Abstract
These studies investigated interactions taking place at the mitochondrial membrane in neonatal rat cerebellum following ethanol exposure, and focused on interactions between pro-apoptotic Bax and proteins of the permeability transition pore (PTP), voltage-dependent anion channel (VDAC), and adenine nucleotide translocator (ANT), of the outer and inner mitochondrial membranes, respectively. Cultured cerebellar granule cells were used to assess the role of these interactions in ethanol neurotoxicity. Analyses were made at the age of maximal cerebellar ethanol vulnerability (P4), compared to the later age of relative resistance (P7), to determine whether differential ethanol sensitivity was mirrored by differences in these molecular interactions. We found that following ethanol exposure, Bax pro-apoptotic associations with both VDAC and ANT were increased, particularly at the age of greater ethanol sensitivity, and these interactions were sustained at this age for at least two hours post-exposure. Since Bax:VDAC interactions disrupt protective VDAC interactions with mitochondrial hexokinase (HXK), we also assessed VDAC:HXK associations following ethanol treatment, and found such interactions were altered by ethanol treatment, but only at two-hours post-exposure, and only in the P4, ethanol-sensitive cerebellum. Ethanol neurotoxicity in cultured neuronal preparations was abolished by pharmacological inhibition of both VDAC and ANT interactions with Bax, but not by a Bax channel blocker. Therefore, we conclude that at this age, within the constraints of our experimental model, a primary mode of Bax-induced initiation of the apoptosis cascade following ethanol insult involves interactions with proteins of the PTP complex, and not channel formation independent of PTP constituents.
Keywords: Ethanol, fetal alcohol syndrome, apoptosis, mitochondria, cerebellum
INTRODUCTION
Exposure to ethanol during development of the central nervous system (CNS) leads to disruption of an array of fundamental developmental processes, leading to widespread neuropathological changes, collectively termed fetal alcohol spectrum disorder (FASD). The most extreme manifestation of this exposure, fetal alcohol syndrome (FAS) was first described nearly forty years ago (Jones and Smith, 1973) and despite recognition of the danger of such exposure, the incidence of FASD/FAS has continued to escalate and currently represents the dominant known cause of intellectual impairment worldwide (Medina, 2011). Certain regions of the developing CNS are selectively vulnerable to ethanol insult, and within these regions, peak periods of sensitivity have been experimentally defined in animals models of FASD/FAS. In the developing cerebellum for example, massive loss of Purkinje and granule cells results from even a single ethanol exposure rendered during the first few postnatal days in the neonatal rat (postnatal days 4-5 [P4-5]), a developmental period corresponding to the human third trimester (Goodlett et al., 1990; Bonthius and West, 1991; Hamre and West, 1993). Only slightly later in the neonatal period, however, this region becomes relatively resistant to such exposure, with neuronal populations able to withstand similar treatment (Goodlett and Eliers, 1997; Pierce et al., 1999; Light et al., 2002).
It has become clear in recent years that much of ethanol-mediated disruption of the developing CNS is related to stimulation of apoptotic processes, with strong involvement of the Bcl-2 family of survival-regulatory proteins (e.g., Moore et al., 1999; Inoue et al., 2002; Heaton et al., 2003a,b,c; Ge et al., 2004; Lee et al., 2008). Within this gene family, the pro-apoptotic Bax protein appears to be particularly important for ethanol-induced cell death in certain developing populations. This has been most dramatically demonstrated in experiments with mutant mice lacking the Bax gene, in which neuronal loss is greatly attenuated following ethanol exposure even during periods of maximal vulnerability (Young et al., 2003; Heaton et al., 2006). In previous studies, we have found that following neonatal ethanol exposure, particularly at ages of peak sensitivity, Bax migrates to the mitochondrial membrane, where it plays a key role in triggering apoptosis (Siler-Marsiglio et al., 2005; Heaton et al., 2012). There are currently two primary models of the mechanisms underlying this Bax-initiated apoptosis. In the first model, Bax is hypothesized to function in collaboration with integral components of the mitochondrial permeability transition pore (PTP) complex, such as the voltage-dependent anion channel (VDAC) proteins of the outer mitochondrial membrane, and the adenine nucleotide translocator (ANT) proteins of the inner membrane, with such interactions leading to loss of the mitochondrial membrane potential, release of mitochondrial contents such as cytochrome-c, and initiation of the apoptosis cascade. Such associations have been demonstrated in some experimental systems, and within these setting, Bax-PTP interactions have been found to promote apoptosis, while their inhibition protects against cell death (Marzo et al, 1998; Narita et al., 1998; Shimizu et al., 1999; Cao et al., 2001). In the second model, it is proposed that Bax functions apart from the PTP, by independent mitochondrial membrane pore formation (Antonsson et al., 2000; Hetz et al., 2005; Bras et al., 2005). There is also considerable experimental support for this position, with blockage of such pore forming capacities providing protection against Bax-mediated cell death in certain systems (e.g., Hetz et al., 2005; Kromer et al., 2007).
The present series of experiments was designed to examine ethanol-mediated interactions taking place at the mitochondrial membrane in neonatal cerebellum, and the role of these interactions in cell death within this region. These interactions were examined at the age of peak ethanol vulnerability in this developing region, and the later ethanol-resistant age, to further determine whether differential regulation of such events contributes to the differential ethanol sensitivity at the two ages. Specific in vivo experiments evaluated Bax:VDAC and Bax:ANT interactions, both of which have been shown to be pro-apoptotic, in P4 and P7 cerebellum following ethanol exposure via vapor inhalation. Since Bax interactions with VDAC can disrupt protective VDAC-mitochondrial hexokinase (HXK) associations, we also examined the relative stability of VDAC:HXK complexes following ethanol exposure. In addition, cerebellar granule cell culture preparations were used to determine the extent to which inhibition of certain of these interactions would blunt ethanol neurotoxicity, assessments that could not be readily made in vivo. For these analyses, we cultured granule cells with ethanol in the presence of a specific inhibitor of Bax:VDAC interactions, and an inhibitor of ANT conformational changes which enable this PTP component to bind with Bax. We also assessed the possible role of Bax channel formation to ethanol-mediated cell death in these culture preparations, using a Bax channel blocker with these neuronal cultures.
METHODS
Ethanol Vapor Inhalation Exposure Paradigm
All procedures used in this study were carried out in compliance with the rules and regulations for the experimental use and care of laboratory animals at the University of Florida, and were approved by the Institutional Animal Care and Use Committee. For the ethanol inhalation treatment, neonatal Long Evans male and female rats (Charles River Co., Portage MI), were acutely exposed to ethanol via the vapor inhalation procedure, as previously described (e.g., Heaton et al. 2000). Briefly, litters were culled to 10 male and female pups, and at P4 or P7, pups were placed, in their home cage along with the nursing dam, in an inhalation apparatus. The Plexiglas inhalation chamber was fitted with intake and exhaust hoses, and air flow was provided by a pump which delivered approximately 0.8-1.0 liter/minute. For the ethanol groups, air flowed into an Erlenmeyer vacuum flask containing 95% ethanol. The air passed through an air stone submerged in the ethanol, and the ethanol-laden vapor was carried into the chamber. The exhaust hose led ethanol vapor into a fume hood. For control litters, the air was pumped into the chamber without exposure to ethanol. Both ethanol and control exposure periods were 2 hours, 45 minutes each day. This acute exposure protocol mimics a “binge” pattern of ethanol consumption, which is a common consumption pattern in women who drink during pregnancy (Stephens, 1985).
For the molecular analyses, tissue was harvested at two time points. Tissue was first collected after two-hours of exposure to ethanol or control conditions (i.e., during the exposure interval), and the second collection was made two hours after completion of the 2 hour, 45 minute exposure. Analyses at the first time point enabled us to assess events taking place as the exposure was ongoing, since it has been demonstrated that ethanol-mediated changes in molecular events in the developing CNS can occur rapidly (e.g., Siler-Marsiglio et al., 2004; Ge et al., 2004). The second time point enabled us to determine whether such changes may be transient, or whether they were sustained during the post-exposure period. The pups were sacrificed via rapid decapitation, the brains removed, and the cerebellum was taken for protein analyses. Blood ethanol concentrations (BECs) were determined 2 hours following completion of the inhalation exposure, using trunk blood from animals exposed in parallel to those used for the various assays, via the QuantiChrom Ethanol Assay kit (DIET-5000) from BioAssay Systems (Haywood, CA). For all molecular analyses, a minimum of six P4 litters and six P7 litters were exposed to control conditions and an equivalent number of litters were exposed to ethanol conditions. For ELISA analyses cerebellae from three to four animals from each exposed litter (control and ethanol) were pooled, and placed in microwells in triplicate; N=12-20 animals were used for each plate with one condition/plate. Three-four replicate plates were run/protein. Since no gender-related differences in the effects of ethanol at this developmental stage in the CNS of rats have been found (Andrews et al., 1999; Bonthius and West, 1991), tissues from male and female pups were pooled.
