Abstract
Studies of CD8 T cell responses to vaccination or infection with various pathogens in both animal models and human subjects have revealed a markedly consistent array of age-related defects. In general, recent work shows that aged CD8 T cell responses are decreased in magnitude, and show poor differentiation into effector cells, with a reduced arsenal of effector functions. Here we review potential mechanisms underlying these defects. We specifically address phenotypic and numeric changes to the naïve CD8 T cell precursor pool, the impact of persistent viral infection(s) and inflammation, and contributions of the aging environment in which these cells are activated.
Keywords: Aging, CD8 T cells, immunity, infection, homeostasis
1. Introduction
The world is rapidly aging. According to the WHO, the proportion of people over 60 years of age is increasing faster than any other population in nearly every country, and is expected to surpass 2 billion people worldwide by the year 2050 [1]. Infectious diseases remain a major cause of morbidity and mortality for the elderly, with influenza and pneumonia combined to rank as the 6th leading cause of death in the United States for those over 65 [2]. The economic impact of these infections is staggering; in 2000, combined billing to Medicare and Medicaid for in-patient treatment of pneumonia was nearly $14 billion [3]. Immunization is one of our most powerful tools to prevent infectious disease, yet the elderly respond poorly to current vaccine platforms [4,5].
Age-associated immune senescence is a catch-all phrase that has been used to describe a plethora of changes to the immune system over a lifetime, including decreased thymic output following puberty, alterations in lymphocyte population dynamics in late life, and reduced intracellular signaling capacities within those cells as a consequence of increased age, all of which are addressed by expert reviews in this volume. In fact, when measured in isolation, there are very few components of the immune system that seem to escape the negative effect of aging. An integrated and comprehensive picture describing how all of these cells, microenvironments, soluble factors, membrane-bound signaling molecules and processes within a given immune response result in “decreased immunity with aging” is still not available. Are there common defects that underlie immune responses to multiple pathogens or is each infection unique? In this review, we will focus on factors contributing to effective CD8 T cell responses to infection, and how disturbances of several of these factors likely collaborate to give impaired protection to infectious disease in old age. In the accompanying review, Haynes and Swain address many of the similar issues for CD4 T cells.
2. Age-associated T cell defects in response to pathogens
Impaired T cell immunity to pathogens as a consequence of increased age has been demonstrated in mice, birds, dogs, monkeys and man, and in response to bacterial, viral, fungal, and parasitic infections. Like so many other biologic processes, the coordinated efforts of an immune response become less effective in later life. What has emerged in the last ~10 years is a remarkable similarity in the CD8 T cell defects observed in response to varying intracellular pathogens, regardless of pathogenesis or host cell reservoir (Figure 1). Research to identify universal defects underlying impaired immunity to multiple pathogens is essential for new therapeutic strategies to improve immune defense in late life, and we shall review these efforts, highlight the general principles discovered so far, and discuss areas where we still lack conclusive information as to how aging impacts pathogen-specific CD8 T cell responsiveness.
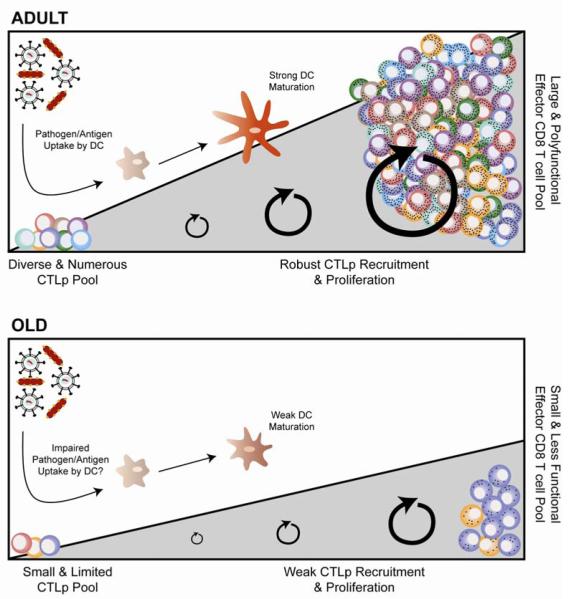
Figure 1. Components that contribute to age-related impaired CD8 T cell responses.
Figure depicts the features of the CD8 T cell immune response in adult (top) and old (bottom) mice, although several of the features have also been also found in humans (see Table 1). Note that the reduced naïve CD8 T cell pool (lower left corner, bottom panel) is faced with potentially reduced stimulation by old DC which can exhibit decreased uptake of Ag and impaired maturation; that and cell-intrinsic defects in proliferation and differentiation of old naïve CD8 T cells result in a reduced effector pool with insufficient effector function, which is evident both in the total pool and at an individual cell basis.
2.1 Multiple infection models identify similar age-associated CD8 T cell defects
West Nile virus (WNV) first appeared in the United States in 1999, and it was quickly apparent that patients of either increased age or with compromised immune status were in greater danger of severe disease or death [6]. Our initial studies showed that across multiple mouse strains, and with different viral isolates and routes of infection, aged mice succumbed to WNV infection at a rate ~4-6x greater than young animals [7-9]. In this infection, we characterized an assortment of functional defects within the antiviral CD8 (described in Section 2.2) and CD4 T cell populations. We have also conclusively shown that transferred CD8 and CD4 T cells isolated from unimmunized old mice show drastically inferior ability to protect RAG-deficient young adult mice from WNV infection[7]. Therefore, at a minimum, there is a clear defect attributable to the aged CD8 and CD4 T cell pool, which, even when the remainder of the immune system is young, still results in inferior resistance to infection. As multiple cell subsets participate in the adaptive immune response to WNV - with CD4 T cells, CD8 T cells, IFN, complement and B cells all contributing significantly to viral control and clearance [7] - it is difficult to precisely dissect the relative extent and importance of defects in specific CD8 T cellfunction. Cell, antibody,cytokine and complement transfers, individually and in combination, would be necessary to address the individual and joint roles of immune system components [7-10], however, properly controlledserial reconstitution of all components of the old immune system in adult host mice is difficult to achieve, leaving us with an incomplete picture.
To study age-related effector T cell defects in a model system dependent on CD8 T cells for pathogen clearance, we exploited systemic infection of mice with Listeria monocytogenes (Lm). Following infection with this intracellular bacterium, CD8 T cell-mediated bacterial clearance can occur in the absence of both CD4 T cell [11] and B cell populations [12], although both subsets contribute to the generation of CD8 memory [12,13]. Following Lm infection of aged mice, we again observed greater mortality and an identical array of impairments in effector CD8 T cell proliferation, differentiation and function as found in the WNV infection model [14].
