults (21–40 yo) | Passive viewing of faces | Threat stimuli (fearful faces vs. neutral faces) | Amygdala: Adolescents > Adults | None | None | Fusiform face area: Adolescents > Adults Amygdala-Hippocampus functional connectivity: Adolescents < Adults |
| Hare et al., 2008 | 12 children (7 – 12 yo); 24 adolescents (13 – 18 yo); 24 adults (19–32 yo) | Emotional Go/No-Go paradigm (faces) | Threat stimuli (fearful faces vs. neutral faces) | Amygdala: Adolescents > Adults & Children; Repeated stimulus exposure reduced amygdala activity to baseline or below in all three age groups; Greater amygdala habituation was associated with lower trait anxiety in adolescents and adults | None | None | Failure to habituate was associated with reduced vPFC-Amygdala functional connectivity |
| Lau et al., 2011 | 15 adolescents (Mean age (SD) = 13.33 (2.35)); 20 adults (Mean age (SD) = 28.90 (8.77)) | Passive discriminative fear-conditioning task | Threat stimuli (neutral faces paired with UCS (CS+) vs. neutral faces not paired with UCS (CS-) | Hippocampus : Adolescents > Adults; Amygdala: Adolescents > Adults | None | DLPFC: Activation positively correlated with fear ratings in adults, but not adolescents | None |
| Reward Context | |||||||
| Ernst et al., 2005 | 16 adolescents (9 – 17 yo); 14 adults (20 – 40 yo) | Wheel of Fortune (WOF) task | Feedback phase | Left & Right Amygdala: Adolescents < Adults | Left N. Accumbens: Adolescents > Adults | None | None |
| Eshel et al., 2007 | Same sample as Ernst et al., 2005 | Wheel of Fortune (WOF) task | Choice Selection phase (risky decisions) | None | None | OFC/VLPFC & dACC: Adolescents < Adults | None |
| Galvan et al., 2006 | 13 children (7 – 11 yo); 12 adolescents (13 – 17 yo); 12 adults (23 –29 yo) | Delayed response two-choice task with parametrically manipulated reward values | Earned reward | None | N. Accumbens: Adolescents > Adults & Children; Orbital FC: Children & Adolescents > Adults | None | None |
| Cognitive Control in the Context of Reward | |||||||
| Geier et al., 2010 | 18 adolescents (13–17 yo); 16 young adults | Monetary Incentive Antisaccade Task | Reward trials vs. Neutral trials (cue appraisal, saccade preparation, saccade execution) | None | Cue Appraisal V. Striatum during Reward trials: Adolescents < Adults Saccade Preparation V. Striatum during reward trials: Adolescents > Adults Saccade execution OFC during Neutral trials: Adolescents > Adults | Cue Appraisal sPCS, inferior frontal gyrus, precuneus, & IPL during Neutral trials: Adolescents < Adults, no group difference on Reward trials Saccade Preparation sPCS, MFG/SFG during both Neutral and Reward trials: Adolescents > Adults; iPCS & MFG/ACC during Reward trials: Adolescents > Adults Saccade execution ACC during Neutral trials: Adolescents > Adults | None |
| Hardin et al., in preparation | 15 children (9–11 yo); 15 adolescents (14–16 yo); 15 adults (20–25 yo) | Saccade Reward Task | Reward trials vs. Neutral trials (antisaccade-prosaccade) | None | Striatum during Reward vs Neutral trials (antisaccade-saccade): Adolescents > Adults | rIFG during Reward vs. Neutral trials (antisaccade-saccade): Adolescents > Children | None |
| Smith et al., 2011 | 35 adolescents (10–17 yo); 35 adults (18–43 yo) | Rewarded Continuous Performance Task | Rewarded target trials vs. Non-rewarded target trials | Positive correlation between age and activation in the left anterior insula | Positive correlation between age and activation in the striatum | Positive correlation between age and activation in DLPFC, inferior PFC, ventromedial OFC | Positive correlation between age and activation in the SMA, brainstem, thalamus, middle temporal and occipital cortices and cerebellum. Negative correlations between age and activation in the inferior temporal gyrus and posterior cingulate |
Neuroimaging Studies Examining the Triadic Neural Systems across Development
The Threat System
Functionally, neuroimaging work in humans is beginning to identify age-related differences in threat system functioning. Overall, the amygdala seems to be more responsive in adolescents than in adults during exposure to threatening social stimuli (Guyer et al., 2008; Killgore and Yurgelun-Todd, 2004; Monk et al., 2003). For example, Guyer and colleagues (2008) examined developmental differences in amygdala activation and neural connectivity to passively viewing fearful faces in 30 adults (21–40yo; 17 male) and 31 adolescents (9–17 yo; 16 male). Adolescents had greater amygdala and fusiform gyrus activation relative to adults when viewing adult fearful faces. In addition to having an increased amygdala response, adolescents showed weaker amygdala-hippocampus connectivity than adults. These findings suggest that the neural resources used for processing social-emotional stimuli differ with development. Adolescents may place greater emphasis on perception and identification of the emotional face, inferred from stronger recruitment of the amygdala, whereas adults rely more on memory of emotional information, inferred from the tighter amygdala-hippocampal link.
