Abstract
Advances in the understanding of the endogenous cannabinoid system have led to several therapeutic indications for new classes of compounds that enhance cannabinergic responses. Endocannabinoid levels are elevated during pathogenic conditions, and inhibitors of endocannabinoid inactivation promote such on-demand responses. The endocannabinoids anandamide and 2-arachidonoyl glycerol have been implicated in protective signaling against excitotoxic episodes, including seizures. To better understand modulatory pathways that can exploit such responses, we used the new generation compound AM6701 that blocks both the anandamide-deactivating enzyme fatty acid amide hydrolase (FAAH) and the 2-arachidonoyl glycerol-deactivating enzyme monoacylglycerol lipase (MAGL) with equal potency. Also studied was the structural isomer AM6702 which is 44-fold more potent for inhibiting FAAH versus MAGL. When applied before and during kainic acid (KA) exposure to cultured hippocampal slices, AM6701 protected against the resulting excitotoxic events of calpain-mediated cytoskeletal damage, loss of presynaptic and postsynaptic proteins, and pyknotic changes in neurons. The equipotent inhibitor was more effective than its close relative AM6702 at protecting against the neurodegenerative cascade assessed in the slice model. In vivo, AM6701 was also the more effective compound for reducing the severity of KA-induced seizures and protecting against behavioral deficits linked to seizure damage. Corresponding with the behavioral improvements, cytoskeletal and synaptic protection was elicited by AM6701, as found in the KA-treated hippocampal slice model. It is proposed that the influence of AM6701 on FAAH and MAGL exerts a synergistic action on the endocannabinoid system, thereby promoting the protective nature of cannabinergic signaling to offset excitotoxic brain injury.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-011-0100-y) contains supplementary material, which is available to authorized users.
Keywords: Amphiphysin I, AM6701, Endocannabinoid modulation, Excitotoxicity, GluA1, Neuroprotection
Introduction
Seizures impose a heavy burden of illness and decreased quality of life on affected patients, thus it is important to identify targeted therapies to reduce seizure pathology. Neuronal degeneration and death arising from excessive excitatory activity reflect the vulnerability of the brain to excitotoxic insults. In the excitotoxicity field, much attention has been applied to potential strategies to preserve learning and memory and other brain functions, including the neuroprotective abilities of specific signaling lipids that make up the family of endocannabinoids (for more detail see Hwang J, et al. [1] Zanettini C, et al. [2], Pacher and Hasko [3], and Janero DR, et al. [4]). Multiple studies have linked the principal endocannabinoids in the central nervous system, anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), with protection against numerous diseases, including Alzheimer's, Parkinson's, and Huntington's disease, epileptic seizures, and stroke/ischemic brain damage [5–14]. In the nervous system, endocannabinoids AEA and 2-AG are synthesized and released on demand from membrane-bound phospholipids, and act at presynaptic Gi-coupled CB1 and CB2 receptors [15–18]. CB1 receptors are localized predominantly to nerve terminals and function to suppress neurotransmitter release and excitotoxic signaling [13, 14, 19–22]. Consequently, promoting cannabinergic signaling by inhibiting endocannabinoid inactivation is a neuroprotective strategy that is being intensively pursued.
Endogenous levels of AEA and 2-AG can be elevated through inactivation of their metabolizing enzymes: fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively [23–26]. In the brain, FAAH has been localized postsynaptically within the somata of hippocampal pyramidal cells, granule cells of the dentate gyrus, Purkinje cells of the cerebellum, and neocortical neuronal cell bodies [23, 27, 28]. MAGL, however, is localized to nerve terminals in many brain regions [29, 30]. Interestingly, studies with selective FAAH inhibitors and FAAH-knockout mice have described CB1 receptor-mediated signaling without the adverse psychotropic effects associated with direct and chronic activation of the CB1 receptor [4, 14]. Therefore, utilizing inhibitors of the endocannabinoid-degrading enzymes to indirectly enhance site-specific protective responses may be an ideal strategy to promote endogenous repair pathways. Studies have shown that systemically administered kainic acid (KA) treatment elevates AEA levels in the brain [14, 31–33]. Related to this response to injury, FAAH inhibition enhanced the salutary effects of cannabinergic signaling in the rat hippocampus, mediated predominantly via CB1 receptors [1, 14, 34, 35]. It has been suggested that CB1 receptor activation reduces excessive glutamatergic signaling and excitotoxic progression thereby protecting hippocampal cells from cytoskeletal and synaptic damage [13, 14, 32, 33, 36]. These findings suggest a key role of endocannabinoids in attenuating excitotoxicity in the brain.