In addition to our studies with WNV and Lm, poorCD8 T cell responses in aged mice have been observed in other model systems. CD8 responses to influenza are decreased in magnitude and show altered kinetics in aged mice following both intravenous and intranasal inoculation, as well as across different mouse strains[15,16]. ImpairedT cell responses in aged mice infected with E. cuniculimicrosporidia have also been demonstrated, particularly following oral inoculation[17].Yet others have reported defective primary CD8 expansion to LCMV in aged mice[18-20]. We have also observed impaired pathogen-specific CD8 T cell responses in aged mice infected with cowpox virus (CPXV) (J.L.U. et al., unpublished observations) and HSV-1[21] (M.J.S. et al., unpublished observations). By contrast, preliminary analysis of CD8 priming in response to vaccinia virus (VACV) infection appears comparable betweenadult and old mice (J.L.U., M.J.S. & J.N-Z.,unpublished observations). Whether these changes are due to the nature of the pathogen(s), or reflect differences in the unique T cell pool recruited into each response, is currently under investigation.
The two model pathogens we have characterized in greater detail – one viral (WNV), one bacterial (Lm-OVA) – are markedly different in routes of infection, host cell tropism, disease progression, and mechanisms of innate immune sensing of the pathogens. WNV is a positive-sense, single-stranded RNA arbovirus belonging to the Japanese Encephalitic Virus serocomplex of the Flavivirus family. It is transmitted through an enzootic cycle between birds and mosquitoes, with Culex pippiens and Aedes albopictus strains being responsible for much of the transmission [22]. In mice, following mosquito bite or subcutaneous infection, the primary site of replication is likely to include keratinocytes[23], although the exact early replication and pathogenesis remain to be comprehensively elucidated. Nonetheless, it is clear that the virus spreads through many parenchymal organs in mice[24], although the viral replication is modest and viremia does not reach the levels that would allow retransmission via mosquito bite. Around day 3 in mice,the virus reaches the central nervous system[24], where it has the potential to cause fatal meningoencephalitis, the cause of age-related increased mortality in both old mice [7] and humans [25]. Competent adaptive immunity in young and adult animals effectively combats the virus in the brain and controls it by day 10; failure to do so in old mice results in death, mostly between days 10-16 [7]. At the present, it is unclear whether WNV shows true persistence in mice and humans; evidence for and against delayed clearance and possible persistence has been presented[26-28].
In contrast, nearly 50 years of research has led to a very dynamic picture of the kinetics of Lm pathogenesis. Systemic infectionleads to rapid delivery of the bacteria to the spleen and liver, the primary tissues of infection. Both neutrophils and inflammatory monocytesare recruited to these sites within the first hours-days [29,30], and IFNγ production by innate cells is important for early control of the pathogen[31,32]. Lm is rapidly internalized into host cells, and bacterial by-products – but very few viable bacteria - are found within neutrophils and inflammatory monocytes cells within hours of infection[33], suggesting acritical role for phagocytic uptake and killing of bacteria in controlling the early stages of infection[30,34]. In contrast, viable Lm isalmost uniquely found within the CD8α+ dendritic cell (DC) subset within hours to days following infection[33,35], a reservoir that appears hospitable to intracellular Lm.
Once inside host cells, Lm escapes the phagosome and enters the cytosol via production of several virulence factors, then spreads from cell to cell by hijacking the host actin polymerization machinery and “propelling itself” into adjacent cells, avoiding exposure to humoral immunity (reviewed in [36]). Nearly ~50% of bacteria within CD8α+ DC have already escaped to the cytosol within 6 hours of infection [37].Because of this intracellular lifecycle, bacterial clearance is dependent on cytolytic CD8 T cells. In the face of a robust, intact immune system, Lm infection is generally cleared within 7-10 days, although there is some evidence that low numbers of bacteria can persist in both the gall bladder and bone marrow of infected animals [38,39].
Of note,the priming of CD8 T cells against Lm is drastically compromised in the absence of either productive intracellular infection of the CD8α+ DC subset[40], or following infection with pathogens unable to escape the phagocytic vacuole into the cytosol [37,41]. Collectively, these data suggest that intracellular pathogen recognition within the CD8α+ DC subset plays an important role in facilitating and/or directing the subsequent priming of effector CD8 T cell populations.
Upon pathogen recognition inside the host cell, there is convergence in the downstream innate signaling pathways for both WNV and Lm (as well as countless other pathogens), leading to induction of inflammatory cytokines and Type I interferons (IFN-I), which in turn activate a network of interferon-stimulated genes [42,43]. Sensing of WNV is mediated by pathogen-recognition receptors including TLR3, TLR7/8, and the cytosolic RIG-I-like receptors [44]. Innate sensing of intracellular Lm is also accomplished through TLR/MyD88 pathways, as well as the cytosolic NOD-like receptors (NLR), although MyD88-mediated recognition is dispensable (and perhaps inhibitory) for the development for adaptive immunity to Lm [41,42,45].
Signal 3 cytokines, chiefly IL-12 & IFN-I, have been increasingly recognized as important in regulating effective cellular immunity. IL-12 has been implicated as a major contributor to effective CD8 T cell priming (reviewed in [46]). Although very little has been published to date on WNV, in the context of Lm infection, IL-12 appears to predominantly serve to fine-tune the effector and memory differentiation programs[47,48], contributing to both clonal expansion [49], as well as the development of short-lived effector cells [50]. Interestingly, the CD8α+ DC subset appears to be a primary producer of IL-12 following in vivo Lm infection [35,51]. However, much redundancy exists in innate sensing pathways (at least in young mice) – in the absence of both IFN-I receptor and IL-12, a strong CD8 T cell response develops following Lminfection [48], and young CD8α+ DC lacking bothMyD88 and TRIF are still able to produce IL-12 [51].
Our preliminary (and coarse) analysis found no difference in the serum levels of IFN-I between adult and old mice following WNV infection [7], although we did not test the efficacy of IFN-I signaling in aged mice. A decreased efficacy of these pathogen-recognition signaling pathways and the “potency” of these signals in aging has been suggested by in vitro studies of WNV infection of aged human dendritic cells [52]. Further studies in this area to clarify how different innate pathways involved in early pathogen sensing may direct the subsequent development of protective CD8 T cell populations with aging are essential, and may help identify age-related mechanistic defects that can be targeted for intervention.
2.2 Functional defects in aged CD8 T cells
There are six common properties frequently measured as indicators of CD8 T cell function: the proliferative capacity of responding T cells, the magnitude of the antigen-specific CD8 response at the peak of expansion, the up-regulation of activation markers, the number of different effector molecules produced on a per-cell basis (including cytokines and lytic proteins, termed ”polyfunctionality”), the quantity of each effector molecule(s) produced, and the ability of the effector cells to lyse targets bearing cognate antigen. Our analyses of primary CD8 T cell responses in mice and monkeys, to viral and bacterial pathogens suggest that all of these properties are compromised to some degree in aged animals (Table 1).
