Abstract
BACKGROUND & AIMS
Patients with systemic sclerosis (SSc) have impairments in gastrointestinal smooth muscle function. The disorder has been associated with circulating antibodies to cholinergic muscarinic type-3 receptor (M3-R). We investigated whether it is possible to neutralize these antibodies with pooled human immunoglobulin (Ig)Gs (pooledhIgG).
METHODS
We studied the effects of IgGs purified from patients with SSc (SScIgGs) on cholinergic nerve stimulation in rat colon tissues. We also examined the effects of SScIgGs on M3-R activation by bethanechol (BeCh), M3-R occupancy, and receptor binding using mmunofluorescence, immunoblot, and ELISA analyses of human internal anal sphincter (IAS) smooth muscle cells (hSMCs), before and after administration of pooledhIgG. Functional displacement of M3-R occupancy by the SScIgGs was compared with that of other IgGs during the sustained phase of BeCh-induced contraction of intact smooth muscles from rats.
RESULTS
SScIgG significantly attenuated neutrally mediated contraction and acetylcholine release in rat colon as well as BeCh-induced sustained contraction of the IAS smooth muscle. In immunofluorescence analysis, SScIgG co-localized with M3-R. In immunoblot and ELISA analyses, M3-R loop-2 peptide and human IAS SMC membrane lysates bound significant amounts of SScIgG, compared with IgGs from healthy individuals and pooledhIgG. Binding was significantly attenuated by application of pooledhIgG, which by itself had no significant effect. Incubation of samples with pooledhIgG, or mixing pooledhIgG with SScIgG before administration to tissues, significantly reduced binding of SScIgG, indicating that pooledhIgG prevents SScIgG blockade of M3-R.
CONCLUSIONS
In studies of rat and human tissues, pooled human IgGs prevent and reverse the cholinergic dysfunctions associated with the progressive gastrointestinal manifestations of SSc, by neutralizing functional M3-R antibodies present in the circulation of patients with SSc.
Keywords: connective tissue disease, smooth muscle atrophy, fibrosis, animal model
Introduction
Scleroderma or systemic sclerosis (SSc) is a progressive autoimmune connective tissue disease associated with progressive fibrosis of skin and numerous internal organs.1 The gastrointestinal (GI) tract is the most common internal organ system affected in SSc with as many as 90% of the patients developing esophageal symptoms.2,3 Interestingly, a large percentage of SSc patients display involvement of the anorectum, causing alterations of the IAS function and frequent fecal incontinence.4–6
GI dysmotility in SSc is believed to be neuropathic in origin, with subsequent smooth muscle atrophy and fibrosis.2 The early stages may involve neuropathic changes in the cholinergic innervation, and in the latter stages there are myopathic changes.2,7 Following earlier studies by Bacman and colleagues on the presence of circulating M3-R in patients with Sjögren’s syndrome,8 Goldblatt et al.9 demonstrated the presence of such antibodies in SSc patients. These antibodies caused significant dysfunction in the mouse colonic smooth muscle contraction induced by the M3-R agonist, carbachol. These studies suggested that neuropathic gastrointestinal dysfunction caused by circulating antibody interference with cholinergic innervation was responsible for GI dysmotility in SSc. Our recent studies using isolated SMCs of the rat IAS demonstrated that SScIgGs cause ~50% inhibition of M3-R activation and suggested that this mechanism in part is responsible for the GI smooth muscle myopathy in SSc.10 None of the above studies, however, neither examined the status of the actual cholinergic innervation and its effects in human SMCs, nor investigated the possible reversal of SScIgGs’ effects.
Because of the lack of clear understanding of the SSc pathophysiological mechanism/s, there is no effective disease modifying treatment for SSc. Pooled human IgG (pooledhIgG) also known as intravenous immunoglobulin (IVIG) has been clinically employed as a potent immunomodulating agent in various immune-mediated disorders.11–13 The use of pooledhIgG for the treatment of SSc has been supported by studies in the tight skin (TSK) animal model of SSc demonstrating that pooledhIgG may cause beneficial effects14 either by counteracting antifibroblast antibodies or decreasing expression of the type I collagen gene,15 as well as by modulating the secretion of profibrotic cytokines TGFβ1 and IL-4.14,16 However, the exact mechanisms of action of pooledhIgG in SSc are not known. Thus, owing to the potential pathogenetic role of circulating antibodies in SSc-related GI dysmotility it will be important to determine the effect of pooledhIgG in SSc in inhibition of the effect of SScIgGs against M3-R.
In the present study we determined the site of action of the M3-R autoantibodies present in SScIgGs using rat colon to assess a cholinergic involvement, and then assessed the myopathic changes using human IAS SMC and rat IAS smooth muscles. The studies also investigated the role of pooledhIgG in reversing the cholinergic blockade by SScIgGs in these systems.
Materials and Methods
Isolation and Purification of IgGs from SSc Patients and Normal Volunteers
Six female patients with SSc fulfilling the criteria for SSc classification of the American College of Rheumatology17 were studied. The studies were approved by the Institutional Review Board of Thomas Jefferson University. Disease duration varied from 4 to 42 years. All patients had typical esophageal manometric features and two had fecal incontinence.18 Total IgGs were purified from plasma of the six SSc patients (SScIgGs) and two normal volunteers (NIgGs), by use of recombinant protein G-Sepharose 4B conjugate (Zymed Laboratories, San Francisco, CA) and disposable spin-out columns (G Biosciences, Maryland Heights, MO) as published previously.19
Human IAS Tissue Samples and SMCs Isolation
Human IAS smooth muscle tissue samples were obtained from five subjects who underwent surgical removal of the anorectal region. SMCs from the circular smooth muscle layer of the IAS were isolated as described previously.10 Isolated cells were resuspended in DMEM growth medium with 5% fetal bovine serum and antibiotics on Lab-Tek II chamber slides (Nulgene Nunc International, Naperville, IL) at 37°C and 5% CO2 in an incubator with regulated humidity.
Experiments in Human SMCs
Effect of SScIgGs, NIgGs and PooledhIgG on Human IAS SMC Contraction
The SMCs isolated as described above were divided into different aliquots, and their responses to bethanechol (BeCh; 10−9 to 10−4 M) before and after SScIgG, NIgG, pooledhIgG (0.1 to 1 mg/ml) and the combination of either pooledhIgG and SScIgG or pooledhIgG and NIgG (0.1 to 1 mg/ml each) for 10 min, were determined. In some experiments, we also examined the effects of higher concentrations (10 mg/ml) of pooledhIgG. The cells were then fixed with acrolein (final concentration 1%) and cell length was measured by using digital micrometry.10 To determine the selectivity of action of the SScIgG on the M3-R activation by BeCh, we compared the effects of different IgGs and their combinations on α1-adrenoceptor (α1-AR) activation by phenylephrine (10−9 to 10−4 M), and K+-depolarization with KCl (2.5 mM to 40 mM).