Subcellular Fractionation
For the analyses of associations between Bax and mitochondrial membrane constituents, cerebellar tissue was fractionated, in order to separate cytosolic and mitochondrial contents. For this procedure, P4 or P7 cerebellae were harvested at the two time points noted above. The tissues were homogenized and isolated into fractions using the Mitochondrial/Cytosol Fractionation Kit and protocol provided by BioVision Inc. (Mountain View, CA). Tissues were suspended in 700μl of 1X Cytosol Extraction Buffer containing protease inhibitor cocktail and dithiothreitol (DTT). Tissue samples were incubated for 30 minutes on ice and homogenized by 80-100 passes in an ice-cold dounce tissue grinder. The homogenates were transferred to new tubes and centrifuged at 2000 rpm for 10 minutes at 4°C to remove nuclei, membranes, and whole cells. A 550μl portion of the supernatant was transferred to a new tube, with care being taken to leave the pellet undisturbed. The supernatant was centrifuged at 14,000 rpm for 30 minutes at 4°C, then retained as the cytosolic fraction. The remaining supernatant on the pellet was removed, and the pellet was rinsed by adding 300μl of 1X PBS. This mixture was than vortexed and centrifuged at 14,000 rpm for 30 minutes at 4°C. Supernatant was removed from the vortexed pellet, and 195μl of Mitochondrial Extraction Buffer added. The suspension was sonicated with an ultrasonic cell disruptor (from Misonix Inc., Farmingdale, NY) to release the mitochondrial contents, and this constituted the mitochondrial fraction. Protein content of cytosolic and mitochondrial fractions was quantified using bicinchoninic acid (BCA) protein assays (Pierce, Rockford, IL). Protein concentrations of fractions exposed to each condition were normalized for molecular analyses. The quality of the separation of the fractions using this procedure was determined by Western blot of mitochondrially-specific cytochrome oxidase subunit Vb (Cox IV), using a polyclonal rabbit antibody (Cell Signaling, Boston, MA). No COX IV was detected in the cytosolic fraction, while abundant levels were found in the mitochondrial fractions, as previously reported using this fractionation procedure (Heaton et al., 2012).
ELISA Assessment of Bax:VDAC, BAX:ANT, and VDAC:HXK Interactions
Changes in native protein-protein interactions between Bax and VDAC, BAX and ANT, and VDAC and HXK following ethanol exposure were determined using an ELISA-based approach (Siler-Marsiglio et al., 2005). Our approach utilizes capture antibodies which recognize native protein for the detection of endogenous protein-protein interactions. It should be noted that this technique detects protein-protein associations, but does not necessarily delineate direct functional interactions. Thus, for the present study, we combined these observations with the manipulative culture studies outlined below, in which the consequences of pharmacological disruption of these associations could be assessed. For the ELISA analyses, mouse monoclonal antibody to VDAC1 (the VDAC isoform that binds to Bax; Abcam, Cambridge, MA; Ab-16816; diluted 1:200 in pH 9.6 bicarbonate buffer [capture antibody]) was bound to 96-well microplates overnight at 4°C. Plates were blocked for 1 hour at room temperature, with 1% Blotto non-fat dry milk in pH 7.2 PBS and then washed 3X using PBS. Each cerebellum exposed to ethanol or control conditions was suspended in 400μl of PBS with 1% protease inhibitor. The suspension was sonicated to lyse the cells to release the endogenous protein complexes. Protein content was determined using the BCA assay. Mitochondrial tissue extracts were then prepared from ethanol and control animals. Equal amounts of protein (125μg/well) were placed in the VDAC antibody-bound 96-well microplates at a final volume of 100μl/well. Plates were incubated overnight at 4°C and then washed 3X with PBS. Primary antibody was added to each plate to detect VDAC-bound Bax. Rabbit polyclonal anti-Bax (Cell Signaling; Ab-2772; 1:200) was added to anti-VDAC plates to detect Bax:VDAC complexes. Following an overnight incubation at 4°C, plates were washed 3X with PBS. Anti-mouse secondary antibody conjugated to alkaline phosphatase (diluted 1:250; Sigma, St. Louis, MO) was added to the anti-VDAC plates. Plates were incubated overnight at 4°C and washed 3X with TBS to avoid inhibition of alkaline phosphatase by inorganic phosphate. All buffers used, including the dilution and wash buffer, were detergent-free with 1% protease inhibitor cocktail. To colorimetrically detect signal from Bax:VDAC, p-nitrophenyl phosphate (pNPP; 2mg/ml), an alkaline phosphatase substrate, in pH 10.4 glycine buffer with 1 mM ZnCl2 was added. Absorbance was read at 415nM on a BioRad microplate reader (Hercules, CA). Absorbance readings from background wells (obtained from wells excluding primary antibody) were subtracted from experimental wells to determine specific binding. This same procedure was also used for detecting Bax:ANT and VDAC:HXK complexes, with ANT and HXK polyclonal goat antibodies from Santa Cruz (Santa Cruz, CA; ANT, SC-9299; 1:200; HXK, SC-6517, 1:200).
This ELISA-based approach has the advantage of minimizing the disruption of native protein-protein interactions. It is important that our method circumvents the need for detergents: Protein interactions with Bax, for example, are induced or inhibited by detergents commonly used in immunoprecipitation procedures including Nonidet P-40, Triton X-100 and CHAPS (Hsu and Youle, 1997; 1998). Cross-linking agents, which can also adjust binding characteristics of proteins, are also avoided by our approach.
Cerebellar Granule Cell Cultures
Cerebellar granule cell cultures were used to assess the importance of the Bax:VDAC, and the BAX:ANT associations, and the role of Bax channel formation in ethanol neurotoxicity. For these determinations, we cultured granule cells with ethanol and inhibitors of VDAC, ANT, and a Bax channel blocker (BCB). For these preparations, P8 pups were sacrificed by rapid decapitation. The cerebellum was removed, minced, and placed in Hank's balanced salt solution (HBSS) containing trypsin and DNAse (Sigma). The tissue was incubated at 37°C for 15-20 minutes, washed with Dulbecco's modified Eagle's medium (DMEM; Cellgro, from Fisher Scientific; Pittsburgh, PA) with 15% fetal bovine serum (Invitrogen; Camarillo, CA), penicillin/streptomycin (Fisher), and trypsin inhibitor (Sigma), then triturated using a flame-narrowed borosilicate pipette to yield a homogeneous suspension. The cells were centrifuged, the supernatant removed, and fresh medium added to the pellet. After gentle aspiration, the cell suspension was filtered through a Nitrex filter (Sefar America, Kansas City, MO) to enrich the preparations for the small granule cells. For the assessments of neuronal survival via the MTT assay following inhibition of Bax associations with VDAC and ANT, and blocking of Bax channel formation, aliquots of the cell suspension were pipetted into 96-well plates coated with poly-L-lysine (0.05mg/ml; Sigma). All cultures were established for 24 hours in a tissue culture incubator at 37°C, with 5% CO2-95% air, in the serum-containing medium. Cell densities in these preparations average 35,000-39,000 neurons/cm2, as determined by a hemocytometer. We have found cells prepared in this manner to be ~95% neuronal, as determined by immunochemical detection of glial fibrillary acidic protein (GFAP) and type III β-tubulin.
Application of Experimental Conditions
After the initial 24-hour plating period, the serum-containing medium was replaced with serum-free, modified N2 medium consisting of DMEM/F12 medium at a 3:1 ratio, 5 mM KCl, and 1% N2 supplement, containing transferrin, insulin, progesterone, putrescine, and selenite (Invitrogen). Addition of Penicillin/Streptomycin was equivalent to that of the serum-containing medium. Use of the serum-free defined medium eliminates contamination from unknown serum components, and discourages proliferation of any non-neuronal cells which survive the filtration procedure (Muller at al., 1997). The experimental conditions then applied included ethanol at a concentration of 400mg/dl, which represent a relatively high, challenging level, but is well within the range seen both in awake humans following binge consumption, and in animal studies of ethanol toxicity (e.g., Lindblad and Olsson, 1976; Young et al., 2003). We have previously found that this ethanol concentration elicits cell death in this neuronal population at this age, preceded by robust Bax mitochondrial translocation (Heaton et al., 2011). The following inhibitors were combined with ethanol: (1) The VDAC inhibitor TAT48-57-β-Ala-Bcl-xl BH44-23, a cell-permeable peptide that binds directly to VDAC and blocks its interactions with Bcl-2 family proteins. This inhibitor was from Calbiochem (Cat. No. 197217), and was applied at a concentration of 20μM. (2) Bongkrekic acid (BA), a specific inhibitor of ANT, which binds to the ANT ATP-binding site, and changes it to a conformation locked in the “m-state” facing the matrix. This conformation prevents Bax-ANT interactions, and the subsequent induction of the mitochondrial permeability transition (MPT; Marzo et al., 1998). BA was from Sigma (Cat. No. B6179), and was applied at a concentration of 2μM. (3) Bax channel blocker (BCB), a cell-permeable dibromocarbazolo-piperazinyl derivative that specifically blocks Bax channel-forming activity. BCB was from Calbiochem (cat. No. 196805), and was applied at a range of concentrations (2.5μM, 5μM or 10μM). The following experimental conditions were applied, for an interval of 24 hours: (1) Control, in which the N2 medium was replaced without further supplementation; (2) Ethanol, in which 400mg/dl ethanol was added to the N2 medium; (3) VDAC inhibitor, BA or BCB, at one of the concentrations noted above; and (4) one of the inhibitors + ethanol, with the various combinations of conditions, as above. In addition, in the BCB cultures, sorbitol was used as a positive control for Bax channel formation. This substance is known to induce rapid apoptosis involving Bcl-2 family proteins (e.g., Maroney et al., 1998; Koyama et al., 2000; Criollo et al., 2007). The culture dishes were sealed with sterile breathable plate sealer, to minimize ethanol evaporation. Ethanol concentrations were confirmed by the QuantiChrom microenzymatic assay, and evaporation during the short exposure time was minimal.