Table 1. Age-related impaired CD8 T cell effector functions.
| Age-related Effector CD8 Defects |
Animal References | Human References |
|---|---|---|
| Proliferative capacity | [14,17,20,56,123] | [5,83,124] |
| Magnitude of primary response to infection/vaccination |
[7,14-20,86] | [5,125] |
| Up-regulation of activation markers |
[7,14,20] | [89] |
| Polyfunctional production of multiple effector proteins |
[7,14,20] | |
| Quantity of effector proteins produced |
[7,14] | [5,83,124-126] |
| Target cell lysis | [7,14,17-19,21] |
It is generally believed that most, if not all, of these T cell qualities are linked to each other, such that the acquisition of effector function follows several rounds of division, although there is surprisingly little hard evidence to support this prevalent view. In fact, at least some TCR transgenic CD8 T cells can acquire cytolytic function as soon as 96 hours post-infection and in the absence of division [53]. Whether this is also true for endogenous, polyclonal CD8 cells, and whether that process is affected by aging, remains unclear, mostly because of technical difficulties in identifying and isolating the very small antigen-specific T cell populations at early time points post-infection. Nonetheless, experiments utilizing the new tetramer enrichment protocol [54,55]to isolate rare, antigen-specific T cell populations are now feasible and could answer this question formally. Regardless, there is ample evidence that the aged T cells in an aged environment cannot proliferate in a sustained manner in response to infection to the same level as adult cells[17,56] (Fig. 1). Utilizing BrdU incorporation assays following Lm infection, we found that old CD8 T cells in an old environment initiated their proliferation at the same time in vivo as adult CD8 T cells in adult mice. However, the amount of BrdU incorporated was significantly lower in old cells, and their proliferationbegan lagging behind adult CD8 cells around days 4-6 post infection, at which time they also exhibited increased apoptosis [14]. Bcl-2 expression was not responsible for these differences, as protein levels were, in fact, higher in old CD8 T cells (M.J.S. and J.N-Z., unpublished observations). It remains to be tested by transfer experiments to what extent this difference in proliferation is cell-autonomous, although similar proliferative defects in aged memory CD8 T cells transferred into young adult recipients prior to re-challenge have been reported [20], suggesting this defect has a cell-intrinsic component. In parallel, aged effector CD8 T cells show decreased differentiation and function when compared to adult counterparts. The relationship between proliferation and differentiation in the course of effector T cell differentiation is incompletely understood, and it is possible, and in fact likely, that these two are indelibly coupled so that fewer divisions in old CD8 T cells result in partial or incomplete differentiation. One could further speculate that antigen-specific CD8 T cells that survive the proliferative burst in old animals are the ones that have proliferated fewer times, and that this results in their impaired differentiation.
However, one should bear in mind that other factors may contribute to the impaired proliferation potential of aged naïve CD8 T cells. External factors may include persistent elevation/dysregulation of the inflammatory cytokine environment [17], defects in recruitment and maturation of antigen presenting cells [56], and the altered density and duration of antigenic display to support T cell expansion [56]. Cell intrinsic factors such as altered signal transduction (mentioned above, and reviewed in [57] and by Goronzy’s group in this issue) and energy metabolism (reviewed in [58]) likely further impact the activation and differentiation of aging T cell populations.
Polyfunctional cytokine production has been suggested to be an important property of T cells that successfully control pathogens. HIV+ patients with slow disease progression often maintain a strong population of antiviral CD8 T cells that exhibit polyfunctionality [59,60] – although the cause and effect relationship between low viral burden (i.e. less chronic stimulation) and the maintenance of highly functional T cells remains unclear. Differences in TCR “signal strength”, as gauged by the response of cells to altered peptide ligands, have been shown to change the functional outcome following T cell stimulation – for example cytokine production vs. proliferation [61] – in long-term in vitro stimulated cell lines. However, old naïve T cells are likely very different from, and therefore should not be compared to, either long-term in vitro stimulated cells, or to antiviral effector or memory cells chronically exposed to Ag. Therefore, it remains to be determined whether old age per se leads to tuning down of naïve T cell thresholds in response to peptide:MHC (pMHC) abundance, and whether that, or the number of cell divisions, as discussed above, may influence polyfunctional responses.
When we examined polyclonal responses to both WNV and Lm, we found no difference in the functional TCR sensitivity of primary CD8 T cell effectors in old mice relative to young adults at the peak of the response, yet the old CTL effectors showed consistently reduced polyfunctionality as well as reduced quantities of the individual cytokines produced per cell [7,14]. While more work is required to fully understand the relationship between T cell proliferation, TCR sensitivity, differentiation and effector function, at a minimum this result tells us that the CD8 T cells that make it into the peak of the response against both WNV and Listeria are of the same functional avidity as those in adult animals. The most critical future experiments should aim to examine early interactions between naïve old T cells and adult and old APC, and to follow early expansion and differentiation of the old and adult T cells stimulated with different abundance of pMHC.
Functional defects in CD8 T cell memory responses with aging are believed to be less numerous, but are also less well understood - this topic has received far less attention to date, compared to the primary CD8 responses. For a full discussion of that topic, the reader is directed to a recent review [62], and to an excellent discussion of CD4 memory responses in this volume by Haynes & Swain. Only two general points will be made here. First, when considering CD8 memory responses with aging, one must distinguish (and separately examine) those formed in youth from a robust primary response and then maintained into an old age, and those ones formed in old age from suboptimal primary responses. Second, while many memory responses formed in youth can persist for life [63], one also needs to examine their functional coordination at the relevant anatomical site where the microbial pathogen is likely to strike, because such examinations have revealed novel and important tissue-specific defects [64].
3. CD8 T-cell autonomous and environmental changes during aging
There is great theoretical and practical importance in distinguishing where the primary age-related immunity defects lie. Such knowledge is critical to the efforts to rationally improve immunity and outcomes of vaccination in older individuals. It is also important to understand qualitative and quantitative features of individual defects, because treatment strategies will be different if:1) a given cell subset or a given molecule is missing or underproduced as a consequence of aging;2) a required cell subset or molecule is present, but not functioning properly because they become inherently defective with aging;or if 3) such cells/molecules are present, but do not function properly because another cell or molecule is not providing the correct signal/context for a response. Below, we discuss the current state of knowledge on CD8 T cell autonomous age-related defects and on age-related changes in their partners (Fig. 1).
3.1 Aging leads to loss in numbers, diversity and true naïve “quality” of naïve CD8 T cell precursors
A highly diverse T cell population is critical for protection against pathogens. The fundamental unit of the Tcell response is the individual clonotype, defined by the Tcell receptor (TCR) α and β chains expressed on the cell surface. The diversity of the Tcell response to infection (i.e. the number of different clonotypes participating) is a better correlate of protection than the magnitude of the response [65,66]. As little as a 2 to 3-fold reduction in repertoire diversity dramatically impairs antigen-specific responses [67,68].