Similarly, Hare and colleagues (2008) examined the role of development and trait anxiety on neural reactivity and habituation to emotionally evocative facial stimuli. Specifically, 12 children (7–12 years old; 7 male), 24 adolescents (13–18 years old; 14 male), and 24 adults (19–32 years old; 10 male) were exposed to an emotional go-nogo paradigm that involved viewing happy, calm (neutral), and fearful faces. Adolescents showed greater amygdala reactivity to fearful faces relative to children or adults; however, amygdala activity decreased to near or below baseline with repeated exposure to the fearful faces in adults and adolescents. Additionally, the degree of amygdala habituation was negatively associated with severity of trait anxiety, such that both adolescents and adults showing reduced habituation over repeated exposures reported higher trait anxiety. Finally, failure to habituate was also associated with weaker functional connectivity between the ventral PFC and amygdala. These findings suggest that exaggerated emotional reactivity during adolescence may be associated with less efficient top-down control. The lack of efficiency to modulate the amygdala is reflected by the absence of differential local activation in the PFC between adolescents and adults, despite greater activation of the amygdala in adolescents. The modulatory role of the PFC on amygdala function is also suggested by higher anxiety levels and slower habituation both being associated with a weaker amygdala-PFC link. From the Triadic Model perspective, individual differences (i.e., trait anxiety) and environmental context (i.e., exposure to fearful social stimuli) may interact to drive the equilibrium shift toward preferential recruitment of the threat system among adolescents.
More recently, 15 adolescents (Mean age (SD) = 13.33 (2.35); 10 males) and 20 adults (Mean age (SD) = 28.90 (8.77); 13 males) completed a fear learning paradigm to examine the neurodevelopment of discriminative learning between threat and safety cues (Lau et al., 2011). Specifically, during a pre-conditioning phase, participants saw images of two female actresses displaying neutral expressions. Next, participants completed a conditioning phase, during which the neutral expression of one actress was randomly selected as the conditioned stimulus (CS+) by being paired with a fearful expression and the sound of a female scream (unconditioned stimulus; UCS). Conversely, the neutral expression of the second actress was never paired with the UCS. Over the course of conditioning, one neutral expression (CS+) became a threat cue, while the second neutral expression (CS−) became a safety cue. FMRI results showed that adolescents had greater activity in subcortical areas such as the amygdala and hippocampus during discriminant threat/safety learning in the conditioning phase. Furthermore, unlike adolescents, adults’ engagement of the PFC was positively correlated with fear ratings. These findings, suggest that the functional imbalance between regions involved in threat/safety discrimination may cause adolescents to rely upon cruder, subcortically mediated forms of threat discrimination in comparison to adults. This may account for the more generalized and pervasive worries and fears that adolescents tend to experience (Pine et al., 1998; Weems and Costa, 2005).
Taken together, accumulating neuroimaging evidence suggests that adolescents display enhanced recruitment of the amygdala relative to adults in the context of viewing aversive social stimuli (Figure 2), suggesting that the threat node of the Triadic Model is hyperactive to social threat. At the same time, adolescents evidence weaker recruitment of the cognitive control system relative to adults, particularly when learning to discriminate between social threat and safety cues. The pattern of increased amygdala and reduced prefrontal engagement among adolescents compared with adults appears specific to exposure to threatening social stimuli. Indeed, it will be important to (1) examine whether this normative pattern of neural response generalizes to non-social aversive cues, (2) systematically study how the threat system behaves in different contexts (e.g., reward), and (3) examine age-related differences in reward system functioning in the context of social threat (e.g., Ernst and Paulus, 2005; Hardin and Ernst, 2009).