Immunofluorescence Analysis
The human IAS SMCs were quickly rinsed with Dulbecco’s phosphate-buffered saline (DPBS) and non specific antibody binding sites were blocked with 1% FBS in DPBS. To determine the membrane binding efficiency of SScIgG, vs NIgG and pooledhIgG, SMCs were incubated with 5 μg/ml of these antibodies for different time points (0 to 30 min) at 37°C, and fixed with acetone for 10 min at −20°C. The cells were then washed three times with DPBS and stained with the anti-human IgG-FITC-conjugated antibody as described previously.20 Cells were co-stained with 5μg/ml of anti-M3-R antibody, as an internal control to detect colocalization of M3-R with SScIgG in human IAS SMCs. These cells were rinsed with DPBS and stained with anti rabbit-TR-conjugated antibody. The cells were finally stained with nuclear staining dye DAPI and images were taken employing a Nikon Eclipse 80i microscope as described previously.20
Immunofluorescence Intensity (IFI) Calculation
IFI was calculated by using NIS Elements 3.1 software by two different methods as follow: a). Intensity per unit area: For this purpose, 20 μm2 of cell membrane area was selected at four different sites of the cell membrane and pixel intensity under this area was calculated and plotted as a bar graph (Fig. 2B and E). The above procedure was repeated in 20 randomly selected cells and calculated as means ± SE. b). Line graph method: For this purpose, intensity under a line of 1-μm width across the cell avoiding the nucleus was calculated and plotted for the membrane IFI of different IgGs (Fig. 3A). The Pearson’s colocalization coefficient was determined for each cell and Mean ± SE was calculated and plotted (Fig. 3B).21
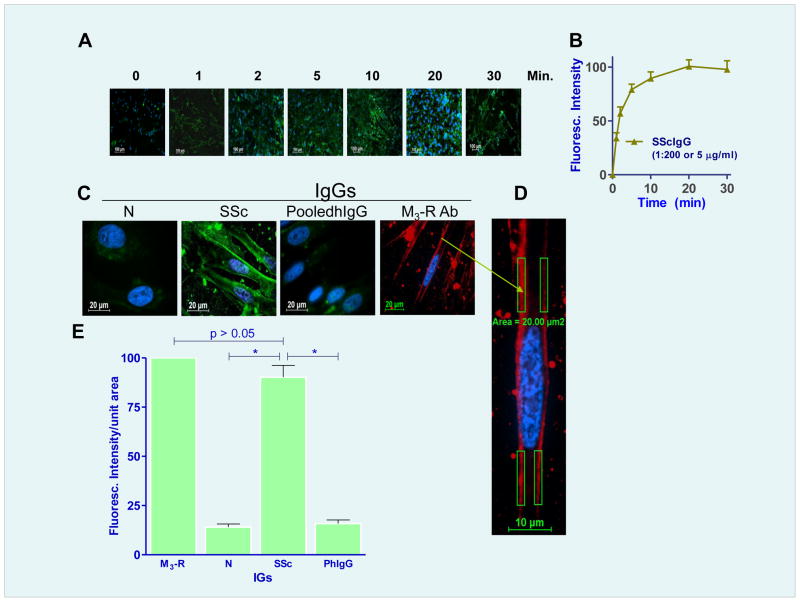
Fig. 2.
A. Time-dependent increase in the M3-R-specifiic IF intensity (IFI) in human IAS SMCs following treatment with (1:200 or 5 μg/ml) of M3-R antibody. B. Maximal IFI of human IAS SMC occurs following 20 min of incubation with the M3-R antibody. C. M3-R occupancy with SScIgG in the SMC membrane is significantly higher (*; p<0.05) as compared with NIgG and pooledhIgG, as determined by IFI/unit area. D. An example of data collection for the intensity/unit area on the membrane. E. Quantitative data showing % maximal IFI/unit area with NIgG, SScIgG, pooledhIgG (on the basis of 100% for M3-R antibody).
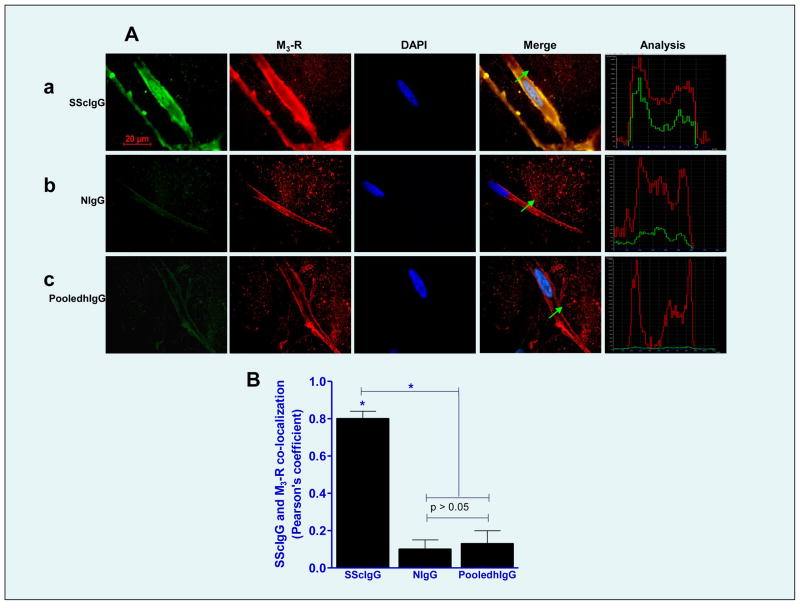
Fig. 3.
A. A. Immunocytochemical colocalization of different IgG preparations (SScIgG, a; NIgG, b; and pooledhIgG, c; (all FITC-conjugated; green)) and M3-R (TR-conjugated; red) and nucleus is stained blue with DAPI. Line graph analyses for the localization of these IgGs on the SMC membrane and their corresponding colocalization with M3-R are shown on the extreme right of each IgG plate. (B) Bar graph data show significant colocalization of SScIgG (vs. NIgG and pooledhIgG) with the M3-R at the SMC membrane.
Western Blot (WB) Analyses for Interaction of SScIgGs vs. NIgGs and pooledhIgG with M3-R
These studies were performed to determine the binding of SScIgG vs. NIgG, and pooledhIgG to M3-R in human IAS SMC membrane fractions (HISMF) prepared following a previously described method.22 The membrane protein was electrophoresed on 7.5% SDS-PAGE and transferred to a PVDF membrane by using iBlot.7 The membrane was subdivided into five equal parts that were kept in blocking buffer (5% bovine serum albumin in Tris buffer saline pH 7.2) for 1 h. These membranes were separately incubated overnight at 4°C with 5 μg/ml of M3-R antibody, SScIgG, NIgG, pooledhIgG, and pooledhIgG+SScIgG, washed three times with TBS containing 0.1% Tween (TBST), and stained with corresponding HRP-conjugated secondary antibodies. The membranes were washed three times for 10 min with TBST, and stained with tertiary antibody against HRP (Chemi IR detection system from LI-COR biosciences) for 1 h at RT in TBST containing 0.002% SDS. The membranes were washed and scanned with a LI-COR Odyssey Imaging System infrared scanner, and relative densities of M3-R and human IgGs were plotted using Image-J (NIH).10 Na+-K+ ATPase (a membrane marker) antibody was used as a loading control.