Following thymic involution, the maintenance of a diverse naïve T cell pool is dependent on homeostatic signals from TCR:self pMHC interactions and from the cytokines IL-7 and IL-15 [69-71](and Haynes and Swain, this volume). As aging progresses and thymic output wanes, homeostatic cycling of naïve T cells increases dramatically once the frequency of naïve cells crosses below a particular threshold [72]. Paradoxically, we have found this to be linked to at least a 66% loss of naïve CD8 T cell precursors, as well as a constriction of diversity within the naïve population [72]. Further examination of this phenomenon in mice revealed that naïve homeostatic proliferation in aging is not an equal opportunity process; rather, some naïve precursors are preferentially maintained at the expense of others, likely due to their higher affinity for self pMHC[73], to the more abundant pMHC ligands, to the better utilization of homeostatic cytokines, or the combination thereof. Many of these precursors no longer have the canonical naïve T cell phenotype, and express high levels of CD44 and other memory markers [73] (Li et al., in preparation), fitting the description of “virtual memory” (VM) cells [74]. Although these cells in at least one study appeared to be the “best-fit”, most competitive clones with high anti-microbial function against a specific model peptide epitope, clonal diversity of such late-life precursors was reduced and would be predicted to further erode immune fitness. Moreover, there is some evidence that the CD44hi VM precursors cannot expand as well as their true naïve counterparts (Li, G. et al, in preparation), contributing, at least in principle, to the reduced accumulation of effector CD8 cells after primary infection. It is interesting that CD4 naïve T cells do not seem to exhibit the same level of numeric attrition with aging (see the review by Haynes and Swain, this volume; Wertheimer A. et al., submitted).
An intriguing additional change in aged murine CD8 T cells was recently described in the entire pool of CD8 cells, affecting both memory and naïve cells: these cells were found to express a variety of inhibitory markers, including PD-1, LAG-3 and 2B4 [75]. Such markers are typically though ofas hallmarks of T cell exhaustion and are found at high levels in chronic viral infections, such as HIV, HCV and LCMV[76-79], but are not seen in repeatedly stimulated CD8 T cells specific for latent persistent herpesviruses[80-82]. While in principle one could see that some, or even many, memory cells in old mice may be repeatedly stimulated by environmental antigens, it is very difficult to understand how or why the naïve CD8 T cells would express such markers unless they were driven to cycle repeatedly and at a high rate, a question that clearly merits further investigation.
Given the strong association of advanced age with susceptibility to numerous infections[7,14,15,18,19,83-85], and diminished responses to vaccination [5,86-89]in different mammals, including humans, the combined consequence of an absolute naïve CD8 T cell loss and the selective maintenance of only the most homeostatically competitive clones with aging likely underlies the “hole in the naïve TCR repertoire” phenomenon, that correlates with increased susceptibility to infection [90,91].
Both anecdotal and specific evidence confirms that TCR clonotype diversity is lost during aging, an issue that was also very thoroughly reviewed recently [92], and therefore will not be addressed here at length. Briefly, decreased protective immunity to influenza in old mice has been attributed to the age-associated loss of naïve CD8 Tcell precursors resulting in limited clonotype diversity [90]. In this response tooit was found that attrition did not affect all responses equally. These studies report a loss of both clonotype frequency and diversity in the more “public” NP366-374-specific CD8 response in old mice (clonotypes shared by individual mice), with less effect on the “private” PA224-233-specific CD8 Tcell response [91]. Recent work from our laboratory has also found a loss of CD8 effector clonotype diversity in old mice responding to the HSV-1 gB495-502 epitope following infection [93]. The other important disturbance in the old T cell repertoire is the appearance and expansion of Tcell clonal expansions (TCE), which have also been reviewed recently [94]. In general, as the TCE increase in number and size, naïve T cell frequency goes down [86], but the causal (homeostatic or other) link between the two still remains elusive.
3.2 Persistent viral infections, inflammation and CD8 T cell changes with aging
Persistent viral infections, particularly with members of the Herpesvirus family (HSV, VZV, EBV and above all CMV) are ubiquitous; nearly every human carries multiple latent infections [95]. Repeated life long interactions between reactivating latent virus and antigen-specific T cells lead to “memory inflation” of the virus specific Tcell populations in both humans and mice[96-99]. Because of that, and the inordinately rich number of epitopes that these viruses present to the immune system, the frequency of memory Tcells dedicated to controlling persistent CMV infection can occupy up to 50% of the human Tcell pool [98].
An extensive account of the role of CMV infection and immune aging has been elegantly presented in other recent reviews [100-103], so our discussion, while substantial, will not be exhaustive. Over a decade ago, the Swedish OCTO and NONA longitudinal studies of elderly humans established a cluster of immune parameters designed to predict mortality, termed the Immune Risk Profile (IRP) [104]. One notable finding of these studies was that the presence of large populations of CMV-specific CD28-CD8+ Tcells inversely correlated with survival time [105](although it must be remembered that the population in question was rather small and ethnically homogenous). How CMV may mediate such an effect remains unclear and is a subject of intense research. Antiviral memory T-cell inflation could come at a cost to the immune system as a whole: competition between memory and naïve T-cells for homeostatic survival signals could impair the maintenance of a diverse naïve T-cell pool. There is evidence that spontaneous TCE in mice result in holes in the naïve T-cell repertoire, particularly for new pathogens whose response would be dominated by T-cells of the same TCR Vβ to which the TCE belong [21], but it is unclear whether such large and restricted TCE in mice have a counterpart in CMV-induced memory inflation in humans. Perhaps a parallel could be found in young adult patients (age 18-26) that were subject to thymectomy within 2 weeks of birth as part of surgery to repair congenital heart defects. A subset of these patients show the typical unbalanced T cell subset distribution and highly restricted CD8 repertoire diversity typically seen in elderly patients [106]. Notably, these changes were predominantly restricted to the CMV-seropositive subset of subjects; moreover, these young patients harbored large populations of CMV-specific T cells [106].