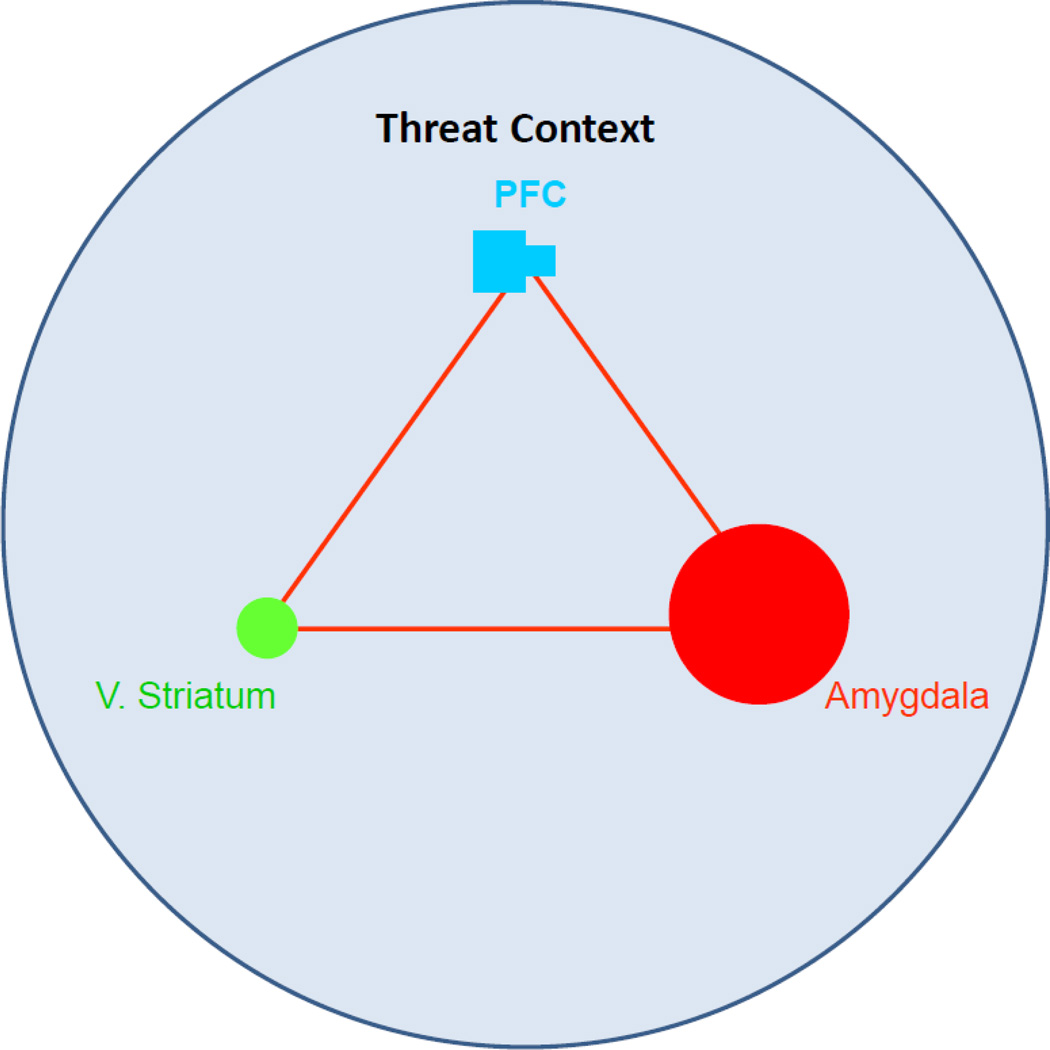
Figure 2.
In the context of threat (e.g., fearful social cues), adolescents display enhanced recruitment of the threat system (localized in the amygdala) and reduced recruitment of the cognitive control system (localized in the prefrontal cortex) relative to adults. This shift in the functional equilibrium among the triadic nodes may contribute to the emergence of affective intensity and lability during this developmental period.
The Reward System
Functional neuroimaging studies have also begun to explore developmental changes in reward and control functioning in the context of appetitive stimuli (e.g., Ernst et al., 2005; Eshel et al., 2007; Galvan, 2006). For instance, 16 adolescents (9–17 yo; 8 male) and 14 adults (20–40 yo; 7 male) completed the Wheel of Fortune task (WOF; Ernst et al., 2004) (Ernst et al., 2005). On each trial, participants saw a wheel divided into two colored slices, each representing the probability of winning a reward. Participants could only win or not win money, and the probability and magnitude of rewards varied across trials. Participants selected one color and if the computer randomly selected this color, the participant won the reward. Conversely, if the computer chose the alternate color, the reward was omitted. Researchers examined blood oxygenation level dependent (BOLD) response during feedback, when participants were informed about reward receipt. Bilateral amygdala and nucleus accumbens were activated in response to win vs. no-win for all subjects. However, group comparisons revealed enhanced activation in the left nucleus accumbens and reduced activation in the left amygdala among adolescents compared to adults during reward receipt. During reward omission, however, adults demonstrated greater reduction of the fMRI BOLD signal in the amygdala relative to adolescents. Taken together, the authors suggested that this pattern of (1) increased activation of the reward system (nucleus accumbens), and (2) decreased activation of the threat system (amygdala) shown by adolescents in the context of reward receipt, may contribute to the increase in risky behaviors and novelty-seeking observed in adolescence.
A subsequent study reported on data from the same study, but focused on the selection, rather than feedback, phase of the Wheel of Fortune task (Eshel et al., 2007). Significant group differences were detected in neural response to risky decisions, with significantly greater activation in the orbitofrontal/ventrolateral PFC and dorsal anterior cingulate cortex in adults than adolescents. Since these regions are involved in the control system and play a role in inhibitory processes, reduced BOLD activation in these regions during risky decision-making among adolescents may also contribute to increased risk-taking during adolescence.
Galvan and colleagues (2006) had similar findings in a study including 37 participants between the ages of 7–29 (22 males), including 13 children (7–11 yo), 12 adolescents (13–17 yo), and 12 adults (23–29 yo). Participants completed a monetary reward paradigm (pirate task) that manipulated reward magnitude. Among adolescents, activation of the nucleus accumbens in response to reward was exaggerated relative to prefrontal activation, a pattern that differed from those seen in children and adults. More specifically, nucleus accumbens activation was greater in adolescents than adults. Conversely, the pattern of activation in the orbital frontal cortex among adolescents was more similar to that of children than that of adults, with more diffuse patterns of activity. The authors suggested that these findings showed a functional imbalance of subcortical reward-related systems relative to top-down cortical control systems, a pattern that may drive elevations in risky behaviors in adolescence. Further, these results support the hypothesis of a developmental shift in the equilibrium among the three nodes of the Triadic Model, with adolescents preferentially recruiting the reward system over the cognitive control system when processing appetitive stimuli (Figure 3).