Enzyme-Linked Immunosorbent Assay (ELISA)
To determine direct binding of SScIgG to the M3-R and its interaction with pooledhIgG in the human IAS SMCs, we performed ELISA studies employing an ELISA kit (NB-E10632 from Novatein Biosciences, Inc., Cambridge, MA) using different concentrations of purified M3-R peptide or HISMF according to the manufacturer’s instructions. These experiments provided the optimal concentrations of M3-R peptide and the HISMF to determine the M3-R binding.
To determine the M3-R binding, 400 μg/ml of HISMF and peptides corresponding to the M3-R second extracellular loop-2 (M3-RL2; KRTVPPGECFIQFLSEPTITFGTAI, amino acids 213–237) and Loop-3 (M3-RL 3; NTFCDSCIPKTFWN, amino acids 514–527) at a concentration of 400 pg/ml were separately dissolved in the carbonate buffer and adsorbed onto separate multiwell plates used for ELISA.23 From 0 to 6 μg/ml of M3-R antibody, SScIgG, NIG, pooledhIgG, and pooledhIgG+SScIgG were added to these multiwell plates and incubated at 37°C for 1 h. Plates were washed three times with DPBST and incubated for 1 h at RT with anti-rabbit-HRP-conjugated for M3-R antibody and anti-human-HRP-conjugated secondary antibodies for SScIgG, NIgG, pooledhIgG and pooledhIgG+SScIgG and washed three times with DPBST and 100 μl of TMB substrate was added and kept for 15 min at RT. Following substrate addition, 100 μl of Stop solution was added to each well and absorbance was recorded at 450nm with ELISA reader.
Neutralization Studies
For this purpose 400 pg/ml M3-R peptide was adsorbed on multiwell plates (used for ELISA) and the effect of pooledhIgG on the maximal binding of the SScIgG (5 μg/ml) to the M3-R peptide was determined by ELISA. We substituted SScIgG alone with the mixture of SScIgG+pooledhIgG (mixed at 37°C for 1h; pooledhIgG concentrations ranged from 0 to 15 μg/ml diluted in PBS). As a negative control for the pooledhIgG effects, we used inactivated pooledhIgG (by proteinase digestion followed by boiling). The OD readings obtained using the combination of pooledhIgG+SScIgG were compared with those of the maximal OD reading obtained with M3-R peptide and SScIgG alone. To determine the neutralization of the M3-R binding with the SScIgGs by pooledhIgG in the IAS SMC membrane, we followed the procedure described above except for substitution of M3-RL2 with 400 μg/ml of HISMF.
Experiments using Intact Rat Colon and IAS Smooth Muscles: Smooth Muscle Strips Preparation and Isometric Tension Recording
Cholinergic Nerve Stimulation Experiments
Male Sprague-Dawley rats (300–350 g) were euthanized by decapitation, and the lower portion of the colon was surgically removed and transferred to oxygenated (95% O2 + 5% CO2) Krebs physiological solution (KPS) at 37°C. The composition of KPS (in mM) was: 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.10 glucose. The colonic smooth muscle strips (~0.5×7 mm) from the circular smooth muscle layer were prepared for the recording of isometric tension using 2 ml organ baths as described previously.24,25 These smooth muscle strips were then transferred to 2 ml organ baths containing oxygenated KPS and allowed to equilibrate for 1–2 h. To determine the effects of cholinergic nerve stimulation, electrical field stimulation (EFS; 10 V, 0.25 to 10 Hz, 4 sec train, each pulse of 0.5 ms) was delivered using a Grass stimulator (model S88; Grass Instruments Co., Quincy, MA). The EFS responses (percent maximal increase in the basal activity) were quantified before and after tetrodotoxin (TTX; 10−6 M), atropine (10−7 M), darifenacin (10−7 M), NIgG and SScIgG (1mg/ml), pooledhIgG (10 mg/ml), and pooledhIgG (10mg/ml)+SScIgG (1mg/ml).
Acetylcholine (ACh) Measurements
Rat colonic smooth muscles were prepared for the EFS as described above. The muscle bath perfusates were collected in the basal state, and following EFS before and after 0Ca2+, TTX (10−6 M), and SScIgG (1mg/ml) before and after pooledhIgG. ACh measurements were made using choline/acetylcholine Quantification kit (BioVision, Milpitas, CA) following manufacturer’s instructions. Data were quantified employing fluorometric (Fluorometer Optima Micropipette Reader; BMG Labtech Ortenberg, Germany) analysis with MARS software.
Functional Displacement of M3-R Experiments using Intact IAS Smooth Muscle
We examined the responses to SScIgG, NIgG and pooledhIgG (0.1 to 1 mg/ml) on the sustained phase of IAS contraction by BeCh (10−4 M). These effects were compared with those of M3-R antagonist darifenacin (10−9 M to 5×10−8 M). The basal tone in each smooth muscle strip of the colon and IAS was determined at the end of each experiment by the administration of EDTA (50 mM) and 0Ca2+.25,26 All experimental protocols were approved by the IACUC of Thomas Jefferson University in accordance with the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
Drugs and Chemicals
BeCh, KCl, phenylephrine, TTX, atropine, and M3-R antibody were purchased from Sigma Aldrich (St. Louis, MO). PooledhIgG (IVIG) was a generous gift from PriviGen (King of Prussia, PA). M3-R loop-2 (M3-RL2) and loop-3 (M3-RL3) peptides were from Peptide 2.0 Inc. (Chantilly, VA), Darifenacin (M3-R selective antagonist) was a gift from Pfizer (Sandwich, Kent, UK). Na+-K+ ATPase monoclonal antibody (a membrane marker) was from Santa Cruz Biotech. (Santa Cruz, CA).
Statistical Analysis
Data are presented as means ± SE of multiple experiments. P values less than 0.05 were considered statistically significant. The concentration-response curves were fitted by nonlinear regression using the computer software Prism (GraphPad Software, San Diego, CA).
Results
Effect of SScIgGs on Cholinergic Nerve Stimulation in the Colon: Studies in Intact Rat Colonic Smooth Muscles
EFS caused a frequency-dependent increase in the contraction of the colonic smooth muscles, that was attenuated by TTX, atropine, and darifenacin (*; p<0.05; n=6; Fig. 1A), suggesting that EFS causes contraction of the colonic smooth muscle via cholinergic nerve stimulation, partly through M3-R activation. The data further showed that SScIgGs (in contrast with pooledhIgG) caused significant suppression of the EFS-induced cholinergic contraction (*; p<0.05; n=6). The latter effect was reversed by 10mg/ml pooledhIgG to the level that was not significantly different from the control values (p>0.05; n=6).