Several recent experiments in mice and humans are currently under review and should be able to shed additional light upon this topic. Three separate mouse studies have experimentally infected young mice with CMV, then followed them for extended periods of time (some of them into bona fide old age) and explored the effects upon T cell homeostasis and immune defense against third-party microbial challenge (Cicin-Sain, L. et al., submitted; Mekkel A. et al., submitted; Smithey M.J. et al., in preparation). All three studies found impairments in CD8 responses to new pathogen challenge over and above these found in aging; survival, however, was not affected or was not tested. These studies, therefore, clearly established that lifelong, but not shorter (minimum 12-15 month) mouse CMV (mCMV) infection was necessary to impair immunity in old mice, and that acute infection with other viruses, such as VACV, did not produce the same effect. They did not fully elucidate the mechanisms by which mCMV accounted for the reduction in CD8 immunity to a novel pathogen, although some evidence for reduced diversity of the antimicrobial CD8 T cell repertoire was presented in at least some of them. These studies also clearly showed that mCMV infection did not affect the numbers of naïve CD8 T cells in mice, while the process of aging did. Concurrently, two large cross-sectional studies have shown that this also holds in humans (Wertheimer, A.M. et al., submitted; Vescovini, R. et al., submitted). Specifically, naïve CD8 T cell numbers (but not CD4 cells) were lost with aging, and effector memory CD8 (and CD4 cells, although this subset is quite modest) were absolutely expanded with CMV infection.However, these two processes were fully independent of one another, inasmuch as aging in CMV-negative subjects did not lead to memory inflation or absolute increase of memory cells, and CMV, conversely, did not mediate naïve cell loss over and above that which happened as a consequence of aging (Wertheimer, A.M. et al., submitted; Vescovini, R. et al., submitted).
What about other persistent or chronic viral infections (VZV, EBV, HSV, HCV, HIV) that may interact with the immune system over years to decades? It’s been estimated that any given individual on the planet is infected with 8-12 different viruses – and those are only the ones we currently know about [95]. One study has compared the impact of infection with HSV (in the presence or absence of simultaneous CMV infection) on the distribution of CD4 and CD8 memory subsets in middle and aged humans, and was unable to find the same disruptive effect as CMV on the “immune profile” [107]. In fact, it is likely that CMV, with its large pool of systemic reservoirs (monocytes, macrophages, endothelial cells and perhaps others), is ideally suited to drive such “aging-related” changes, whereas other herpesviruses tend to have more restricted latency niches. This is supported by findings that systemic, intraperitoneal infection of mice with HSV-1 produces similar changes with time (and aging) to mCMV infection[108].
Chronic viral infections, particularly HCV, HIV and some LCMV strains, are thought to drive immune exhaustion rather than senescence. There is an increasing population of HIV+ patients in which decades of successful therapeutic viral control has perhaps shifted the repeating viral-immune system interactions to more resemble a latent than a chronic infection. Antiretroviral therapy (ART) has markedly prolonged the life of those infected with HIV, and many of these individuals are now 25+ years post-diagnosis. Interestingly, the onset of age-associated diseases linked to systemic inflammation are occurring 10-15 years earlier in HIV+ patients than uninfected patients [109].Although ART helps to control viral replication, serum markers of chronic inflammation (such as C-reactive protein) are elevated in HIV+ patients on long-term ART treatment [110],as is seen in CMV+ elderly patients [111]. As these HIV+ patients continue to age with their persistent/chronic virus, we may discover that the consequences of lifelong interactions between the immune system and a persistent pathogen are not unique to CMV.
3.3 Effector T cell recruitment into peripheral tissues
The coordinated recruitment of effector T cells back to the primary or memory site of infection is an under-explored area of aging research. In our mouse studies of WNV infection we found no evidence for impaired migration of primary CD4 and CD8 effector T cells into the aged brain parenchyma [7], nor did we find problems with aged T cell trafficking into the liver following Lm infection [14]. By contrast, Agius et al. have elegantly shown that impaired cutaneous DTH responses in aged humans is a result of inefficient endothelial activation to facilitate memory CD4 T cell recruitment into the skin [64]. Whether this reflects differences in the challenge model, differences between mice and humans, or differences between primary and memory effector responses remains unclear at the present. As mentioned above, we do not possess extensive data to evaluate tissue-specific immunity in aging in other tissues, and other types of “imprinting” in aging could very well also be impaired, particularly in respect to mucosal or other cutaneous T cell populations
3.4 The role of APC: Pathogen uptake and antigen presentation
Measuring the magnitude and function of CD8 T cells at the peak of their response to a new pathogen is useful for characterizing age-related defects, yet provides no clue as to which compromised immune components underlie the problem. Indeed, only carefully designed transfer experiments can dissect which components are defective. As antigen presentation is at the heart of CD8 T cell responses, investigations of the very early events following infection are paramount. Many articles have failed to find age-related defects in APC when such APC were differentiated from old and adult animals ex vivo with growth factors and cytokines[112-114]. However, such approaches arelimited by potential artifacts due to in vitro selection of the best growing cells, which may also be the most functional (but perhaps in vivo very rare) cells in the old animal. Moreover, this approach may miss subtle problems with APC function in vivo at sites of infection.
Evaluation of human DC function ex vivo has been challenging given the low numbers of DC that are recovered from PBMC samples, and the hazards in interpretating experiments using cells differentiated in vitro. DC populations isolated from the blood of aged human donors have been shown to express lower levels of many TLR receptors, and their stimulation in vitro with TLR ligands results in reduced cytokine production [115].
In the mouse model, studies are emerging that more criticallydissect the in vivo response of aged DC populations to infection. The general picture suggests that age-related dysfunction occurs in pathogen sensing pathways and/or cytokine production.Aged mice infected with influenza were found to suffer impaired activation of the NLRP3 inflammasome, leading to reduced production of mature IL-1β and IL-18, and impaired caspase-1 activation [116]. Further, some of these functional defects could be reversed by treatment of aged mice with nigericin, improving morbidity and mortality from flu [116].FollowingHSV-2 infection, serum IFNα levels in aged mice were significantly lower than those in adults, yet adoptive transfer of adult DC prior to infection could both improve IFNα serum levels and reduce viral load [117].
We have utilized various adoptive transfer strategies in attempt to discriminate the relative roles of aged T cells and the aged environment. In the WNVmodel, transfer of adult and old polyclonal CD4 or CD8 T cells into young RAG−/− recipients revealed clear age-related Tcell pool-intrinsic defects, because young donor cells were protective, while the old were not [7]. However, this does not mean that all other components of the anti-WNV response were intact in old animals. Specifically, we did not examine the early coordination of the immune response and it is not known whether additional innate immunity, APC-inherent, or APC:Tcell communication defects may exist.
We have begun investigating age-related changes in the well-characterized Listeria model, where a specific APC subset – CD8α+ dendritic cells – are essential in both the establishment of the infection, and development of the immune response. As mentioned above, following systemic infection, Lm is rapidly cleared from the bloodstream within minutes to hours by neutrophils and macrophages, while a portion of the bacteria are taken up by the CD8α+ DC in the spleen [33-35,40,118], a process dependent on the deposition of complement C3b protein on the bacterial surface, and its subsequent binding of host platelets [119].