MTT Neuronal Survival Assay
In order to assess neuronal viability, the MTT assay was used. For this procedure, the formazan product is produced as a result of the cleavage of the tetrazolium salt MTT by the inner mitochondrial membrane enzyme succinate-dehydrogenase. Only living cells with functional mitochondria can make the conversion, and therefore the amount of blue formazan production is directly proportional to the number of viable cells present (Mosmann, 1983). The assay is thus a reliable measure of survival in non-proliferative populations such as cerebellar granule cells (Oberdoerster and Rabin, 1999). This MTT reduction has previously been shown to produce highly accurate measurements of neuronal number in non-proliferative preparations such as cerebellar granule cells (Ankarcrona et al., 1995). Such measures are directly proportional to quantification of these cells by light microscopic observations (Manthorpe et al., 1986), and correlate with additional measures of cell viability such as Annexin V, and Propidium Iodide staining, and with indices of apoptotic processes such as cytochrome-c and caspase activation (Siler-Marsiglio et al., 2004). This assay was initiated 24 hours following application of experimental conditions. The protocol for the MTT survival assay was as outlined in detail previously (Mitchell et al., 1999). Briefly, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Sigma) was prepared in phenol red-free Eagle's Minimum Essential Medium. The granule cell cultures were incubated in the MTT solution for 5 hours at 37°C, resulting in production of the formazan product as a result of the mitochondrial cleavage. Following incubation, the plates were centrifuged, and the untransformed MTT solution was removed. Propanol was added to each well and the plates were shaken to uniformly solubilize the blue formazan. The optical density (OD) of the formazan MTT cleavage product was measured in each well using an automated plate reader (BioRad) with a test wavelength of 550 nm. Each plate contained multiple wells of a given experimental condition and multiple control wells, and mean OD readings were compiled for columns of 4-12 wells (fewer wells are use for the ethanol-supplemented cultures to prevent diffusion to control wells; Mitchell et al., 1998). This procedure was replicated a minimum of 8 times, on 2-4 plates/condition.
Statistical Analyses
Statistical analyses were made using StatView software on a Macintosh computer. The effects of ethanol on the formation of Bax:VDAC, Bax:ANT, and VDAC:HXK interactions following ethanol exposure on P4 and P7 were made via the two-way analysis of variance (ANOVA), and posthoc comparisons were made with the Fisher's Protected Least Significant Difference (PLSD) test, where appropriate. Analyses of survival of cerebellar granule cells following experimental treatments were made via a one-way ANOVA, with post hoc testing as above. All data were obtained from at least three independent experiments.
RESULTS
Blood Ethanol Concentrations
The blood ethanol concentrations (BECs) of P4 and P7 animals, measured 2 hours following termination of vapor inhalation, averaged 273±3.92 mg/dl in the P4 pups, and 279±3.42 mg/dl in the P7 pups. We have previously shown that BECs measured at this interval following vapor inhalation represent peak levels (Heaton et al., 2003a).
ELISA Analyses of Bax:VDAC, VDAC:HXK, and Bax:ANT Associations in Neonatal Cerebellum Following Ethanol Exposure at P4 and P7
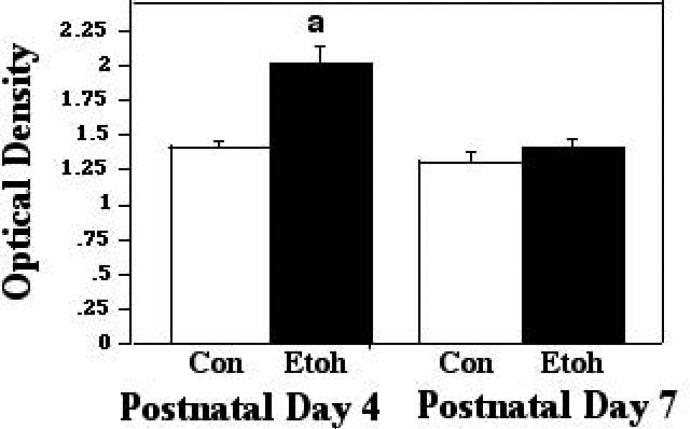
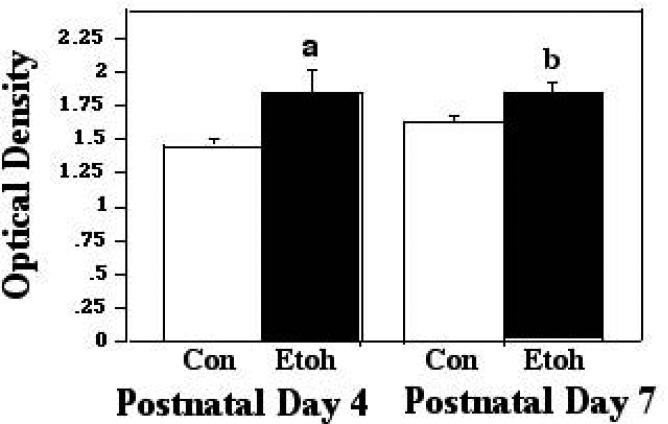
Protein-protein interactions between Bax and VDAC, associations which are conducive to apoptosis, were assessed via our ELISA-based approach, during the 2 hour, 45 minute ethanol vapor inhalation period, at the two-hour time point, and two hours following termination of exposure. The first analytical point was chosen to examine the more rapid molecular changes elicited by the ethanol exposure, and the later sampling point was chosen to determine whether changes detected endured in the absence of the ethanol stimulus, or, if no alterations were detected during exposure, whether there may be a delayed response to the ethanol insult. The analyses of the assessments made during exposure, via the two-way ANOVA, revealed a significant main effect of treatment (F [1,89] 18.10; p<0.0001), age (F [1,89] 18.11; p<0.0001), and a significant treatment × age interaction (F [1,89] 9.03; p = 0.0034). Individual post hoc comparisons indicated differential responsiveness to ethanol at the two developmental ages: At P4, the peak period of ethanol sensitivity, acute treatment resulted in a significant increase in the presence of Bax:VDAC complexes, to 1.43X control levels (p<0.0001). Conversely, in the P7 animals, there was no change in these interactions as a function of ethanol treatment. These data are presented in Figure 1A. When assessed at the 2 hour post-treatment interval, the 2-way ANOVA again indicated a significant effect of treatment (F [1,32] 8.62; p=0.0061), age (F [1,32] 15.81; p=.0004), and a significant treatment X age interaction (F [1,32] 6.889; p = 0.0132). The Post doc tests revealed that the enhanced Bax:VDAC interactions at P4 were sustained at two-hours post treatment (p=.0133). There were again no alterations in these associations in the P7 animals. These data are represented in Figure 1B.
Figure 1A.
Bax:VDAC associations were measured by ELISA in tissue derived from P4 and P7 neonatal rat cerebellum, with tissue collected two hours into exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). a=Significantly greater than P4 controls, p<0.0001.
Figure 1B.
Bax:VDAC associations were measured by ELISA in tissue derived from P4 and P7 neonatal rat cerebellum, with tissue collected two hours following termination of exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). a=Significantly greater than P4 controls, p=0.0133.
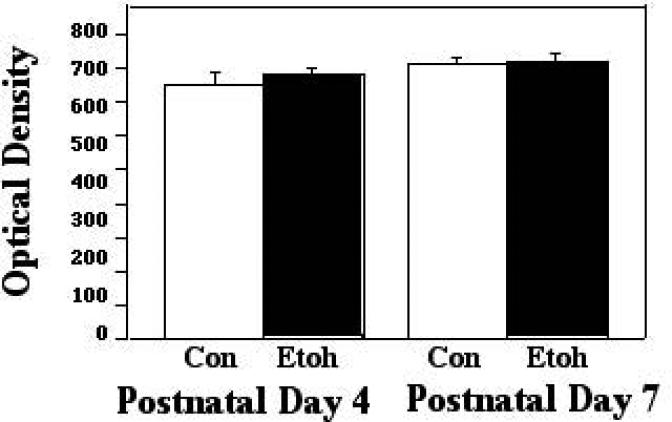
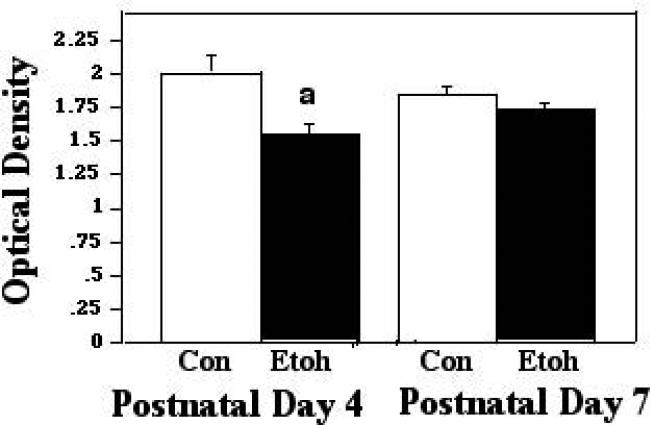
When Bax interacts with VDAC, it can displace HXK from dimerizing with VDAC, an association which protects the mitochondrial membrane potential. Therefore, we examined VDAC-HXK associations during and two hours following ethanol exposure, in order to determine whether such displacement may have taken place. When analyzed during exposure, there was no ethanol effect on these interactions in either P4 or P7 cerebellum. At the two-hour post exposure time point, however, there was a significant effect of treatment (F [1,27] 15.24; p=0.0006), and a significant treatment X age interaction (F [1,27] 5.64; p = 0.0249). The post test indicated a significant decrease in these complexes as a result of ethanol treatment at P4 (p=0.0053), but similar interactions were not significantly changed at P7. These results are depicted in Figures 2A and 2B.