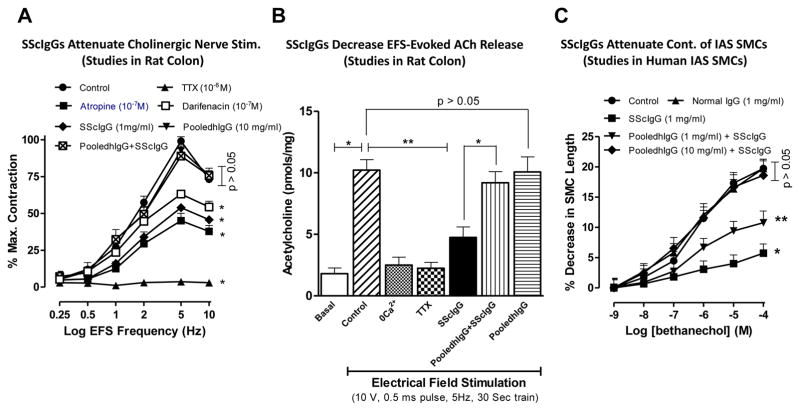
Fig. 1.
A. TTX, atropine, and darifenacin significantly attenuate the EFS-induced contraction of the rat colonic smooth muscle caused by EFS (*; p<0.05; n=6). SScIgG also causes significant (*; p<0.05; n=6) attenuation of these EFS responses that is reversed by pooledhIgG to values not significantly different from those obtained in the control and the pooledhIgG alone experiments (p>0.05). B. EFS causes a significant (*; p<0.05; n=4) increase in ACh release that is significantly (**; p<0.05; n=4) mitigated not only by 0Ca2+ and TTX but also by SScIgG. The effects of SScIgG are reversed by pooledhIgG pre-treatment. C. SScIgG significantly (*; p<0.05; n=6) inhibits M3-R activation-induced human SMC contraction by BeCh. These inhibitor effects of SScIgG are restored by pooledhIgG in a concentration-dependent manner (significantly by 1mg/ml pooledhIgG; *), and almost completely by 10 mg/ml pooledhIgG to the levels that are not significantly different from control, NIG and pooledhIgG experiments (p>0.05).
Effect of SScIgGs on EFS-Evoked ACh Release in Intact Rat Colonic Smooth Muscles
Direct measurements of ACh from myenteric neurons revealed that EFS causes a significant increase in ACh release, which was significantly attenuated by 0Ca2+, TTX as well as by SScIgG (**; p<0.05; Fig. 1B; n=4). A complete obliteration of the EFS-evoked release of ACh by 0Ca2+ and TTX suggests definitive neurotransmitter release of ACh. The suppressant effects of SScIgGs on ACh release were reversed by pooledhIgG (*; p<0.05; n=4), whereas pooledhIgG by itself had no significant effects on basal release of ACh (p>0.05).
Effect of SScIgG vs. NIgG and PooledhIgG on BeCh-Induced Contraction of Human IAS SMCs
SScIgGs significantly attenuated the contraction of BeCh-induced M3-R activation in human IAS SMCs (*; p<0.05; n=6), and that inhibition was significantly reversed by pretreatment of the SMC with 1mg/ml pooledhIgG (**; p<0.05). The maximal effective concentration of BeCh (10−4 M), in control experiments induced a SMC contraction of 19.7 ± 1.5%. SScIgG (1mg/ml) significantly attenuated this response to 5.7 ± 0.1% (~70% inhibition; *; p<0.05; n=6), and pooledhIgG (10 mg/ml) reversed this to 18.6 ± 2.4%, a value not significantly different from controls (p<0.05; n=6; Fig. 1C). In contrast, the contractile effects of phenylephrine (alpha1-adrenoceptor or α1-AR activator) and K+ depolarization by KCl were not modified by SScIgG (data not shown). These data demonstrate the selectivity of the suppressant effects of SScIgG in the M3-R activation in the human IAS SMC.
M3-R Occupancy by SScIgGs vs. NIgGs and PooledhIgG in Human IAS SMC
Data showed that SScIgG binds to the SMCs with M3-R selective immunofluorescence intensity (IFI) saturating at 20 min (IFI of M3-R using M3-R antibody was considered as 100 %; Fig. 2A,B). Significant IFI with SScIg was observed at 5 and 10 min as 79.57 ± 5.43 and 89.47 ± 6.24%, respectively (*; p<0.05; n=6). Therefore, a 10 min time frame was selected for the subsequent experiments with SScIgG. In contrast with the SScIgG, NIgG and pooledhIgG (14 ± 1.5 and 16 ± 2.2%, respectively) had significantly less binding (*; p<0.05; Fig. 2C,D,E). Collectively, these data reveal that SScIgG has significantly higher binding to the IAS SMC membrane than NIgG and pooledhIgG. Immunofluorescence (IF) analysis also showed a significant (*; p<0.05; n=6) colocalization of the M3-R and SScIgG binding to the human SMC membranes, as determined by the secondary antibodies conjugated with TR and FITIC, respectively (*; p<0.05; n=6; Fig. 3A,B). This colocalization was not observed with either NIgG or pooledhIgG.
Functional Displacement of M3-Receptor (M3-R) Occupancy by SScIgG in Rat IAS Smooth Muscle
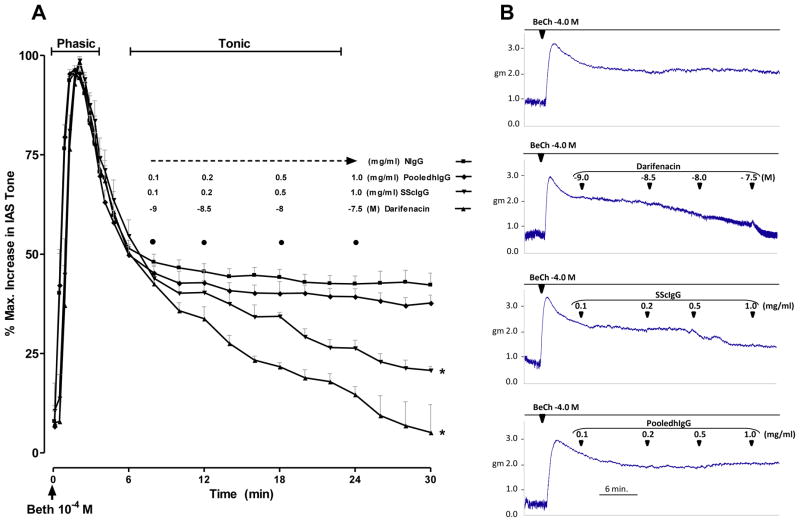
These studies were carried out in the tonic component of the rat IAS smooth muscle contraction induced by 10−4 M BeCh. As shown in Fig. 4A, 10−4 M BeCh caused an initial phasic contraction followed by the sustained or tonic component. The sustained component allowed sufficient time to examine different concentrations of NIgG, SScIgG or pooledhIgG and darifenacin. As shown in Fig. 4A, only SScIgG significantly attenuated the tonic component, because of the interference of M3-R occupancy by the SScIgGs as it resembled the effect of darifenacin. Typical tracings of the effect of the functional occupancy and reversal of the BeCh-induced M3-R activation by SScIgG and darifenacin are shown in Fig. 4B.