To investigate the ability of aging APC to prime naive CD8 T cells, we performed adoptive transfer of indicator adult CD8 TCR transgenic T cells into adult and old recipients, who then were infected with Lm [56]. This approach controls for intrinsic CD8 Tcell deficiencies, comparing the ability of the young and aged environment to stimulate the same transferred (young) cell population, and has been previously used to show that the aged environment can impair the expansion of young transgenic T cells in response influenza [16]. Our experiments extended this result into the Lm infection model, where specific functions for the CD8α+ DC subset have been extensively characterized[33-35,40,118-120].Specifically, we found that the early establishment of Lm infection within splenic CD8α+ DC population was abortive in old mice. Recruitment of additional CD8α+ DC to the spleen for the first 3-5 days post infection was also impaired in aged mice, and these cells failed to up-regulate costimulatory molecules to the same degree as the CD8α+ DC in young Lm-infected mice[56] (illustrated in Fig. 1 – note the impaired and incomplete activation of the DC depicted in the figure). Mechanistic studies are in progress to further characterize this defect.
4. Correcting defects in CD8 T cell responses with aging
Strategies to improve T cell function in late life are in of great importance. In vitro studies have shown that enzymatic pre-treatment with an O-linked glycoprotein endopeptidase (OSGE)that modifies glycosylation of cell surface proteins (including CD43, CD44, and CD45)leads to improved Ca++ flux, up-regulation of activation markers, effector cytokine production and lytic activity in both young and old CD8 T cells following anti-CD3/CD28 stimulation [121]. These studies have been extended with CD4 cells, finding that pre-treatment of old CD4 transgenic T cells prior to adoptive transfer improves their proliferative response and activation phenotype in response to cognate antigen in vivo [122]. These results suggest that cell-intrinsic approaches to improve T cell responsiveness may be of use for improving the decreased T cell responses observed in aging.
We found it was possible to improve the quality of the old CD8 T cell response by repeated stimulation using a live single-replication cycle vaccine [10]. In these experiments, old mice receiving vaccine followed by WNV challenge [10], or two rounds of vaccination with a replication-incompetent virus (J.L.U., unpublished observations) improved both absolute numbers of antiviral CD8 T cells and their production of all relevant antiviral mediators to levels equal to, or often better than, that seen in adults[10]. It is possible that lower recall T cell responses in adult animals in that model reflected better antibody responses that mediate rapid clearance of the virus or vaccine, limiting antigen availability and curtailing the ability to fully stimulate T cells. That notwithstanding, it was clear that secondary effectors in old mice possessed a full complement of functions, which was not the case with primary or memory cells in the same animals; therefore, secondary expansion was crucial to the improved effector function. What is not clear, however, is whether secondary stimulation had managed to finally expand the few competent naïve CD8 precursors that can fully differentiate, or whether it had managed to push many old cells across the critical proliferative/differentiation barrier that they could not cross in the primary response. TCR clonal analysis of the cell populations at the peak of primary, memory and recall responses should provide an answer to these questions.
5. Conclusion – the cumulative nature of age-related CD8 T cell defects in immunity to infection and the implications for intervention
The above review highlights the existence of multiple, parallel and cumulative defects in CD8 T cell immunity to infection in older organisms (Fig. 1; Table 1). Specifically, old animals contain reduced numbers (at least threefold and up to 10-fold) and diversity (not precisely quantified so far) of naïve CD8 Tcell precursors, many of which haveconverted into virtual memory cells that may have lower proliferative potential. These precursors are not adept at full proliferation (~3-4fold reduced expansion) and show increased apoptosis as they differentiate into effector cells; the resulting effectors exhibit reduced polyfunctionality and lower amounts of cytokines per cell. Their differentiation is impaired at least in some infections by suboptimal DC signals. Intense efforts are underway to translate these observations into humans and to then critically evaluate which of these defects may present prime targets for correction and interventions in older adults by immune modulation and rational vaccine design.
Highlights.
CD8 T cell responses become impaired in both magnitude and function as a consequence of age.
Aging dramatically impacts naïve CD8 T cell precursors, both numerically and phenotypically.
Extrinsic factors may further contribute to a decline in CD8 T primingefficacy in late life.
Acknowledgments
We wish to thank the past and present members of the Nikolich laboratory for support and helpful discussions. Supported by NIH awards AG020719, AG035309, AI081680 and N01 AI 00017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO Ageing and Life-Course [Internet] http://www.who.int/ageing/en/
- 2.Older Americans 2010: Key Indicators of Well-Being [Internet] http://www.agingstats.gov/agingstatsdotnet/main_site/default.aspx.
- 3.Gorina Y, Kelly T, Lubitz J, Hines Z. Trends in influenza and pneumonia among older persons in the United States. Aging Trends. 2008;8:1–11. [PubMed] [Google Scholar]
- 4.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 6.Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 7.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206:2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007;37:1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 9.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181:8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhrlaub JL, Brien JD, Widman DG, Mason PW, Nikolich-Zugich J. Repeated in vivo stimulation of T and B cell responses in old mice generates protective immunity against lethal West Nile virus encephalitis. J Immunol. 2011;186:3882–3891. doi: 10.4049/jimmunol.1002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–2063. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 12.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 13.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smithey MJ, Renkema KR, Rudd BD, Nikolich-Zugich J. Increased apoptosis, curtailed expansion and incomplete differentiation of CD8+ T cells combine to decrease clearance of L. monocytogenes in old mice. Eur J Immunol. 2011;41:1352–1364. doi: 10.1002/eji.201041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Po JLZ, Gardner EM, Anaraki F, Katsikis PD, Murasko DM. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123:1167–1181. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Bennett AJ, Fisher E, Williams-Bey Y, Shen H, Murasko DM. Limited expansion of virus-specific CD8 T cells in the aged environment. Mech Ageing Dev. 2009;130:713–721. doi: 10.1016/j.mad.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181:7977–7984. doi: 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effros RB, Walford RL. Diminished T-cell response to influenza virus in aged mice. Immunology. 1983;49:387–392. [PMC free article] [PubMed] [Google Scholar]
- 19.Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. Eur J Immunol. 2002;32:1567–1573. doi: 10.1002/1521-4141(200206)32:6<1567::AID-IMMU1567>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Decman V, Laidlaw BJ, Dimenna LJ, Abdulla S, Mozdzanowska K, Erikson J, Ertl HCJ, Wherry EJ. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol. 2010;184:5151–5159. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- 21.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson JF, Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the Northeastern United States. J Infect Dis. 2006;194:1577–1579. doi: 10.1086/508754. [DOI] [PubMed] [Google Scholar]