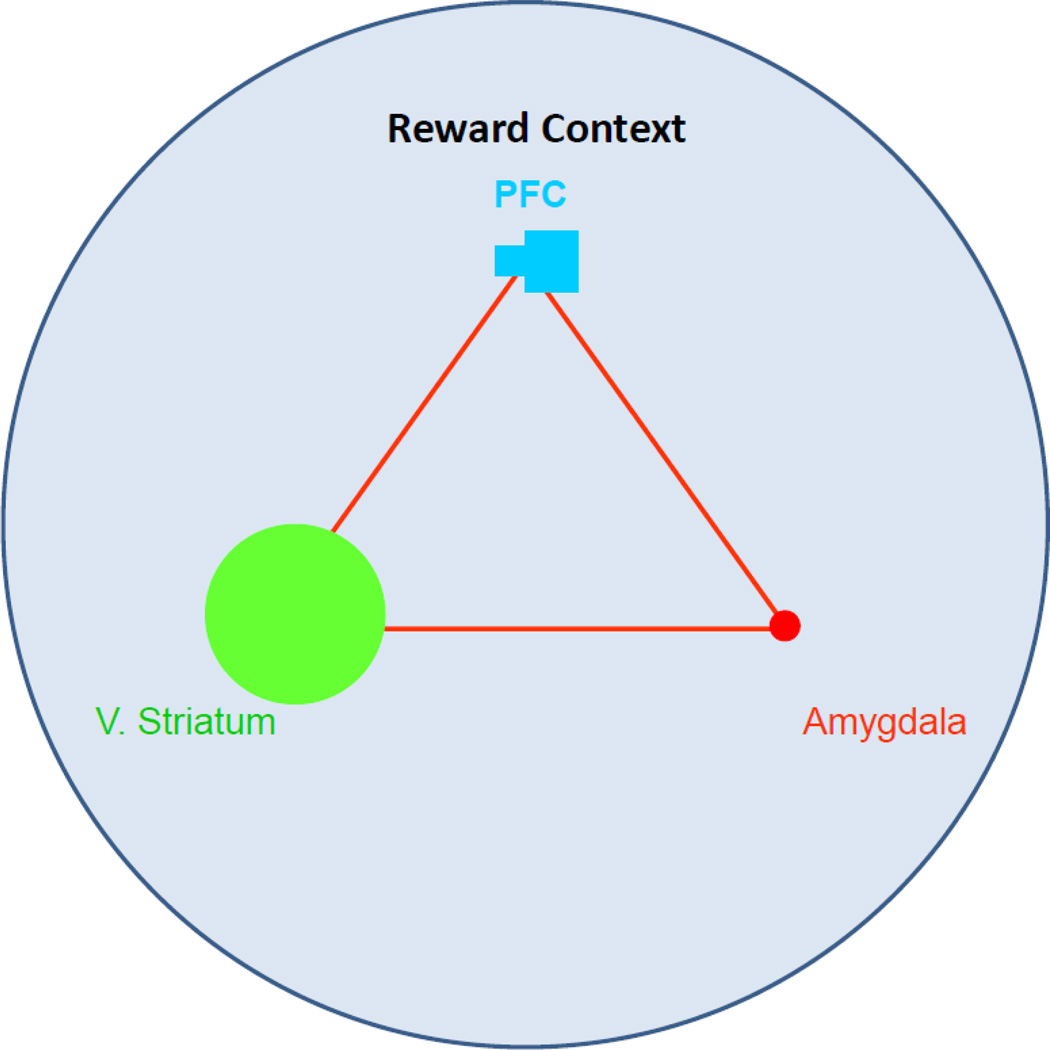
Figure 3.
In the context of reward, adolescents display preferential recruitment of the reward system over the cognitive control system when processing appetitive stimuli. This shift in the functional equilibrium among the triadic nodes may drive typical increases in risk-taking that are commonly seen among adolescents.
Importantly, some studies have reported conflicting evidence, such as reduced recruitment of reward structures in response to rewarding stimuli among adolescents compared to adults (Bjork et al., 2004; Forbes et al., 2010). Specifically, post-pubertal adolescents have displayed reduced striatal activation to monetary reward tasks relative to adults (Bjork et al., 2004; Forbes et al., 2010), and pre-pubertal children (Forbes et al., 2010). Clearly, additional research is needed to resolve these contradictory findings and clarify how developmentally unique patterns of function in reward structures translate into distinct adolescent behavior patterns. In addition, how the amygdala and related threat circuits behave in appetitive contexts among adolescents relative to adults needs further research; however, preliminary data suggest a diminished amygdala response in these contexts among adolescents compared to adults.