Figure 2A.
VDAC:HXK associations were measured by ELISA in tissue derived from P4 and P7 neonatal rat cerebellum, with tissue collected two hours into exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). No significant differences were found in these interactions at this sampling point.
Figure 2B.
VDAC:HXK associations were measured by ELISA in tissue derived from P4 and P7 neonatal rat cerebellum, with tissue collected two hours following termination of exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). a=Significantly less than P4 controls, p=0.0053.
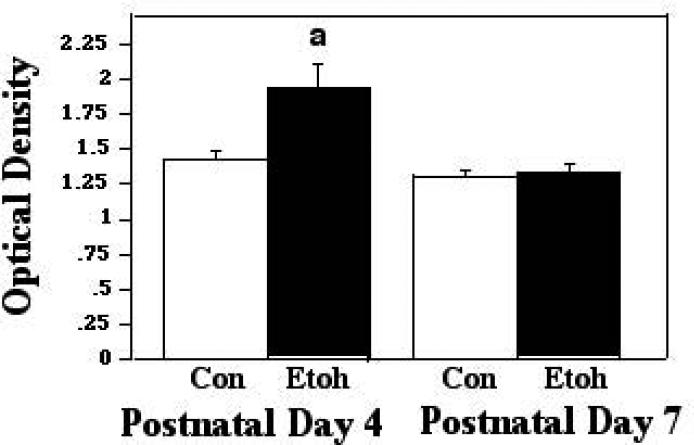
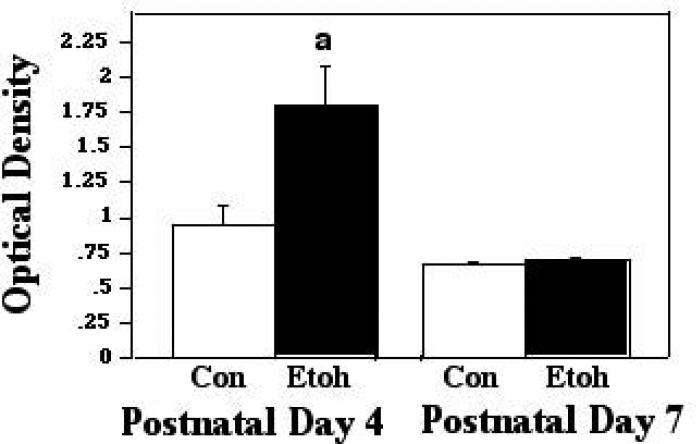
Bax also forms complexes with the ANT proteins of the inner mitochondrial membrane (IMM), and such associations have been found to lead to loss of membrane potential and ultimately, cell death (Marzo et al, 1998). When these interactions were assessed via ELISA assays during ethanol inhalation, we found a significant effect of treatment only (F [1,63] 11.24; p = 0.0014). The post hoc analyses showed that ethanol exposure elicited an increase in these associations at P4, to 1.28X control levels (p=0.0407), and the modest increase at P7 (to 1.14X controls) was also statistically significant (p=0.0218). These comparisons are presented in Figure 3A. When similar protein-protein complexes were examined two hours after termination of exposure, however, the ANOVA revealed a significant effect of treatment (F [1,23] 19.20; p = 0.0002), age (F [1,23] 48.38; p<0.0001), and a treatment X age interaction (F [1,23] 16.81; p = 0.0004). Post hoc assessments indicated that at P4, the increase in these interactions was sustained through this post-treatment interval, and in fact increased to 1.9X that measured in controls (p=.0381), while the ethanol and controls did not differ in the P7 animals at this time point. These data are shown in Figure 3B.
Figure 3A.
Bax:ANT associations were measured by ELISA in tissue derived from P4 and P7 neonatal rat cerebellum, with tissue collected two hours into exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). a=Significantly greater than P4 controls, p=0.0407; b=significantly greater than P7 controls, p=0.0218.
Figure 3B.
Bax:ANT associations were measured by ELISA in tissue derived from P4 and P7 neonatal rat cerebellum, with tissue collected two hours following termination of exposure to ethanol via vapor inhalation (Etoh), or to control conditions (Con). Error bars represent standard error of the mean (SEM). a=Significantly greater than P4 controls, p=0.0381.
Analyses of Neuronal Survival of Cerebellar Granule Cells Co-Cultured with Ethanol, VDAC Inhibitor, Bongkrekic Acid, and Bax Channel Blocker
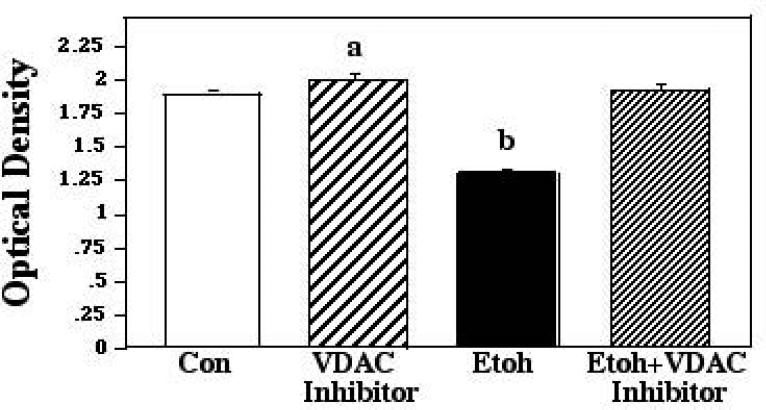
Postnatal day 8 cerebellar granule cell preparations were used to determine the role of the protein-protein interactions implicated by the ELISA assessments in promoting ethanol-induced neuronal apoptosis in developing cerebellum. In each of these analyses, we cultured granule cells with ethanol at a concentration of 400mg/dl. As noted above, this concentration represents a moderately high, challenging ethanol level, but is well within the range seen both in awake humans following binge consumption, and in animal studies of ethanol toxicity (e.g., Lindblad and Olsson, 1976; Young et al., 2003). For the first of these culture studies, ethanol was combined with the VDAC inhibitor TAT48-57-β-Ala-Bcl-xl BH44-23, a cell-permeable peptide that binds directly to VDAC and blocks its interactions with Bcl-2 family proteins. Experimental conditions were applied simultaneously, and exposure was continued for 24 hours, followed by analyses of survival via the MTT assay. The ANOVA applied to these data indicated a significant effect of treatment (F [3,140] 16.81; p = 0.0004). Posthoc assessments showed that while ethanol markedly depressed survival in these preparations, this cell loss was completely prevented by inhibition of VDAC interactions with Bcl-2-related proteins, most probably Bax, with survival in the ethanol + VDAC inhibitor cultures differing significantly from ethanol alone (p<0.0001), but being equivalent to that seen in both controls and VDAC inhibitor-only cultures. The VDAC inhibitor also provided a small but statistically significant survival advantage compared to controls (p=.0066). These results are depicted in Figure 4.
Figure 4.
Postnatal day 8 cerebellar granule cells were cultured in control medium (Con), in medium supplemented with VDAC inhibitor, in medium supplemented with 400mg/dl ethanol (Etoh), and in medium with ethanol plus the VDAC inhibitor. Neuronal survival was measured via the MTT assay. Error bars represent standard error of the mean (SEM). a=Significantly greater than controls, p=0.0066; b=significantly less than controls, VDAC inhibitor alone, and ethanol plus VDAC inhibitor, p<0.0001 in each instance.