- 23.Lim P-Y, Behr MJ, Chadwick CM, Shi P-Y, Bernard KA. Keratinocytes are cell targets of West Nile virus in vivo. J Virol. 2011;85:5197–5201. doi: 10.1128/JVI.02692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AN, Kent KA, Bennett CJ, Bernard KA. Tissue tropism and neuroinvasion of West Nile virus do not differ for two mouse strains with different survival rates. Virology. 2007;368:422–430. doi: 10.1016/j.virol.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DWC, Tesh RB, Fisher-Hoch S. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, Bernard KA. Persistence of West Nile Virus in the Central Nervous System and Periphery of Mice. PLoS ONE. 2010;5:e10649. doi: 10.1371/journal.pone.0010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibney KB, Lanciotti RS, Sejvar JJ, Nugent CT, Linnen JM, Delorey MJ, Lehman JA, Boswell EN, Staples JE, Fischer M. West nile virus RNA not detected in urine of 40 people tested 6 years after acute West Nile virus disease. J Infect Dis. 2011;203:344–347. doi: 10.1093/infdis/jiq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin J, Ferguson TA. Identification of an IFN-gamma-producing neutrophil early in the response to Listeria monocytogenes. J Immunol. 2009;182:7069–7073. doi: 10.4049/jimmunol.0802410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi C, Hohl TM, Leiner I, Equinda MJ, Fan X, Pamer EG. Ly6G+ neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. J Immunol. 2011;187:5293–5298. doi: 10.4049/jimmunol.1101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 32.Dai WJ, Bartens W, Köhler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 33.Campisi L, Soudja SM, Cazareth J, Bassand D, Lazzari A, Brau F, Narni-Mancinelli E, Glaichenhaus N, Geissmann F, Lauvau G. Splenic CD8αζ dendritic cells undergo rapid programming by cytosolic bacteria and inflammation to induce protective CD8ζ T-cell memory. Eur J Immunol. 2011;41:1594–1605. doi: 10.1002/eji.201041036. [DOI] [PubMed] [Google Scholar]
- 34.Waite JC, Leiner I, Lauer P, Rae CS, Barbet G, Zheng H, Portnoy DA, Pamer EG, Dustin ML. Dynamic Imaging of the Effector Immune Response to Listeria Infection In Vivo. PLoS Pathog. 2011;7:e1001326. doi: 10.1371/journal.ppat.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell LM, Brzoza-Lewis KL, Henry CJ, Grayson JM, Westcott MM, Hiltbold EM. Distinct responses of splenic dendritic cell subsets to infection with Listeria monocytogenes: maturation phenotype, level of infection, and T cell priming capacity ex vivo. Cell Immunol. 2011;268:79–86. doi: 10.1016/j.cellimm.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mostowy S, Cossart P. Chapter 3 - Virulence Factors That Modulate the Cell Biology of Listeria Infection and the Host Response. In: Unanue ET, Carrero JA, editors. Advances in Immunology: Immunity to Listeria monocytogenes. Elsevier Inc.; 2012. pp. 19–32. [DOI] [PubMed] [Google Scholar]
- 37.Muraille E, Narni-Mancinelli E, Gounon P, Bassand D, Glaichenhaus N, Lenz LL, Lauvau G. Cytosolic expression of SecA2 is a prerequisite for long-term protective immunity. Cell Microbiol. 2007;9:1445–1454. doi: 10.1111/j.1462-5822.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 38.Hardy J. Extracellular Replication of Listeria monocytogenes in the Murine Gall Bladder. Science. 2004;303:851–853. doi: 10.1126/science.1092712. [DOI] [PubMed] [Google Scholar]
- 39.Hardy J, Chu P, Contag CH. Foci of Listeria monocytogenes persist in the bone marrow. Dis Models and Mech. 2009;2:39–46. doi: 10.1242/dmm.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, Belizaire R, Aoshi T, Schreiber RD, Miller MJ, et al. CD8alpha+ Dendritic Cells Are an Obligate Cellular Entry Point for Productive Infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahjat KS, Meyer-Morse N, Lemmens EE, Shugart JA, Dubensky TW, Brockstedt DG, Portnoy DA. Suppression of Cell-Mediated Immunity following Recognition of Phagosome-Confined Bacteria. PLoS Pathog. 2009;5:e1000568. doi: 10.1371/journal.ppat.1000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witte CE, Archer KA, Rae CS, Sauer J-D, Woodward JJ, Portnoy DA. Chapter 8 - Innate Immune Pathways Triggered by Listeria monocytogenes and Their Role in the Induction of Cell-Mediated Immunity. In: Unanue ET, Carrero JA, editors. Advances in Immunology: Immunity to Listeria monocytogenes. Elsevier Inc.; 2012. pp. 135–156. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Op Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M, Diamond MS. The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system. J Virol. 2010;84:12125–12138. doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Way SS, Kollmann TR, Hajjar AM, Wilson CB. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J Immunol. 2003;171:533–537. doi: 10.4049/jimmunol.171.2.533. [DOI] [PubMed] [Google Scholar]
- 46.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Op Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis MM, Way SS, Wilson CB. IL-23 promotes the production of IL-17 by antigen-specific CD8 T cells in the absence of IL-12 and type-I interferons. J Immunol. 2009;183:381–387. doi: 10.4049/jimmunol.0900939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: Role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur J Immunol. 2009;39:1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 50.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham Q-M, Zickovich JM, Lefrançois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan Y, Xu Y, Seah S, Brady JL, Carrington EM, Cheers C, Croker BA, Wu L, Villadangos JA, Lew AM. Resident and monocyte-derived dendritic cells become dominant IL-12 producers under different conditions and signaling pathways. J Immunol. 2010;185:2125–2133. doi: 10.4049/jimmunol.0903793. [DOI] [PubMed] [Google Scholar]
- 52.Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J Infect Dis. 2011;203:1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu C, Heaps AG, Cerundolo V, McMichael AJ, Bangham CR, Callan MFC. Early acquisition of cytolytic function and transcriptional changes in a primary CD8+ T-cell response in vivo. Blood. 2006;109:1086–1094. doi: 10.1182/blood-2006-03-011643. [DOI] [PubMed] [Google Scholar]
- 54.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li G, Smithey MJ, Rudd BD, Nikolich-Zugich J. Age-associated alterations in CD8a+ dendritic cells impair CD8 T cell expansion in response to an intracellular bacterium. Aging Cell. doi: 10.1111/j.1474-9726.2012.00867.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ-Y, Fulop T. Impact of age on T cell signaling: A general defect or specific alterations? Ageing Res Rev. 2011;10:370–378. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A, Larsen M, Pancino G, Douek DC, Autran B, et al. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113:6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evavold BD, Sloan-Lancaster J, Hsu BL, Allen PM. Separation of T helper 1 clone cytolysis from proliferation and lymphokine production using analog peptides. J Immunol. 1993;150:3131–3140. [PubMed] [Google Scholar]
- 62.Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Op Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 64.Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou A-P, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Messaoudi I. Direct Link Between mhc Polymorphism, T Cell Avidity, and Diversity in Immune Defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 66.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 67.Woodland DL, Kotzin BL, Palmer E. Functional consequences of a T cell receptor D beta 2 and J beta 2 gene segment deletion. J Immunol. 1990;144:379–385. [PubMed] [Google Scholar]