The Control System
Recently, a growing body of work has emerged examining the neurodevelopmental correlates of cognitive control processes, such as inhibition and sustained attention, revealing interesting effects of incentives on both behavioral and neural responses to inhibition-related tasks (Figure 4). For example, Geier and colleagues (2010) examined neural activation during a novel monetary incentive antisaccade task among 18 adolescents (13–17 yo; 10 males) and 16 adults (18–30 yo; 6 males). An antisaccade is an eye movement in the opposite direction of a suddenly appearing target, requiring the inhibition of a prepotent response toward the target, and the execution of an endogenously guided response. Each trial began by cuing the reward contingency of the upcoming saccade execution (i.e., reward versus neutral (non-reward) trials). Reward trials resulted in faster inhibitory responses across groups. During neutral trials, adolescents attenuated activation in oculomotor and cognitive control regions relative to adults; however, this group difference was not observed on reward trials, suggesting that the presence of incentives for engaging inhibitory functions could ‘normalize’ the deficient cognitive control function seen in adolescents. On reward trials, the results of group comparisons varied across task phases, with adolescents showing reduced ventral striatum activation during cue appraisal, but exaggerated ventral striatum recruitment during saccade preparation relative to adults. No group differences were reported during saccade execution. Overall, these findings suggest that adolescents may be particularly sensitive to reward modulation of inhibitory control function, and this sensitivity to reward may lead to improved behavioral inhibition.
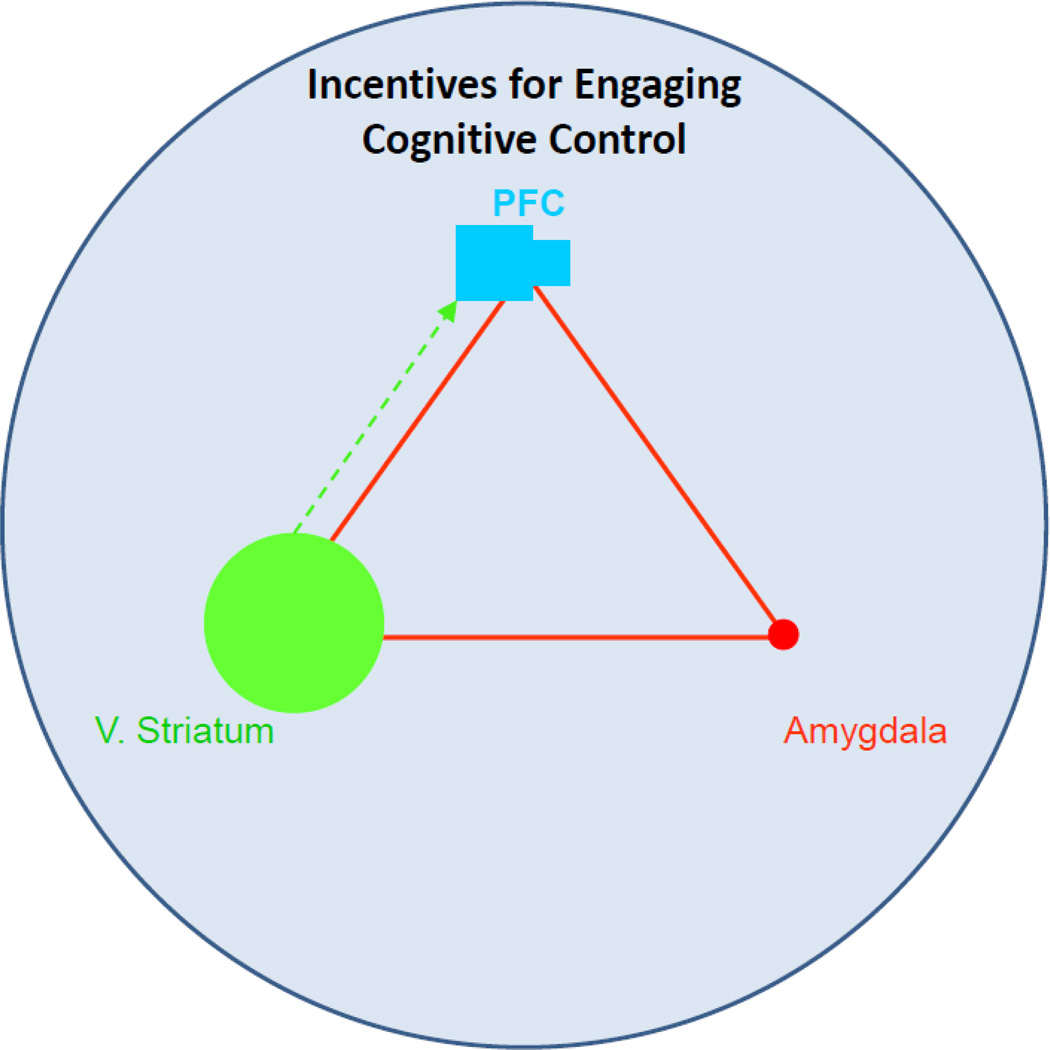
Figure 4.
When adolescents are provided with incentives to engage cognitive control functions, such as inhibitory control and sustained attention, the deficient recruitment of the cognitive control system is ameliorated, and adolescents show marked improvements in the ability to sustain attention and inhibit prepotent responding. Thus, adolescents appear to be uniquely sensitive to the effects of incentives on cognitive control functions.