Fig. 4.
A. In control experiments M3-R activation by BeCh causes a two phase contraction of the IAS; phasic followed by the tonic. SScIgG (but not NIgG and pooledhIgG) in resemblance with darifenacin (M3-R selective inhibitor) causes significant and concentration-dependent decrease in the BeCh-induced sustained contraction (*; p<0.05; n=6). B. Actual tracings of the effect of darifenacin, SScIgG, NIgG, and pooledhIgG on the BeCh-induced sustained contraction of the IAS.
Assessment of SScIgG, NIgG and PooledhIgG Binding to M3-R using Western Blot Analysis in Human IAS SMCs
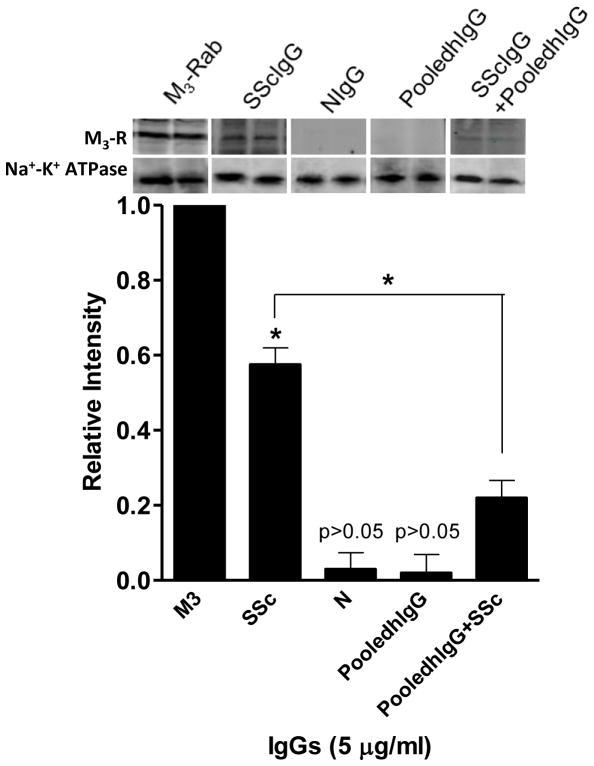
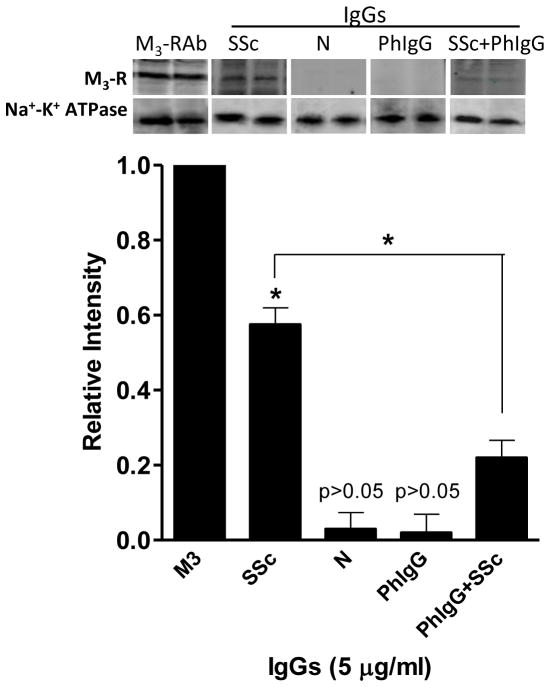
Western blot analysis was used to confirm the binding of SScIgG to M3-R in HISMF, and its reversal by pooledhIgG. When electrophoresed in parallel with the M3-R antibody, SScIgG (and not NIgG and pooledhIgG) revealed a specific protein band coinciding with the M3-R in HISMF (Fig. 5). The western blot analysis further showed that pretreatment of the SMC membranes with pooledhIgG added either 30 min before SScIgG, simultaneously or with the premixed solution of SSCIgG with pooledhIgG (incubated at 37°C for 30 min) significantly decreased binding of SScIgG with M3-R. A relative intensity graph was plotted by considering the M3-R band intensity as 1.0. The Relative intensity of the SScIgG band was significantly higher (0.57 ± 0.04; *; p<0.05) than with NIgG and pooledhIgG (0.03 ± 0.03 and 0.02 ± 0.04, respectively). SScIgG (5 μg/ml) combined with pooledhIgG displayed a significantly decreased binding to the M3-R (from 0.57 ± 0.04 to 0.22 ± 0.04; *; p<0.05, n=6). Thus collectively, these data show the presence of significant binding of SScIgG to the M3-R in the human SMC membranes that is inhibited by pooledhIgG pretreatment.
Fig. 5.
Western blot analysis shows significant (*; p<0.05, n=6) binding of SScIgG with M3-R (p<0.05) in contrast with the other IgGs, in human IAS SMC membrane fractions (HISMF). In addition, combination of pooledhIgG+SScIgG causes significant decrease in this binding (*; p <0.05; n=6).
Assessment of SScIgG, NIgG and PooledhIgG Binding to M3-R and Influence of PooledhIgG employing Human IAS SMC Membrane Lysate and M3-R loop-2 Peptide
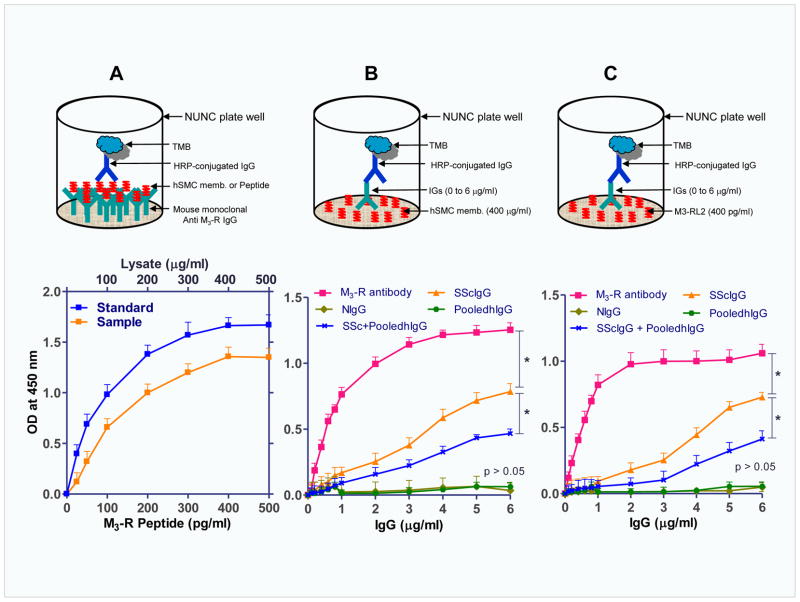
M3-R specific ELISA studies revealed a concentration-dependent increase in the binding with the standard M3-R peptide and the HISMF (Fig. 6A), with the maximal binding at 300 pg/ml and 300 μg/ml, respectively. Following this, we used multiwell plates (used for ELISA) preadsorbed with the maximal concentration (300 μg/ml) of the HISMF and performed ELISA using different concentrations of the IgGs for M3-R binding analysis (Fig. 6B). The data showed that SScIgG (and not NIgG and pooledhIgG) binds to HISMF significantly in a concentration-dependent manner and the maximal OD at 5μg/ml of the lysate was 0.71 ± 0.05 (*; p<0.05; n=6). Pretreatment of the SScIgG with pooledhIgG (SScIgG+pooledhIgG) caused a significant decrease in the binding of the SScIgG to the M3-R as reflected by a decrease in the OD (from 0.71 ± 0.05 to 0.43 ± 0.024; *; p<0.05; n=6; Fig. 6B). These data suggest that the membranes have M3-R domains that selectively bind with the SScIgGs.