- 68.Nanda NK, Apple R, Sercarz E. Limitations in plasticity of the T-cell receptor repertoire. Proc Natl Acad Sci USA. 1991;88:9503–9507. doi: 10.1073/pnas.88.21.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 72.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci USA. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci USA. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haluszczak C, Akue AD, Hamilton SE, Johnson LDS, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HCJ, Goldstein DR, Wherry EJ. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wedemeyer H, He X-S, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 79.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 80.Lang A, Nikolich-Zugich J. Functional CD8 T cell memory responding to persistent latent infection is maintained for life. J Immunol. 2011;187:3759–3768. doi: 10.4049/jimmunol.1100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, Fearon DT, Heath WR, Carbone FR. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol. 2012;188:2173–2178. doi: 10.4049/jimmunol.1102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, Kern F. Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J Virol. 2012;86:1001–1009. doi: 10.1128/JVI.00873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980;2:801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- 84.Bender BS. Infectious disease risk in the elderly. Immunol Allergy Clin North Am. 2003;23:57–64. vi. doi: 10.1016/s0889-8561(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 85.Turner J, Frank AA, Orme IM. Old mice express a transient early resistance to pulmonary tuberculosis that is mediated by CD8 T cells. Infect Immun. 2002;70:4628–4637. doi: 10.1128/IAI.70.8.4628-4637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Čičin-šain L, Smyk-Paerson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, et al. Loss of Naive T Cells and Repertoire Constriction Predict Poor Response to Vaccination in Old Primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gardner EM, Gonzalez EW, Nogusa S, Murasko DM. Age-related changes in the immune response to influenza vaccination in a racially diverse, healthy elderly population. Vaccine. 2006;24:1609–1614. doi: 10.1016/j.vaccine.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 88.Gardner P, Pabbatireddy S. Vaccines for women age 50 and older. Emerg Infect Dis. 2004;10:1990–1995. doi: 10.3201/eid1011.040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagar LE, Gentleman B, Pircher H, McElhaney JE, Watts TH. Influenza-specific T cells from older people are enriched in the late effector subset and their presence inversely correlates with vaccine response. PLoS ONE. 2011;6:e23698. doi: 10.1371/journal.pone.0023698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Op Immunol. 2011;23:537–542. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rudd BD, Venturi V, Davenport MP, Nikolich-Zugich J. Evolution of the antigen-specific CD8+ TCR repertoire across the life span: evidence for clonal homogenization of the old TCR repertoire. J Immunol. 2011;186:2056–2064. doi: 10.4049/jimmunol.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 96.Holtappels R, Podlech J, Geginat G, Steffens HP, Thomas D, Reddehase MJ. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 98.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chidrawar S, Khan N, Wei W, Mclarnon A, Smith N, Nayak L, Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin & Exp Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buchholz VR, Neuenhahn M, Busch DH. CD8+ T cell differentiation in the aging immune system: until the last clone standing. Curr Op Immunol. 2011;23:549–554. doi: 10.1016/j.coi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2011;10:362–369. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 102.Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157:175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 103.Wills M, Akbar A, Beswick M, Bosch JA, Caruso C, Colonna-Romano G, Dutta A, Franceschi C, Fulop T, Gkrania-Klotsas E, et al. Report from the second cytomegalovirus and immunosenescence workshop. Immunity & Ageing. 2011;8:10. doi: 10.1186/1742-4933-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wikby A, Johansson B, Ferguson F, Olsson J. Age-related changes in immune parameters in a very old population of Swedish people: a longitudinal study. Exp Geron. 1994;29:531–541. doi: 10.1016/0531-5565(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 105.Hadrup SR, Strindhall J, Køllgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 106.Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, Ferrand C, Debré P, Sidi D, Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Derhovanessian E, Maier AB, Hahnel K, Beck R, De Craen AJM, Slagboom EP, Westendorp RGJ, Pawelec G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011;92:2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- 108.Lang A, Brien JD, Nikolich-Zugich J. Inflation and long-term maintenance of CD8 T cells responding to a latent herpesvirus depend upon establishment of latency and presence of viral antigens. J Immunol. 2009;183:8077–8087. doi: 10.4049/jimmunol.0801117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 110.Neuhaus J, Jacobs DR, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Derhovanessian E, Maier AB, Beck R, Jahn G, Hahnel K, Slagboom PE, De Craen AJM, Westendorp RGJ, Pawelec G. Hallmark Features of Immunosenescence Are Absent in Familial Longevity. J Immunol. 2010;185:4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- 112.Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, Kaech S, Goldstein DR. Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 113.Lung TL, Saurwein-Teissl M, Parson W, Schönitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–1612. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 114.Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin & Exp Immunol. 1996;105:544–550. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 Inflammasome Function in Elderly Mice during Influenza Infection Is Rescued by Treatment with Nigericin. J Immunol. 2012;188:2815–2824. doi: 10.4049/jimmunol.1103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schröder S, Chakraborty T, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 119.Verschoor A, Neuenhahn M, Navarini AA, Graef P, Plaumann A, Seidlmeier A, Nieswandt B, Massberg S, Zinkernagel RM, Hengartner H, et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol. 2011;12:1194–1201. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 120.Neuenhahn M, Busch DH. Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens. Immunol Lett. 2007;114:66–72. doi: 10.1016/j.imlet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 121.Sadighi Akha AA, Berger SB, Miller RA. Enhancement of CD8 T-cell function through modifying surface glycoproteins in young and old mice. Immunology. 2006;119:187–194. doi: 10.1111/j.1365-2567.2006.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garcia GG, Miller RA. Ex vivo enzymatic treatment of aged CD4 T cells restores antigen-driven CD69 expression and proliferation in mice. Immunobiology. 2011;216:66–71. doi: 10.1016/j.imbio.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang J, Gross D, Elbaum P, Murasko DM. Aging affects initiation and continuation of T cell proliferation. Mech Ageing Dev. 2007;128:332–339. doi: 10.1016/j.mad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Bernstein ED, Gardner EM, Abrutyn E, Gross P, Murasko DM. Cytokine production after influenza vaccination in a healthy elderly population. Vaccine. 1998;16:1722–1731. doi: 10.1016/s0264-410x(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 125.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 126.Rukavina D, Laskarin G, Rubesa G, Strbo N, Bedenicki I, Manestar D, Glavas M, Christmas SE, Podack ER. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood. 1998;92:2410–2420. [PubMed] [Google Scholar]