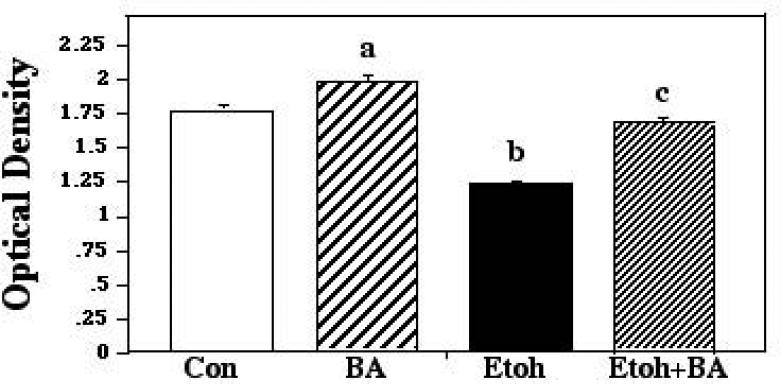
The second of the cultures analyses paired ethanol with PTP inhibitor bongkrekic acid (BA). BA binds to the ATP-binding site of ANT, and changes it to a conformation locked in the “m-state” facing the matrix. This conformation prevents Bax-ANT interactions, and the subsequent triggering of the MPT (Marzo et al., 1998; Cao et al., 2001). The ANOVA applied to these data showed a significant effect of treatment (F [3,110] 113.76; p < 0.0001). Post hoc analyses indicated that while ethanol produced a marked reduction of neuronal survival, inclusion of BA in the ethanol cultures provided significant rescue, with survival in these cultures significantly exceeding that measured in ethanol-only cultures (p<0.0001), and similar to survival seen in control cultures. As with the VDAC inhibitor, cultures supplemented with BA alone fared significantly better than control and ethanol + BA cultures (p=0.0004; p<0.0001, respectively), suggesting that this apoptosis inhibitor suppressed some of the attrition normally found in cultured cells. These results are presented in Figure 5.
Figure 5.
Postnatal day 8 cerebellar granule cells were cultured in control medium (Con), in medium supplemented with bongkrekic acid (BA), in medium supplemented with 400mg/dl ethanol (Etoh), and in medium with ethanol plus BA. Neuronal survival was measured via the MTT assay. Error bars represent standard error of the mean (SEM). a=Significantly greater than controls, p=0.0004; b=significantly less than controls, BA alone, and ethanol plus BA, p<0.0001 in each instance; c=significantly less than BA alone, p<0.0001.
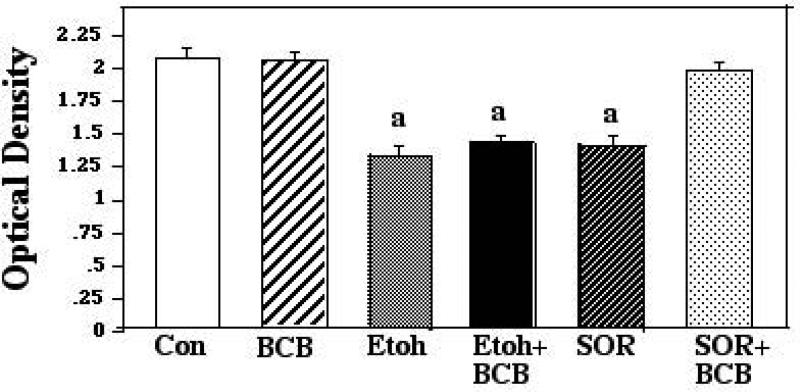
The third culture experiments were designed to assess the role of Bax channel formation in developmental ethanol neurotoxicity and to determine whether direct inhibition of such channel formation would result in a corresponding inhibition of ethanol-mediated cell death. For this portion of the study, granule cells were cultured with ethanol plus a cell-permeable dibromocarbazolo-piperazinyl derivative that specifically blocks Bax channel-forming activity (Hetz et al., 2005). Ethanol and the Bax channel blocker (BCB) were administered simultaneously, as above, and in additional preliminary experiments, cells were pretreated with the BCB for 2 hours prior to addition of ethanol. After a 24-hour survival time, the MTT survival assay was applied, and we found that the BCB did not afford any protection against the ethanol in preparations in which conditions were administered simultaneously, or in pretreated cultures. This experiment was repeated using the BCB at a range of concentrations (2.5μM, 5μM and 10μM) with identical results (data not shown). We then added a positive control to the experimental design, to confirm the efficacy of the blocker. For this control the vendor recommended sorbitol, which has previously been shown to induce rapid Bax-related apoptosis in a number of populations (e.g., Maroney et al., 1998; Koyama et al., 2000; Criollo et al., 2007). For this experiment, cultures were incubated with ethanol or sorbitol (20μM), with and without the BCB (5μM). The ANOVA applied to this data revealed a significant effect of treatment (F [5,66] 22.57; p < 0.0001). The post hoc analyses revealed that ethanol and sorbitol both significantly reduced neuronal survival compared to controls (p<0.0001 for each). BCB did not diminish the cell loss in the ethanol cultures, however, with survival in the ethanol + BCB cultures similar to that in the ethanol-only preparations, and differing significantly from controls (p<0.0001). BCB did prevent neuronal apoptosis in the presence of sorbitol, however, with survival in the sorbitol + BCB cultures indistinguishable from that of control and BCB-only cultures. These results are summarized in Figure 6.
Figure 6.
Postnatal day 8 cerebellar granule cells were cultured in control medium (Con), in medium supplemented with Bax channel blocker (BCB), in medium supplemented with 400mg/dl ethanol (Etoh), in medium with ethanol plus BCB, in medium supplemented with sorbitol (SOR), and in medium supplemented with SOR plus BCB. Neuronal survival was measured via the MTT assay. Error bars represent standard error of the mean (SEM). a=Significantly less than controls, BCB alone, and SOR plus BCB, p<0.0001 in each instance.
DISCUSSION
It has been shown that the neuropathological alterations in the developing CNS as a function of ethanol exposure are accompanied by striking alterations in expression and activities of proteins of the survival-regulatory Bcl-2 gene family (Moore et al., 1999; Ge et al., 2004; Lee et al., 2008). The Bax protein, a 21kD pro-apoptotic member of this family, appears to be particularly critical to ethanol neurotoxicity during these early periods. Bax is robustly upregulated following ethanol insult, both in vitro and in vivo (Moore et al., 1999; Olney et al, 2002: Heaton et al., 2003a; 2011; 2012). The protein subsequently translocates to the mitochondrial membrane, where it heterodimerizes with pro-survival proteins Bcl-2 and Bcl-xl, thus abrogating their protective potential (Siler-Marsiglio et al, 2005; Heaton et al., 2011; 2012). These pro-apoptotic events are particularly pronounced during periods which have been defined as maximally vulnerable to ethanol (Moore et al., 1999; Heaton et al, 2003a,b,c). The present series of studies was designed to further investigate interactions taking place at the mitochondrial membrane following ethanol-mediated Bax translocation, and focused on protein-protein associations between Bax and mitochondrial membrane proteins. In the in vivo portion of the study, neonatal rat cerebellum was used as a model system, and culture studies in which manipulative analyses could be made utilized cerebellar granule cell preparations. Analyses were made at the age of maximal ethanol vulnerability (P4), compared to the later age of relative resistance (P7), to determine whether the differential sensitivity previous defined correlated with differences in these molecular interactions. Our findings demonstrate that following ethanol exposure within this developing region, mitochondrially-localized Bax interacts with PTP proteins of both the outer mitochondrial membrane (VDAC) and the inner mitochondrial membrane (ANT), interactions which are conducive to an apoptotic outcome, with such pro-apoptotic associations seen predominantly at the age of greater ethanol sensitivity, compared to the later resistant age. Specifically, we found that ethanol exposure on P4, the age of peak ethanol vulnerability in developing cerebellum, results in enhanced interactions between Bax and VDAC, when assessed both during the exposure period, and two hours after termination of exposure, suggesting that such changes were sustained during this post-treatment interval. Conversely, at P7, the age at which this developing region is less ethanol-sensitive, no such changes were detected. Since Bax:VDAC interactions may be the result of Bax disruption of VDAC interactions with the HXK kinase, we assessed VDAC:HXK associations following ethanol treatment. This analysis revealed that such protective interactions were indeed decreased by ethanol treatment, but only after termination of the ethanol exposure period, and only in the P4, ethanol-sensitive cerebellum. We also found that Bax interacts with ANT proteins of the IMM, and while ethanol exposure resulted in small increases in such associations in both P4 and P7 cerebella when assessed during exposure, robust increases in these interactions were found at the two-hour post treatment time point, but only in the P4 animals. Ethanol neurotoxicity in the cultured neuronal preparations was abolished by pharmacological inhibition of both the VDAC and the ANT interactions with Bax, but not by a Bax channel blocker. Therefore, we conclude that at this age, within the constraints of our experimental model, a primary mode of Bax-induced initiation of the apoptosis cascade following ethanol exposure involves interactions with proteins of the PTP complex, and not channel formation independent of PTP constituents. The following sections consider these findings in the context of prior investigations.