Fig. 6.
A. ELISA binding studies for M3-R peptide and human IAS SMC membrane fraction (HISMF). A. OD concentration-curves using M3-R standards and HISMF. B. Data show that M3-R antibody and SScIgG bind with HISMF in a concentration-dependent manner (*; p<0.05; n=6), and pooledhIgG significantly decreases this binding. C. Similar data were obtained when M3-R peptide (M3-RL2) instead of HISMF is used.
To further confirm these observations, we used multiwell plates (used for ELISA) preadsorbed with the maximum concentration of M3-R second loop peptide (M3-RL2; 5μg/ml), followed by the different concentrations of the IgGs. The data show a concentration-dependent increase in M3-R binding with SScIgG (*; p<0.05; Fig. 6C) and not with NIgG or pooledhIgG (p>0.05). However, pretreatment with pooledhIgG (SScIgG+pooledhIgG), caused a significant decrease in the binding of the SScIgG to the M3-R (*; p<0.05). In contrast when M3-RL2 peptide was replaced with the M3-R third loop peptide (M3-RL3), there was no significant binding (data not shown). These data validate the results obtained with the intact cell membrane studies, and suggest that SScIgGs display significant and specific binding to the M3-R in the IAS SMC and further confirm a significant attenuation of this binding by pooledhIgG.
PooledhIgG Neutralizes the SScIGs in vitro: Studies using Human IAS SMC Membrane Extracts
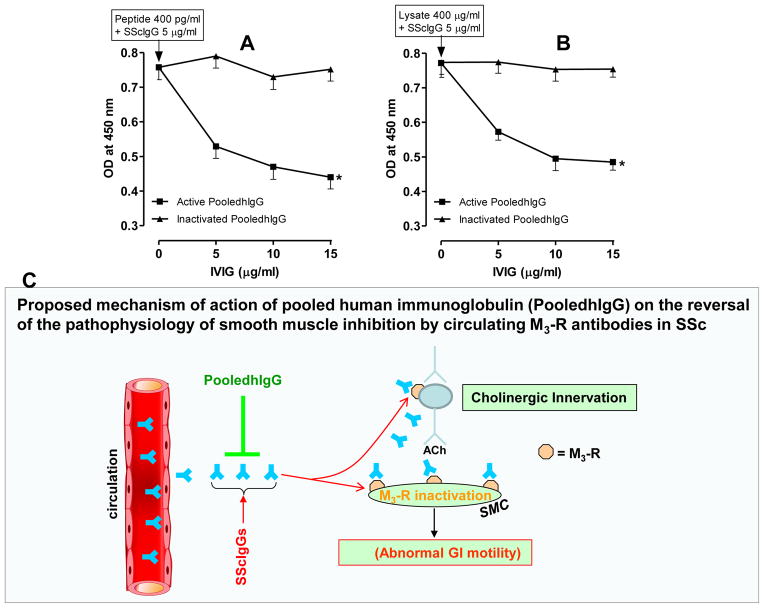
In these experiments, we determined the maximal OD following incubation of 5 μg/ml of SScIgGs from different patients in multiwell plates (used for ELISA) preadsorbed with 400 pg/ml of M3-RL2 (Fig. 7). Pretreatment with pooledhIgG caused a significant and concentration-dependent decrease in this maximal OD; 15 μg/ml of pooledhIgG decreased the OD from 0.77 ± 0.34 to 0.41 ± 0.05 (*; p<0.05; n=6; Fig. 7A). However, inactivated pooledhIgG failed to cause any decrease in the interaction between SScIgG and M3-RL2. Interestingly, similar data were obtained when HISMF (400 mg/ml; Fig. 7B) instead of M3-RL2 was used. Thus, these data show that neutralization of the SScIgG with pooledhIgG causes a decrease in the M3-R binding by the SScIgGs.
Fig. 7.
A. Neutralization studies via ELISA show that pooledhIgG (active and not inactivated pooledhIgG) causes significant and concentration-dependent decrease (*; p<0.05) in the binding of SScIgG at the M3-RL2. B. Similar data were obtained when M3-RL2 was replaced with the membrane lysates. In these experiments, maximal OD is first observed following the incubation of 400 μg of HISMF with 5 μg/ml of SScIgGs. C. A working model suggests that SScIgGs lead to gastrointestinal motility dysfunction by blocking M3-R at the cholinergic neurotransmission at the neural and the SMC levels. Data further suggest that pooledhIgG neutralizes the SSc autoantibodies in the circulation thus attenuating these effects of SScIgGs on M3-R inhibition. This neutralization may in part reverse the pathophysiology of SSC gastrointestinal smooth muscle dysfunction.
Discussion
These studies indicate several important points. Firstly, sera from patients with SSc contain antibodies of the IgG class that act both at the myenteric nerves and at the smooth muscle cell membrane receptors. Secondly, these antibodies reversibly block cholinergic nerve and muscle functions. Thirdly, the effects of these antibodies can be inhibited by pooledhIgG. Finally, the studies show that one of the prominent sites of action of these antibodies is at M3-R and that this interaction can be effectively reversed by pooledhIgG. The proposed model of the pathophysiological actions of SScIgGs and their reversal by pooledhIgG (via neutralization of SScIgGs) in the gastrointestinal tract are given in Fig. 7.
The exact pathogenesis of GI dysmotility in SSc remains elusive. SSc patients are known to harbor various anti-nuclear antibodies including anti-DNA-topoisomerase I (anti-topo I), anti-centromere (CENPs-A, -B, -C and -D), and anti-RNA-polymerase III antibodies.1 Although, the specific nature of antibodies present in the sera of GI symptomatic SSc patients antibodies that may recognize epitopes present in the GI tract is not known, earlier studies from mouse colon smooth muscle9 and rat IAS SMCs10 suggested these antibodies interfere with M3-R activation.