Likewise, Smith and colleagues (2011) employed a continuous performance task (CPT), which involved presenting participants with a series of letters and instructing them to push a button when they saw rare “target letters” (i.e., X and O) and to inhibit their response when they saw any other “non-target letters” (i.e., all other letters). Half of the participants were informed that they would be rewarded for responding to the letter X, while the other half were rewarded for responding to the letter O. Thus, the task yielded three types of trials: non-targets, rewarded targets and non-rewarded targets. Behaviorally, adolescents evidenced significantly slower responding to nonrewarded targets relative to adults, and responded significantly faster to rewarded targets relative to nonrewarded targets. No difference in reaction time across target-types was found among adults, suggesting that adolescents are more sensitive to the effects of incentives on sustained attention compared to adults. The comparison of neural activation in response to rewarded vs. non-rewarded targets revealed positive linear relationships between age and reward-induced activation in regions implicated in sustained attention (e.g., dorsolateral PFC and ventromedial orbitofrontal cortex), but negative linear relationships between age and reward-induced activation in regions coding for visuospatial attention (e.g., putamen, posterior cingulate cortex, and inferior temporal gyrus). These findings are in line with previous work suggesting that adolescents display a particular sensitivity of the cognitive control system to reward modulation, which may contribute to improvements, or “normalization,” of control functions such as inhibition or sustained attention.
Implications for Prevention
Data from fMRI studies largely support a neurodevelopmental context-specific shift in the equilibrium between the three nodes of the Triadic Model. Adolescents tend to display preferential recruitment of threat or reward systems, and diminished modulation of these systems by the cognitive control system when processing aversive or appetitive stimuli (Figures 2 and 3). However, providing incentives to adolescents can reduce deficiencies relative to adults in the cognitive control system, and improve cognitive performance (Figure 4). Based on this interpretation and supporting data, it is feasible to apply available prevention approaches to specifically target the neurodevelopmental vulnerabilities that are typical of normative adolescent development and exploit adolescent sensitivity to rewards in ways that incentivize control functions in order to reduce risk-taking.
One approach may directly target the cognitive control system with cognitive and/or behavioral strategies designed to enhance cognitive functioning and inhibitory control. For example, cognitive remediation approaches, including working memory training, have impacted behavioral and neural functioning. Although specific training paradigms have varied across studies, there are a few general similarities. First, the training paradigms generally include combinations of visuospatial and auditory modalities, training generally occurs on a daily basis for 15–45 minutes per session, depending on the population being trained (i.e., shorter for children), and the tasks are designed to adapt to participant performance, becoming increasingly difficult as participant performance improves (e.g., Jaeggi et al., 2008; Klingberg et al., 2002; Olesen et al., 2004).
Working memory training has already yielded promising results among clinical samples characterized by deficits in cognitive control, namely those with attention-deficit/hyperactivity disorder (e.g., Klingberg et al., 2005; Klingberg et al., 2002). Moreover, neuroimaging studies have revealed changes in neural structure and function among adults following working memory training, including increased activation in prefrontal and parietal regions while engaged in working memory tasks (Olesen et al., 2004; Westerberg and Klingberg, 2007) and increased white matter integrity in frontoparietal circuits (Takeuchi et al., 2010). As such, cognitive remediation may be useful for enhancing cognitive control functions and facilitating the recruitment of prefrontal regions implicated in cognitive control among adolescents, and ultimately reduce risk behavior; however, more research is needed to test this hypothesis. Given the particularly important role of context in determining developmental differences in the equilibrium of the Triadic Model, novel approaches to working memory training or other forms of cognitive remediation that specifically aim to strengthen cognitive control functions in the face of aversive or appetitive stimuli may prove particularly fruitful. Moreover, adolescents may be particularly sensitive to approaches that incentivize engagement in working memory training, thus leading to even more pronounced training-induced changes in behavioral and neural functioning among adolescents relative to adults. Again, these hypotheses are speculative and are in need of empirical testing.
Alternatively, prevention efforts may yield positive results by directly targeting the increased reactivity in the threat system, which has been reported among adolescents, particularly in contexts involving negative social-emotional stimuli. For example, acceptance-based strategies, which generally involve helping individuals to accept negative thoughts and emotions when they occur and engage in activities that are in line with their personal values (i.e., “where they want to go in life”) may be particularly beneficial for adolescents. Specifically, Biglan, Hayes, and Pistorello (2008) propose that training adolescents to accept negative thoughts and feelings about peer rejection and to define valued life directions that they would like to pursue can help them to resist pressures from deviant peers to engage in unhealthy behaviors, such as smoking cigarettes. Given the particular sensitivity of the adolescent threat system to social threat cues, acceptance-based approaches may provide the means to overcome this particular vulnerability. Neuroimaging evidence indicates a key role of the amygdala in maintaining avoidance and escape behavior (Schlund and Cataldo, 2010), and that amygdale reactivity to social threat cues can habituate over the course of repeated cue exposure (Hare et al., 2008); therefore, by training adolescents to accept rather than avoid negative emotions (i.e., by caving in to peer pressure), it may be possible to reduce recruitment of the amygdala in the context of social threat. Similarly, mindfulness meditation has been proposed to work as a treatment for psychiatric disorders including drug dependence and depression by reducing stress reactivity (i.e., decreasing recruitment of the threat system, including the amygdala), while simultaneously strengthening cognitive control circuits (i.e., increasing recruitment of the prefrontal cortex) (e.g., Brewer et al., 2009). In this way, acceptance-based and mindfulness-based approaches may reduce the incidence of both risk-taking and avoidance behaviors in the face of negative affect during adolescence, thus reducing the risk of deleterious outcomes.