The Role of the Mitochondria in Apoptosis
A wide range of apoptotic signals converge on the mitochondrion to initiate the apoptotic cascade via disruption of the mitochondrial membrane potential (MMP). Maintenance of this potential is critical for cellular survival; its loss renders the membrane permeable, leading to release of apoptogenic contents such as cytochrome-c (cyto-c) and apoptosis-inducing factor (AIF; Kroemer, 1998; Susin et al., 1999). Mitochondrial membrane integrity is regulated by the permeability transition pore (PTP), a multicomponent protein complex which spans both outer and inner mitochondrial membranes (Pastorino et al., 2002; Bras et al., 2005; Kromer et al., 2007). The exact makeup of the PTP is in some dispute, but it is generally accepted that the minimal constituents are the VDAC proteins of the outer mitochondrial membrane (OMM), ANT of the inner mitochondrial membrane (IMM), and cyclophilin D of the matrix (Kroemer et al., 2007). In addition, HXK is loosely attached to the OMM, and interacts with VDAC (Gottlob et al., 2001). The outer membrane is semi-permeable due to VDAC, which allows passage of solutes of up to 5kD, while the inner membrane is relatively impermeable (Kumarswamy and Chandna, 2009). Sustained opening of the PTP of the inner membrane results in a sudden increase in permeability, leading to osmotic pressure on the matrix, swelling and rupture of the OMM, release of mitochondrial contents into the cytosol, and eventual cell death (Polster and Fiskum, 2004; Kumarswamy and Chandna, 2009). This lethal sequence defines the mitochondrial permeability transition. The mitochondrial membrane appears to be a primary target of ethanol, with exposure shown to disrupt the mitochondrial membrane potential and lead to cyto-c release and caspase activation in a number of neuronal populations (e.g., hippocampal neurons, cerebellar granule cells; de la Monte and Wands, 2002; Vaudry et al., 2002; Li et al., 2002; Koch et al., 2004; Heaton et al., 2011).
Mechanisms of BAX-Mediated Apoptosis
Although it is widely recognized that Bax plays a critical role in disruption of the mitochondrial membrane and initiation of the cell death cascade in a variety of conditions, the exact mechanisms underling this process are a matter of some controversy. As noted above, there are currently two primary models of Bax-induced apoptosis, one in which Bax functions in collaboration with proteins of the PTP, and the second in which Bax functions apart from the PTP, by independent mitochondrial membrane pore formation. The following sections will consider previous evidence supportive of each of these models, and the present data as they relate to each.
Bax Interactions with PTP Proteins: BAX-VDAC Associations
VDAC, a 30kD β-barrel protein, also known as porin, is the most abundant protein in the outer mitochondrial membrane (Cesar and Wilson, 2004). It forms a voltage-regulated channel in the OMM, and mediates exchange of ATP/ADP, succinate, and citrate between mitochondria and cytosol under physiological conditions (Colombini, 1983; Pastorino et al., 2002). VDAC is critical for a range of cellular processes, including ATP rationing, calcium homeostasis, and apoptosis (Galganska et al., 2010). At low membrane potential, VDAC assumes a conformation which exposes contact sites that either Bax or HXK can bind with high affinity (Belzaqc et al., 2003). Of the three VDAC isoforms, only VDAC1 binds Bax, and it has been speculated that it may in fact serve as a Bax receptor (Kroemer et al., 2007; Messina et al., 2012). Bax heterodimeric interactions with VDAC have been well documented, and there is compelling evidence that such associations can lead to mitochondrial membrane depolarization, and cyto-c release, initiating the apoptotic cascade: Narita et al. (1998) found that introduction of recombinant Bax directly onto isolated mitochondria resulted in loss of the membrane potential, and release of cyto-c, coincident with formation of Bax:VDAC dimers. This effect could be blocked by application of PTP inhibitors cyclosporine-A and bongkrekic acid. In yeast cells deficient in VDAC, Bax did not cause loss of membrane potential or cyto-c release, but when the cells were transfected with the VDAC1 gene, Bax was once again able to depolarize the membrane and induce cyto-c loss, strongly suggesting that the Bax-VDAC interaction is critical for initiating the apoptosis cascade (Shimizu et al., 1999; Tsujimoto and Shimizu, 2000). Single Bax:VDAC channels formed in a planar lipid bilayer have also been shown to be permeable to cyto-c (Tsujimoto and Shimizu, 2000). Since the pore diameter normally formed by VDAC (~3nm) is not sufficient to accommodate cyto-c, it was important to determine how such passage was possible in the Bax-VDAC channels. While this is still a matter of some controversy, it appears that the channels formed by Bax:VDAC heterodimers are much larger than homotypic channels formed by either Bax or VDAC alone, and are thus cyto-c permeable (Shimizu et al., 1999; 2000; Tsujimoto and Shimizu, 2000; Pastorino et al., 2002; Ly et al., 2003).
An additional protection against membrane depolarization is afforded by the interactions between VDAC and HXK. HXK is a 100kD protein kinase located in the cytosol, loosely attached to the OMM. This protein functions in catalyzing the first commitment step of glucose metabolism (Gottlob et al., 2001). Under apoptotic conditions, the hydrophobic N-terminal domains of HXK can interact with VDAC to suppress loss of the membrane potential and cell death (Pastorino et al., 2002; Zaid et al., 2005). Bax directly competes with HXK for the same contact sites on the VDAC molecule (Pastorino et al., 2002; Kroemer et al., 2007). Thus, Bax displacement of VDAC from association with HXK promotes apoptosis, while VDAC:HXK interactions are protective (Kroemer et al., 2007).
Our findings of enhanced interactions between Bax and VDAC as a result of ethanol exposure are consistent with previous studies in which such associations were found to increase following insult or injury. In the rat CNS, for example, transient cerebral ischemia results in rapid Bax mitochondrial translocation, followed by elevations in the formation of Bax:VDAC heterodimers (Cao et all., 2001). Of particular relevance to the present study, is an investigation using rat hepatocytes, in which ethanol treatment resulted in loss of mitochondrial membrane potential, release of cyto-c and subsequent apoptotic cell death, along with enhanced interactions between Bax and VDAC (Adachi et al., 2004). These cells could be rescued by PTP inhibitor cyclosporine A, or by microinjection of VDAC antibodies, which prevented formation of the Bax:VDAC complexes. In the present study, we also found that in neonatal cerebellum, Bax:VDAC associations appear to be critical to the ethanol-induced apoptotic process. We found these interactions were enhanced following ethanol treatment, and this effect was found only at the age at which the neonatal cerebellum has been shown to be maximally sensitive to ethanol (P4). In addition, in the granule cell preparations, application of the VDAC inhibitor, which prevents conformational changes in this protein which would enable it to dimerize with Bax, prevented ethanol-mediated cell death, with survival in these cells being restored to control levels.
In our assessments of the protective interactions between VDAC and HXK, we found that these associations were unaffected by ethanol exposure at either age when assessed during the exposure period, but by two-hours post-exposure, such interactions were significantly decreased, but only at the age of maximal ethanol vulnerability (P4). These data together with our observations of increases in Bax:VDAC interactions in the P4 animals both during exposure and at the two hours post-exposure assessment point, suggest that VDAC is being effectively displaced from HXK by mitochondrially translocated Bax as a function of ethanol treatment. This displacement is further facilitated by the ethanol-mediated enhancement of Bax expression and of mitochondrially-localized Bax seen following exposure during these sensitive neonatal periods (e.g., Moore et al., 1999; Siler-Marsiglio et al., 2005; Heaton et al., 2012).
Bax Interactions with PTP Proteins: BAX-ANT
ANT is a 30kD mitochondrial inner membrane protein, belonging to a large family of structurally-related proteins which share a capacity to convert into non-specific pores (Kroemer et al., 2007). Under physiological conditions, ANT imports ADP into the mitochondrial matrix and exports ATP to the cytoplasm, a process critical to normal cellular metabolism (Shimizu et al., 2000). Under apoptotic conditions ANT can switch its vital function to a lethal one, in which it undergoes conformational change that is conductive to Bax binding (Fiore et al., 1998; Marzo et al., 1998). Upon Bax interaction, ANT loses its transporter function and membrane permeability is induced (Green and Kromer, 2004; Kroemer et al., 2007). The importance of these interactions has been effectively demonstrated in yeast cells, in which the formation of Bax-ANT complexes resulted in channel formation, and triggered the cell death cascade. Conversely, ANT-deficient yeast cells are resistant to Bax-mediated apoptosis (Marzo et al., 1998). This Bax-ANT channel formation can be inhibited by Bcl-2, and by pharmacological agents such as bongkrekic acid (BA) which shifts ANT to the m-conformation, with the adenosine binding site facing the matrix, preventing interactions with Bax and opening of the PTP (Ly et al., 2003; Verrier et al., 2003; Vyssokikh and Brdiczka, 2003). As with VDAC, Bax:ANT heterodimerization is increased following imposition of transient ischemic conditions in the rat CNS, with such interactions facilitating cyto-c release, and caspase activation (Cao et al., 2001). Application of the ANT inhibitor BA effectively interrupts Bax:ANT interactions and caspase activation in these ischemic preparations, in vitro, and this ANT inhibition has also been shown to protect against ischemia-induced cell death in vivo (Cao et al., 2001). The present results are consistent with a role for Bax:ANT associations in ethanol-mediated call death in the developing CNS. We found that at both ages assessed, such interactions were increased, although more marked increases were seen at the earlier age, and these increases were sustained for at least two hours post-exposure. Compelling evidence confirming the importance of Bax:ANT interactions was provided by the granule cells culture experiments in which BA was demonstrated to effectively inhibit ethanol-mediated cell death. As noted above, BA alters the conformation of the ANT protein in a manner that prevents dimerization with Bax, since these interactions are conformation-specific (Marzo et al., 1998; Cao et al., 2001).