The present studies provide evidence in favor of cholinergic blockade by M3-R inactivation at the neural as well as at the muscular levels and show that SScIgGs cause significant impairment of cholinergic contraction of the colon. Importantly, these SScIgG-impaired responses are significantly and selectively reversed by pooledhIgG. An effect of SScIgGs on the cholinergic myenteric neurons was demonstrated by the significant decrease in the actual release of ACh in these preparations, which is reversible by pooledhIgG.
Evidence for the partial effects of the SScIgGs at the myogenic level comes firstly from studies showing that the SScIgGs (and not NIgGs and pooledhIgG) attenuate the IAS SMC contractility caused by BeCh-induced M3-R activation, while SScIgGs do not modify SMC contraction by other agonists. Secondly, IF studies in the SMCs show that SScIgGs colocalize with M3-R. Thirdly, the binding of M3-R by the SScIgGs is further evident by the WB studies where SMC membranes incubated with the SScIgGs reveal a M3-R-specific band. Fourthly, the binding of SScIgGs and interference with M3-R by SScIgGs is further evident from the functional displacement of M3-R occupancy. Herein, SScIgGs (and not NIgGs) inhibit BeCh-induced sustained contraction of intact IAS smooth muscle of rat in a concentration-dependent manner. Finally, HISMF causes concentration-dependent increase in the M3-R binding when multiwell plates (used for ELISA) are preadsorbed with M3-R-specific antibody. In addition, SScIgGs cause selective and concentration-dependent increase in the binding when multiwell plates pre-adsorbed with either HISMF or M3-RL2 are used. The studies reveal that M3-RL2 is critical for the binding of the SScIgGs to the M3-R as the substitution of M3-RL2 with M3-RL3 produces no significant binding. Studies by Scarselli et al.27 corroborate these findings for the critical role of M3-RL2 in the M3-R activation.
Thus, the results described here provide novel information demonstrating that the M3-R inactivation caused by SScIgGs is reversible by pooledhIgG, via interference with the M3-R occupancy. Pretreatment of SScIgGs with pooledhIgG significantly decreases the binding of SScIgGs to purified M3-R peptide. This suggests the presence of anti-idiotypic antibodies in pooledhIgG that block the pathophysiological activity of anti-M3-R antibodies in SScIgGs. Functional displacement of M3-R occupancy by pooledhIgG is indicated by its ability to reverse the SScIgGs-induced inhibition of BeCh-induced sustained contraction of intact IAS smooth muscle. ELISA studies confirm that pooledhIgG reverses the SScIgGs-induced decrease in the binding with purified M3-R. Such data are similar whether multiwell plates are preadsorbed with the HISMF or the M3-RL2 peptide.
Several possible mechanisms may explain the reversal of SScIgGs-induced M3-R inactivation by pooledhIgG including neutralization,28,29 desensitization, internalization or degradation of the IgGs.30–32
Present studies provide further evidence that pooledhIgG reverses M3-R inactivation whether the cells are incubated with pooledhIgG followed by SScIgG or with the premixed SScIgGs and pooledhIgG. This is shown by IF, WB, ELISA, neutralization, and functional displacement of M3-R occupancy studies. Therefore we suggest that the effects of pooledhIgG occur primarily by binding with the SScIgG thus neutralizing the effects of these antibodies in the circulation. This hypothesis is further supported by the observations that pooledhIgG forms an immune complex with IgGs interacting with Fcγ receptors on dendritic cells thus reducing the severity of autoimmune diseases12,33 From the present data, we postulate that neutralization of these pathogenic antibodies by pooledhIgG may represent a novel approach for the treatment of SSc GI motility disorders by blocking cholinergic dysfunction induced by SScIgG. However, the exact mechanism, and the role of other pathways including possible removal of the SSc pathogenic antibodies in the therapeutic efficiency and efficacy of pooledhIgG remain to be determined. Previously reported studies on the effect of pooledhIgG in SSc patients11 have primarily focused on cutaneous and articular manifestations. Therefore, the present studies that identify a novel therapeutic and potentially mechanistic role of pooledhIgG in the GI dysfunction of SSc may represent a major step forward for the treatment of SSc.
In summary, the present studies provide evidence for the proposed mechanism for the M3-R-mediated cholinergic dysfunction in SSc-related GI manifestations and its restoration by pooledhIgG. Collectively, we posit that neutralization of these pathogenic antibodies by pooledhIgG may in part relieve SSc-associated GI motility disorders by blocking cholinergic inhibition. Based on the long-term use of pooledhIgG in other autoimmune diseases, we suggest that pooledhIgG may provide a novel and safe therapy for the SSc-related GI motility disorders.
Acknowledgments
Grant Support: The work was supported by Grant Number RO1DK035385 from the National Institutes of Diabetes and Digestive and Kidney Diseases, and an institutional grant from Thomas Jefferson University.