Finally, prevention strategies designed to target the preferential recruitment of the reward system in the context of appetitive stimuli during adolescence may also help to reduce negative outcomes. For example, behavioral activation (BA) treatments (Lejuez et al., 2011; Reynolds et al., 2011) provide adolescents with alternative behavioral options that are reinforcing (e.g., prosocial, yet rewarding activities such as sports, volunteering, and hobbies) and less likely to lead to detrimental long-term consequences compared to risk-taking behaviors (e.g., substance use, unprotected sex, and delinquency). In general, BA involves identifying specific activities that are enjoyable and important to individuals, and then providing strategies to increase engagement in these activities in order to improve mood and reduce engagement in unhealthy behaviors. Given the importance of peer affiliation to adolescents, incorporating peer-oriented activities that are enjoyable, yet healthy, may be particularly effective. Additionally, BA often also involves the development of ‘behavioral contracts’ which can be made with friends or family members in order to modify the environmental contingencies that maintain unhealthy behaviors. In their simplest form, behavioral contracts primarily involve an agreement from a loved one to remove incentives for engaging in unhealthy behavior, and provide incentives for healthy behaviors instead. For example, adolescents participating in a targeted BA substance use prevention program may develop a behavioral contract with their parents to earn socially-oriented incentives (e.g., opportunities to go to the movies with their friends) for engaging in healthy behaviors, such as completing their homework. Simultaneously, the contract may also outline specific incentives that can be lost (e.g., opportunities to text or chat with friends online) for engaging in risky behaviors, such as staying out past curfew or lying.
BA has already shown promising results in reducing alcohol-related problems among late-adolescent college freshmen. BA was recently integrated into a college orientation program to address common adjustment difficulties among incoming college students (Reynolds et al., 2011). Compared to a contact-matched control group, participants who received BA displayed significant reductions in problem drinking by the end of the semester. As such, BA may be an effective approach to capitalize on the specific balance of the functional equilibrium within the Triadic Model during adolescence, particularly in terms of adolescent sensitivity to social orientation and rewards by (1) helping them to identify and implement alternative rewarding, yet non-substance related activities with their peers (e.g., playing sports, going to the movies) and (2) developing behavioral contracts to provide clear incentives for engaging in healthy, adaptive behaviors. In this way, the preferential recruitment of the reward system over the cognitive control system may be exploited to prevent deleterious outcomes among adolescents.
Conclusions and Future Directions
The current review emphasizes significant effects of neurodevelopment and context on the functional equilibrium among the nodes of the Triadic Neural Systems Model of Motivated Behavior. We suggest that shifts in this equilibrium may contribute to the emergence of typical adolescent characteristics, including affective intensity and lability, peer-oriented social engagement, and increased risk-taking. Although mainly used in the current review as a framework to aid in the interpretation of neuroimaging findings among adolescents, the Triadic Model can serve as a tool for guiding future research on the neurobiology of motivated behavior in adolescents. For example, there is a great need for more research on reward system functioning in the context of threat, and threat system functioning in the context of reward. Similarly, research on cognitive control system development could examine the effect of threat, instead of rewards, as a motivator for control system engagement. Additionally, studies of resting state functional connectivity across development are emerging (Fair et al., 2009; Stevens et al., 2009), and may provide insight into the functional balance across the Triadic nodes in different contexts. Finally, neuroimaging methods yielding greater temporal resolution, such as ERP or MEG, could examine the temporal sequence of activation across the Triadic nodes, further refining our understanding of mechanisms driving functional equilibrium shifts in different contexts.
Other directions for future research warrant mentioning. First, future researchers could examine the effects neuroendocrine factors, such as pubertal changes in sex hormones, as well as acute reactivity of stress hormones, on the Triadic functional equilibrium. Second, longitudinal studies are needed to directly examine the causal effects of individual differences in Triadic functioning on affective, behavioral, and social outcomes. Third, genetic impact on individual variations of the dynamics of the Triadic Model may provide knowledge on molecular contributors to the mechanisms underlying individual changes in the neural functions associated with motivated behavior. Fourth, additional research examining the effectiveness of novel incentive-based prevention and intervention efforts to reduce adolescent risk-taking and psychopathology is greatly needed. Finally, efforts are needed to examine the extent to which these novel preventions and interventions, particularly incentive-based approaches, lead to functional changes in the Triadic Model equilibrium. Once Triadic functioning is better understood among normatively developing populations, research can be replicated and extended to examine the same processes among individuals suffering from psychopathology, expanding our knowledge of the neurobiology of adolescent motivated behavior even further.
Table 1.