In our analyses of both Bax:ANT and Bax:VDAC interactions, it is notable that at the age of greatest ethanol vulnerability, these protein associations were particularly long-lasting, persisting for at least two hours post-exposure, while similar increases at the older age returned to control levels during this period in which exposure was suspended. Such sustained neurotoxicity, characteristic of the more ethanol-sensitive age, would likely continue to disrupt normal developmental processes. These persistent apoptotic-related protein associations may be related to the fact that peak blood ethanol concentrations achieved with the inhalation procedure persist for two-three hours following treatment, although the pattern of BEC resolution is similar for both neonatal ages (Heaton et al., 2003a). It will be important in future studies to determine the relationship between these sustained apoptotic events and BEC, whether they subside as blood ethanol declines (i.e., in the younger animals), or whether at the most ethanol-sensitive age, the apoptotic processes may continue for a considerable time, even in the absence of the proximate stimulus. It will also be important to determine the mechanisms underlying both the sustained pro-apoptotic conditions at the earlier age, and the more rapid recovery at the later age.
Bax-Induced Apoptosis Independent of PTP Proteins
The second major model of Bax mitochondrial disruption is the PTP-independent process of direct channel formation by homotypic Bax oligomers. This model is based on the observation that certain Bcl-2 family proteins, including Bax, Bcl-2 and truncated Bid, possess structural similarities to pore-forming domains of bacterial toxins (Gross et al., 1998; Verrier et al., 2003; Bras et al., 2005). This model calls for the direct formation of OMM pores by Bax alone, sufficient for cyto-c release, with no involvement of PTP proteins, or permeability transition events (e.g., mitochondrial swelling and rupture; Tafani et al., 2001). This hypothesis has been supported in studies in which recombinant Bax rendered oligomeric by treatment with octyl glucoside formed channels in liposomes and elicited cyto-c release from isolated mitochondria, whereas monomeric Bax was ineffective in this regard (Antonsson et al., 2000). This channel formation and subsequent release of cyto-c was not prevented by PTP inhibitors (e.g., cyclosporine A; Antonsson et al., 2000). Specific pharmacological inhibitors of Bax channel formation have been synthesized and their application has been shown to prevent cyto-c release and protect against cell death in some preparations. In staurosporin-challenged HeLa cells, for example, inhibition of Bax channel formation suppresses cyto-c release and cell death, and in vivo, similar inhibition affords significant protection against global ischemia in gerbil hippocampal neurons (Hetz et al., 2005).
In addition to the channels formed by Bax alone (sometimes referred to as “pure” Bax channels; Pavlov et al., 2001), oligomeric Bax and/or Bak, another pro-apoptotic member of the Bcl-2 family, appear to be components of the so-called mitochondrial apoptosis-induced channel (MAC). This channel was first characterized in the outer mitochondrial membrane of hematopoietic FL5.12 and yeast cells using patch clamp procedures on cells in the early stages of apoptosis (Pavlov et al., 2001). The MAC is quite similar to the Bax channels first described, but with differences in certain physiological properties found in patch clamping analyses (e.g., greater peak conductance in MAC channels; Pavlov et al, 2001; Dejean et al., 2005; 2006). The pore size formed by MAC is also greater than that typically found in Bax-only channels, and is sufficient to allow passage of proteins even larger than cyto-c which are normally sequestered within the mitochondria, such as SMAC/Diablo (Saito et al., 2000). These channels can be induced by Bax, and their formation is blocked by Bcl-2 (Dejean et al., 2006; Kinnally and Antonsson, 2007). Their activities, e.g., facilitation of cyto-c release, are not affected by PTP inhibitors, but are suppressed by Bax channel blockers (Dejean et al., 2006; Kinnally and Antonsson, 2007).
Based on the evidence currently available, it appears that ethanol-induced apoptosis may not involve Bax or MAC channel formation independent of PTP proteins, at least in some systems. In the investigation by Adachi et al., for example, ethanol-mediated apoptosis of rat primary hepatocytes was a function of Bax-VDAC interactions, and could be blocked by inhibition of these associations (Adachi et al., 2004). Bax homotypic oligomerization was not found following ethanol treatment in these cells, however, although such complexes were elicited in tumor necrosis factor-alpha (TNFα) treated hepatocytes (Adachi et al., 2004). Similarly, in the present study, ethanol-induced death of cerebellar granule cells was not prevented by application of Bax channel blocker, across a range of concentrations, even if the cells were pretreated with the blocker. We were, however, able to block this neuronal loss completely with PTP inhibitors. Thus, our results also are not supportive of the Bax channel or MAC formation as primary mechanisms of ethanol neurotoxicity, within this neuronal model system, at this developmental age. We cannot rule out a role for Bax channel formation entirely, however. It is possible in our experimental paradigm, for example, that Bax or MAC channels contribute to early phases of ethanol-induced toxicity, and that over the 24-hour exposure time, this effect was supplanted by Bax-PTP interactions in cultures with the Bax channel blocker. Such dual or sequential Bax functionality has in fact been proposed previously, suggesting that the potential for both PTP-dependent and independent cell death may co-exist within a given cell type, and that the pathway activated may depend on conditions, and possibly, the nature of the apoptotic signal (Kroemer et al., 2007; Kumarswamy and Chandna, 2009).
CONCLUDING REMARKS
This study has defined molecular processes occurring at the mitochondrial membrane which are induced by ethanol exposure during developmental periods, and has shown that these processes are differentially affected at ages of maximal ethanol sensitivity, compared to later ages in which the neonatal cerebellum is able to withstand ethanol insult. The results of the present study suggest that Bax-PTP interactions are critical for ethanol neurotoxicity in the neonatal cerebellum at the ages assessed, with our observations of ethanol-induced formation of Bax:VDAC and Bax:ANT associations, and with such associations correlated with differential temporal ethanol sensitivity within developing cerebellum. We also found that inhibition of such interactions in vitro was sufficient to block ethanol neurotoxicity. These results provide strong evident for the importance of the PTP to developmental ethanol toxicity. These molecular processes may represent points which can be targeted in future studies concerned with designing possible therapeutic strategies for minimizing devastative effects of developmental ethanol exposure.
Acknowledgments
This research was supported by NIAAA grants AA012151 and AA016327.