Abbreviations used in this Paper
- BeCh
bethanechol (M3-R agonist)
- EFS
electrical field stimulation
- ELISA
enzyme-linked immunosorbent assay
- HISMF
human IAS SMC membrane fractions’ lysate
- IAS
internal anal sphincter
- IF
immunofluorescence
- IFI
IF intensity
- IgG
immunoglobulin
- pooledhIgG
pooled human immunoglobulin
- NIgG
IgGs from normal subjects
- M3-R
muscarinic type3 receptor
- M3-RL2
second extracellular loop peptide of M3-R
- M3-RL3
third loop peptide of M3-R
- SMC
smooth muscle cells
- SSc
systemic sclerosis or scleroderma
- SScIgG
IgGs from scleroderma patients
Footnotes
Disclosures: The authors have nothing to disclose
Involvement of Authors with the Manuscript:
Jagmohan Singh in data acquisition, analysis and interpretation; and statistical analysis; Sidney Cohen in the enrolment and follow up of scleroderma patients; Vaibhav Mehendiratta in the human blood samples’ collection and ms preparation; Fabian Mendoza in the isolation and purification of SScIgGs; Sergio A. Jimenez in isolation and purification of scleroderma IgGs and data interpretation; Anthony DiMarino in the follow up of patients; and Satish Rattan in funds procurements, concept and design, interpretation of data, and study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Mechanisms of disease: Scleroderma. New Eng J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S. The gastrointestinal manifestations of scleroderma: pathogenesis and management. Gastroenterology. 1980;79:155–166. [PubMed] [Google Scholar]
- 3.DiMarino AJ, Carlson G, Myers A, et al. Duodenal myoelectric activity in scleroderma. Abnormal responses to mechanical and hormonal stimuli. New Eng J Med. 1973;289:1220–1223. doi: 10.1056/NEJM197312062892304. [DOI] [PubMed] [Google Scholar]
- 4.Alper D, Ram E, Stein GY, et al. Resting anal pressure following hemorrhoidectomy and lateral sphincterotomy. Dis Colon Rectum. 2005;48:2080–2084. doi: 10.1007/s10350-005-0165-y. [DOI] [PubMed] [Google Scholar]
- 5.Trezza M, Krogh K, Egekvist H, et al. Bowel problems in patients with systemic sclerosis. Scand J Gastroenterol. 1999;34:409–413. doi: 10.1080/003655299750026434. [DOI] [PubMed] [Google Scholar]
- 6.Koh CE, Young CJ, Wright CM, et al. The internal anal sphincter in systemic sclerosis. Dis Colon Rectum. 2009;52:315–318. doi: 10.1007/DCR.0b013e31819a5d59. [DOI] [PubMed] [Google Scholar]
- 7.Engel AF, Kamm MA, Talbot IC. Progressive systemic sclerosis of the internal anal sphincter leading to passive faecal incontinence. Gut. 1994;35:857–859. doi: 10.1136/gut.35.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacman S, Sterin-Borda L, Camusso JJ, et al. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjögren’s syndrome. Clin Exp Immunol. 1996;104:454–459. doi: 10.1046/j.1365-2249.1996.42748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldblatt F, Gordon TP, Waterman SA. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology. 2002;123:1144–1150. doi: 10.1053/gast.2002.36057. [DOI] [PubMed] [Google Scholar]
- 10.Singh J, Mehendiratta V, Del Galdo F, et al. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1206–G1213. doi: 10.1152/ajpgi.00286.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baleva M, Nikolov K. The role of intravenous immunoglobulin preparations in the treatment of systemic sclerosis. Int J Rheumatol. 2011;2011:1–4. doi: 10.1155/2011/829751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballow M. The IgG molecule as a biological immune response modifier: mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J Allergy Clin Immunol. 2011;127:315–323. doi: 10.1016/j.jaci.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Bayry J, Thirion M, Misra N, et al. Mechanisms of action of intravenous immunoglobulin in autoimmune and inflammatory diseases. Neurol Sci. 2003;4:S217–S221. doi: 10.1007/s10072-003-0081-7. [DOI] [PubMed] [Google Scholar]
- 14.McGaha T, Saito S, Phelps RG, et al. Lack of skin fibrosis in tight skin (TSK) mice with targeted mutation in the interleukin-4Rα and transforming factor-β genes. J Invest Dermatol. 2001;116:136–143. doi: 10.1046/j.1523-1747.2001.00217.x. [DOI] [PubMed] [Google Scholar]
- 15.Blank M, Levy Y, Amital H, et al. The role of intravenous immunoglobulin therapy in mediating skin fibrosis in tight skin mice. Arthritis Rheum. 2002;46:1689–1690. doi: 10.1002/art.10363. [DOI] [PubMed] [Google Scholar]
- 16.Sempowski GD, Beckman MP, Derdak SD, et al. Subsets of murine lung fibroblasts express membrane-bound and soluble IL- 4 receptors: role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994;152:3606–3614. [PubMed] [Google Scholar]
- 17.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 2009;15:202–205. [PubMed] [Google Scholar]
- 18.Ouyang A, Locke GR., III Overview of neurogastroenterology-gastrointestinal motility and functional GI disorders: classification, prevalence, and epidemiology. Gastroenterol Clin North Am. 2007;36:485–498. doi: 10.1016/j.gtc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Grodzki AC, Berenstein E. Antibodypurification:affinitychromatography-proteinA andproteinGSepharose. Methods Mol Biol. 2010;588:33–41. doi: 10.1007/978-1-59745-324-0_5. [DOI] [PubMed] [Google Scholar]
- 20.Singh J, Maxwell PJ, IV, Rattan S. Immunocytochemical evidence for PDBu-induced activation of RhoA/ROCK in human internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G317–G325. doi: 10.1152/ajpgi.00084.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J Microsc. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1747–G1756. doi: 10.1152/ajpgi.00438.2006. [DOI] [PubMed] [Google Scholar]
- 23.Koo NY, Li J, Hwang SM, et al. Functional epitope of muscarinic type 3 receptor which interacts with autoantibodies from Sjogren’s syndrome patients. Rheumatology. 2008;47:828–833. doi: 10.1093/rheumatology/ken064. [DOI] [PubMed] [Google Scholar]
- 24.Chakder S, McHugh KM, Rattan S. Inhibitory neurotransmission in lethal spotted mutant mice: A model for Hirschsprung’s disease. Gastroenterology. 1997;112:1575–1585. doi: 10.1016/s0016-5085(97)70039-8. [DOI] [PubMed] [Google Scholar]
- 25.Rattan S, De Godoy MAF, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology. 2006;131:108–116. doi: 10.1053/j.gastro.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Biancani P, Walsh JH, Behar J. Vasoactive intestinal polypeptide: a neurotransmitter for relaxation of the rabbit internal anal sphincter. Gastroenterology. 1985;89:867–874. doi: 10.1016/0016-5085(85)90585-2. [DOI] [PubMed] [Google Scholar]
- 27.Scarselli M, Li B, Kim SK, et al. Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. J Biol Chem. 2007;282:7385–7396. doi: 10.1074/jbc.M610394200. [DOI] [PubMed] [Google Scholar]
- 28.Cavill D, Waterman SA, Gordon TP. Antiidiotypic antibodies neutralize autoantibodies that inhibit cholinergic neurotransmission. Arthritis Rheum. 2003;48:4597–3602. doi: 10.1002/art.11343. [DOI] [PubMed] [Google Scholar]
- 29.Smith AJ, Jackson MW, Wang F, et al. Neutralization of muscarinic receptor autoantibodies by intravenous immunoglobulin in Sjögren Syndrome. Hum Immunol. 2005;66:411–416. doi: 10.1016/j.humimm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Dawson L, Tobin A, Smith P, et al. Antimuscarinic antibodies in Sjögren’s syndrome: where are we, and where are we going? Arthritis Rheum. 2005;52:2984–2995. doi: 10.1002/art.21347. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt M, Frings M, Mono ML, et al. Protein-coupled receptor-induced sensitization of phospholipase C stimulation by receptor tyrosine kinases. J Biochem. 2000;275:32603–32610. doi: 10.1074/jbc.M004784200. [DOI] [PubMed] [Google Scholar]
- 32.Van Koppen CJ. Multiple pathways for the dynamin-regulated internalization of muscarinic acetylcholine receptors. Biochem Soc Trans. 2001;29:505–508. doi: 10.1042/bst0290505. [DOI] [PubMed] [Google Scholar]
- 33.Siragam V, Crow AR, Brinc D, et al. Intravenous immunoglobulin ameliorates ITP via activating Fcγ receptors on dendritic cells. Nat Med. 2006;12:688–692. doi: 10.1038/nm1416. [DOI] [PubMed] [Google Scholar]