Summary of the three nodes of the Triadic Model: The role, anatomy, and function of the three systems.
| Role in Triadic Model | ||
|---|---|---|
| Threat | Reward | Cognitive Control |
| Anatomy | ||
| Amygdala Hippocampus Insula |
Striatum Orbitofrontal Cortex |
Dorsolateral PFC Ventromedial/Orbital PFC Anterior Cingulate Cortex |
| Function | ||
| Aversive Stimuli Fear Responses Threat Avoidance |
Appetitive Stimuli Approach Motivation Motor Response Positive Affect |
Salience Detection Executive Attention Motor Control Conflict Detection Conflict Monitoring Conflict Resolution |
Highlights.
Adolescence is marked by increases in risky behavior and psychopathology
The Triadic Model is a framework to inform the neural basis of adolescent behavior
Three neurocircuits encoding threat, reward, and control drive motivated behavior
Development and context modulate the functional triadic equilibrium
Understanding triadic functioning can inform incentive-based prevention
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The opinions and assertions contained in this paper are the private views of the authors and are not construed as official or as reflecting the views of the NIMH or the Department of Health and Human Services.
Conflicts of Interest Statement
The authors declare that there are no conflicts of interest.
Contributor Information
Jessica M. Richards, Email: jessic1@umd.edu.
Rista C. Plate, Email: rista.plate@nih.gov.
Monique Ernst, Email: ernstm@mail.nih.gov.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Biglan A, Hayes SC, Pistorello J. Acceptance and commitment: implications for prevention science. Prev Sci. 2008;9:139–152. doi: 10.1007/s11121-008-0099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knuton B, Fong GW, G W, Caggiano DM, Bennett SM, Hommer DW. Incentive-Elicited Brain Activation in Adolescents: Similarities and Differences from Young Adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, Grier A, Bergquist KL, Reis DL, Potenza MN, Carroll KM, Rounsaville BJ. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30:306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008 doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Hardin M. Neurodevelopment underlying adolescent behavior: A Neurobiological Model. In: Press P, editor. Developmental Social Cognitive Neuroscience. 1st ed. 2009. pp. 165–169. [Google Scholar]
- Ernst M, Mueller SC. The adolescent brain: insights from functional neuroimaging research. Dev Neurobiol. 2008;68:729–743. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson E, Jazbec S, McClure E, Monk C, Leibenluft E, Blair J, Pine D. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Spear LP. Reward Systems. In: de Haan M, Gunnar MR, editors. Handbook of developmental social neuroscience. New York: Guilford Press; 2009. p. 558. [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a "local to distributed" organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Mayles DL, Tarr JA, Sciarrillo SR, Dahl RE. Healthy adolescents' neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Galvan A. Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in Reward Processing and Its Influence on Inhibitory Control in Adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Ernst M. Functional brain imaging of development-related risk and vulnerability for substance use in adolescents. J Addict Med. 2009;3:47–54. doi: 10.1097/ADM.0b013e31819ca788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept Mot Skills. 2004;99:371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Dev. 2002;73:1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav Modif. 2011;35:111–161. doi: 10.1177/0145445510390929. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. NeuroImage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Sawyer SM, Viner RM, Haller DM, Bose K, Vos T, Ferguson J, Mathers CD. Global patterns of mortality in young people: a systematic analysis of population health data. Lancet. 2009;374:881–892. doi: 10.1016/S0140-6736(09)60741-8. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Reynolds EK, Macpherson L, Tull MT, Baruch DE, Lejuez CW. Integration of the brief behavioral activation treatment for depression (BATD) into a college orientation program: Depression and alcohol outcomes. J Couns Psychol. 2011 doi: 10.1037/a0024634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund MW, Cataldo MF. Amygdala involvement in human avoidance, escape and approach behavior. NeuroImage. 2010;53:769–776. doi: 10.1016/j.neuroimage.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents' emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Smith AB, Halari R, Giampetro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spear LP. The Psychobiology of Adolescence. In: Kline K, editor. Authoritative communities: The scientific case for nurturing the whole child (the search institute series on developmentally attentive community and society) New York, NY: Springer Publishing; 2007. pp. 263–280. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Belsky J. A sociobiological perspective on psychopathology in adolescence. In: Cicchetti D, Toth S, editors. Rochester Symposium on Developmental Psychopathology. Rochester, NY: University of Rochester Press; 1996. pp. 93–124. [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou SY, Kawasaki Y, Takahashi T, Matsui M, Seto H, Ono T, Kurachi M. Male-specific volume expansion of the human hippocampus during adolescence. Cereb Cortex. 2005;15:187–193. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, Costa NM. Developmental differences in the expression of childhood anxiety symptoms and fears. J Am Acad Child Adolesc Psychiatry. 2005;44:656–663. doi: 10.1097/01.chi.0000162583.25829.4b. [DOI] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein RJ, Hankin BL, Hedeker D, Flay BR. Longitudinal Patterns of Daily Affect and Global Mood During Adolescence. J Res Adolesc. 2007;17:587–600. doi: 10.1111/j.1532-7795.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg H, Klingberg T. Changes in cortical activity after training of working memory--a single-subject analysis. Physiol Behav. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Yakovlev P. The mylogenetic cycles of regional maturation of the brain. Blackwell Scientific Publishing, Boston. 1967 [Google Scholar]