REFERENCES
- Adachi M, Higuchi H, Miura S, Azuma T, Inokuchi S, Saito H, Kato S, Ishii H. Bax interacts with the voltage-dependent anion channel and mediates ethanol-induced apoptosis in rat hepatocytes. Amer J Physiol Gastrointest Liver Physiol. 2004;287:G695–705. doi: 10.1152/ajpgi.00415.2003. [DOI] [PubMed] [Google Scholar]
- Andrews DL, Williams GS, Mahoney JC, West JR. DNA fragmentation during exposure of rat cerebella to ethanol under hypoxia imposed in vitro. J Neurobiol. 1999;8:82–92. doi: 10.1002/(sici)1097-4695(199901)38:1<82::aid-neu6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou J. Bax oligomerization is required for channel-forming activity in lipsomes and to trigger cytochrome c release from mitochondria. J Biochem. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- Belzacq AS, Vieira Helena LA, Verrier F, Vandecasteele G, Cohen I, Prevost MC, Larquet E, Pariselli F, Petit PX, Kahn A, Rizzuto R, Brenner C, Kroemer G. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 2003;63:541–546. [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochem (Mosc) 2005;70:231–239. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- Cao G, Minami M, Pei W, Yan C, Chen D, O'Horo C, Graham SH, Chen J. Intracellular Bax translocation after transient cerebral ischemia: implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab. 2001;21:321–33. doi: 10.1097/00004647-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Cesar MC, Wilson JE. All three isoforms of the voltage-dependent anion channel (VDAC1, VDAC2, and VDAC3) are present in mitochondria from bovine, rabbit, and rat brain. Arch Biochem Biophys. 2004;422:191–196. doi: 10.1016/j.abb.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Colombini M. Purification of VDAC (voltage-dependent anion-selective channel) from rat liver mitochondria. J Membr Biol. 1983;74:115–121. doi: 10.1007/BF01870500. [DOI] [PubMed] [Google Scholar]
- Criollo A, Galluzzi L, Maiuri MC, Tasdemir E, Lavandero S, Kroemer G. Mitochondrial control of cell death induced by hyperosmotic stress. Apoptosis. 2007;12:3–18. doi: 10.1007/s10495-006-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. Cell Mol Life Sci. 2002;59:882–893. doi: 10.1007/s00018-002-8475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, Kinnally KW. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Molec Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Kinnally KW. Is MAC the knife that cuts cytochrome c from mitochondria during apoptosis? Cell Death Differen. 2006;13:1387–1395. doi: 10.1038/sj.cdd.4401949. [DOI] [PubMed] [Google Scholar]
- Fiore C, Trézéguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noel F, Lauquin GJ, Brandolin G, Vignais PV. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie. 1998;80:137–150. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- Galganska H, Karachitos A, Wojtkowska M, Stobienia O, Budzinska M, Kmita H. Communication between mitochondria and nucleus: putative role for VDAC in reduction/oxidation mechanism. Biochim Biophys Acta. 2010;1797:1276–1280. doi: 10.1016/j.bbabio.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Ge Y, Belcher SM, Pierce DR, Light KE. Altered expression of Bcl-2, Bad and Bax mRNA occurs in the rat cerebellum within hours after ethanol exposure on postnatal day 4 but not on postnatal day 9. Mol Brain Res. 2004;129:124–134. doi: 10.1016/j.molbrainres.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;2:738–744. [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M, Walker DW. Ethanol-induced alterations in the expression of neurotrophic factors in the developing rat central nervous system. Devel Brain Res. 2000;121:97–107. doi: 10.1016/s0165-3806(00)00032-8. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Moore DB, Paiva M, Madorsky I, Mayer J, Shaw G. The role of neurotrophic factors, apoptosis-related proteins, and endogenous antioxidants in the differential temporal vulnerability of neonatal cerebellum to ethanol. Alcohol Clin Exp Res. 2003;27:657–669. doi: 10.1097/01.ALC.0000060527.55252.71. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Shaw G. Ethanol effects on neonatal rat cortex: comparative analyses of neurotrophic factors, apoptosis-related proteins, and oxidative processes during vulnerable and resistant periods. Devel Brain Res. 2003;145:249–262. doi: 10.1016/j.devbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Mayer J, Moore DB. Effects of ethanol on neurotrophic factors, apoptosis-related proteins, endogenous antioxidants, and reactive oxygen species in neonatal striatum: relationship to periods of vulnerability. Devel Brain Res. 2003;140:237–252. doi: 10.1016/s0165-3806(02)00610-7. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Siler-Marsiglio K, Shaw G. The effect of Bax deletion on ethanol sensitivity in the neonatal rat cerebellum. J Neurobiol. 2006;66:95–101. doi: 10.1002/neu.20208. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Siler-Marsiglio K. Ethanol influences on Bax translocation, mitochondrial membrane potential, and reactive oxygen species generation are modulated by vitamin E and brain-derived neurotrophic factor. Alcohol Clin Exp Res. 2011;35:1122–1133. doi: 10.1111/j.1530-0277.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Kubovec S, Kotler A, Rogozinski J, Swanson E, Madorsky V, Posados M. Differential effects of ethanol on c-jun N-terminal kinase, 14-3-3 proteins, and Bax in postnatal day 4 and postnatal day 7 rat cerebellum. Brain Res. 2012;1432:15–27. doi: 10.1016/j.brainres.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, Schwarz MK, Church DJ, Korsmeyer SJ, Martinou JC, Antonsson B. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005;280:42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nakamura K, Iwahashi K, Ameno K, Itoh M, Suwaki H. Changes of Bcl-2 and Bax mRNA expressions in the ethanol-treated mouse brain. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2002;37:120–129. [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. The Lancet. 1973:999–1000. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- Koch OR, Pani G, Borrello S, Colavitti R, Cravero A, Farre S, Galeotti T. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol Aspects Med. 2004;25:191–198. doi: 10.1016/j.mam.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Koyama AH, Arakawa T, Adachi A. Characterization of apoptosis induced by sorbitol: a unique system for the detection of antiapoptotic activities of viruses. Microbes Infect. 2000;2:599–606. doi: 10.1016/s1286-4579(00)00366-x. [DOI] [PubMed] [Google Scholar]
- Kroemer G. The mitochondrion as an integrator/coordinator of cell death pathways. Cell Death Differ. 1998;19:525–547. doi: 10.1038/sj.cdd.4400387. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kumarswamy R, Chandna S. Putative partners in Bax mediated cytochrome-c release: ANT, CypD, VDAC or none of them? Mitochondrion. 2009;9:1–8. doi: 10.1016/j.mito.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Lee HY, Naha N, Kim JH, Jo MJ, Min KS, Seong HH, Shin DH, Kim MO. Age- and area-dependent distinct effects of ethanol on Bax and Bcl-2 expression in prenatal rat brain. J Microbiol Biotech. 2008;18:1590–1598. [PubMed] [Google Scholar]
- Li Y, Meyer EM, Walker DW, Millard WJ, He Y-J, King MA. Alpha7 nicotinic receptor activation inhibits ethanol-induced mitochondrial dysfunction, cytochrome c release and neurotoxicity in primary rat hippocampal neuronal cultures. J Neurochem. 2002;81:853–858. doi: 10.1046/j.1471-4159.2002.00891.x. [DOI] [PubMed] [Google Scholar]
- Light KE, Belcher SM, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience. 2002;114:327–337. doi: 10.1016/s0306-4522(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Lindblad B, Olsson R. Unusually high levels of blood alcohol? JAMA. 1976;236:1600–1602. [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- Manthorpe M, Fagnani R, Skaper SD, Varon S. An automated colorimetric microassay for neuronotrophic factors. Brain Res. 1986;390:191–198. doi: 10.1016/s0006-8993(86)80227-x. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Glicksman MA, Basma AN, Walton KM, Knight EJ, Murphy CA, Bartlett BA, Finn JP, Angeles T, Matsuda Y, Neff T, Dionne CA. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HLA, Prevost M-C, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- Medina AE. Fetal Alcohol Spectrum Disorders and Abnormal Neuronal Plasticity. Neuroscientist. 2011;17:274–287. doi: 10.1177/1073858410383336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, Reina S, Guarino F, De Pinto V. VDAC isoforms in mammals. Biochim Biophys Acta. 2012:2011. doi: 10.1016/j.bbamem.2011.10.005. Oct. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Paiva M, Heaton MB. Optimal 96-well plate set up to avoid ethanol volatility when assessing ethanol cytotoxicity. Alcohol. 1998;15:137–139. doi: 10.1016/s0741-8329(97)00124-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Paiva M, Walker DW, Heaton MB. BDNF and NGF afford in vitro neuroprotection against ethanol combined with acute ischemia and chronic hypoglycemia. Devel Neurosci. 1999;21:68–75. doi: 10.1159/000017368. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid calorimetric assay for cellular growth and survival: Applications to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moore DB, Walker DW, Heaton MB. Neonatal ethanol exposure alters Bcl-2 family mRNA levels in the rat cerebellar vermis. Alcohol Clin Exp Res. 1999;23:1251–1261. doi: 10.1111/j.1530-0277.1999.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Muller Y, Tangre K, Clos J. Autocrine regulation of apoptosis and Bcl-2 expression by nerve growth factor in early differentiating cerebellar granule neurons involves low affinity neurotrophin receptor. Neurochem Internatl. 1997;31:177–191. doi: 10.1016/s0197-0186(96)00147-7. [DOI] [PubMed] [Google Scholar]
- Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerster J, Rabin RA. Enhanced caspase activity during ethanol-induced apoptosis in rat cerebellar granule cells. Eur J Pharmacol. 1999;385:273–282. doi: 10.1016/s0014-2999(99)00714-1. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D'Sa C, Roth A. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- Pavlov EP, Priault M, Pietkiewicz D, Cheng E, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA, Kinnally KW. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J Cell Biol. 2001;155:725–731. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DR, Williams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcohol Clin Exp Res. 1999;23:1650–1659. [PubMed] [Google Scholar]
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Shinohara Y, Tsujimoto Y. Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator. Oncogene. 2000;19:4309–4318. doi: 10.1038/sj.onc.1203788. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio, Shaw G, Heaton MB. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J Neurobiol. 2004;59:261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Paiva M, Madorsky I, Pan Q, Shaw G, Heaton MB. Functional mechanisms of apoptosis-related proteins in neonatal rat cerebellum are differentially influenced by ethanol at postnatal day 4 and 7. J Neurosci Res. 2005;81:632–643. doi: 10.1002/jnr.20591. [DOI] [PubMed] [Google Scholar]
- Stephens CJ. Alcohol consumption during pregnancy among southern city women. Drug Alcohol Depend. 1985;16:19–29. doi: 10.1016/0376-8716(85)90078-x. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–6. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tafani M, Minchenko DA, Serroni A, Farber JL. Induction of the mitochondrial permeability transition mediates the killing of HeLa cells by staurosporine. Cancer Res. 2001;61:2459–2466. [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differen. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fontaine M, Fournier A, Vandry H, Gonzalez BJ. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci USA. 2002;99:6398–6403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier F, Mignotte B, Jan G, Brenner C. Study of PTPC composition during apoptosis for identification of viral protein target. Annals NY Acad Sci. 2003;1010:126–142. doi: 10.1196/annals.1299.022. [DOI] [PubMed] [Google Scholar]
- Vyssokikh MY, Brdiczka D. The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim Polonica. 2003;50:389–404. [PubMed] [Google Scholar]
- Young C, Klocke BJ, Tenkova T, Choi J, Labruyere J, Qin YQ, Holtzman DM, Roth KA, Olney JW. Ethanol-induced neuronal apoptosis in vivo requires Bax in the developing mouse brain. Cell Death Differen. 2003;10:1148–1155. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]
- Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]