Selective and potent inhibition of FAAH and MAGL is an important consideration for targeted neuroprotection against excitotoxic insults. Carbamate inhibitors in particular, have been documented to be highly selective for FAAH and MAGL by irreversibly binding to active-site serine residues on those enzymes [4, 37]. In the present study, we used new-generation carbamate inhibitors of the endocannabinoid-degrading enzymes and demonstrate in vitro and in vivo that a dual FAAH/MAGL inhibitor (i.e., AM6701) is more neuroprotective than the inhibitor AM6702, which is more selective for FAAH than MAGL. Our results indicate that after KA-induced excitotoxicity, modulation of the endocannabinoid system with AM6701 protects against neuronal compromise in hippocampal slices, and reduces seizure activity, cytoskeletal damage, synaptic decline, and behavioral deficits in rats. Our experiments provide further evidence for the therapeutic potential of endocannabinoid responses against excitotoxic brain injury.
Materials and Methods
Animals
Male Sprague–Dawley rats for in vivo work and a litter of rat pups (postnatal days 11–12) for hippocampal slice culture experiments were purchased from Charles River Laboratories (Wilmington, MA). The animals were housed in a temperature- and humidity-controlled room with a cycle of 12 h light to 12 h dark, with access to food and water ad libitum. All animal experiments were carried out in compliance with procedures approved by the Institutional Animal Care and Use Committees at the University of Connecticut and the University of North Carolina at Pembroke.
Chemicals and Antibodies
The carbamoyl tetrazole compounds 5-([1,1'-biphenyl]-4-ylmethyl)-N,N-dimethyl-2H-tetrazole-2-carboxamide (AM6701) and 5-([1,1'-biphenyl]-4-ylmethyl)-N,N-dimethyl-1H-tetrazole-1-carboxamide (AM6702) were synthesized at Northeastern University (Boston, MA). KA was purchased from Tocris (Ellisville, MO). Affinity-purified antibodies to the amino-terminal of calpain-mediated spectrin breakdown product (BDPN), and to the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) subunit glutamate receptor 1 ([GluA1], also known as GluR1) were used as previously described [13, 33, 38, 39]. A phosphorylation state-specific antibody against the AMPA receptor GluA1 subunit at serine-845 (pGluA1-Ser-845) was obtained from Millipore (Bedford, MA). Anti-amphiphysin I serum was purchased from Synaptic Systems (Goettingen, Germany), anti-synapsin II serum from Calbiochem (San Diego, CA), and anti-actin from Sigma (St. Louis, MO). All other reagents were obtained from Sigma.
FAAH and MAGL Fluorometric Assays
Human-recombinant FAAH and MAGL were expressed in Escherichia coli as previously described [40, 41]. A high-throughput fluorometric screening assay for recombinant FAAH inhibition using the fluorescent substrate arachidonoyl 7-amino-4-methylcoumarin amide was performed as previously reported [40]. The assays for assessing MAGL activity followed similar procedures using the fluorescent substrate arachidonoyl, 7-hydroxy-6-methoxy-4-methylcoumarin ester [42]. Concentration-response curves were obtained by measuring the percent fluorescence after 3 h incubation against increasing concentrations of AM6701 or AM6702 using excitation and emission wavelengths of 360 nm and 460 nm, respectively. IC50 values were determined and reported as means ± SD.
Organotypic Hippocampal Slice Cultures
Hippocampus sections were rapidly removed from Sprague–Dawley rat pups 11 to 12 days postnatal and transversely sectioned into 400-μm thick slices, as previously described [13, 33, 43]. Nine hippocampal slices were then carefully transferred to each of 6 Millicell-CM membrane inserts (Millipore Corporation, Bedford, MA) containing 50% basal medium Eagle, 25% Earle’s balanced salts, 25% horse serum, and defined supplements [39]. Slices were maintained at 37°C in 5% CO2-enriched atmosphere for a 15 to 20 day maturation period before experiments were initiated.
Model of Excitotoxicity in Vitro
Cultured hippocampal slices were pretreated with either 1 μM AM6701 or 1 μM AM6702 in 0.1% dimethyl sulfoxide in serum-free media at 37°C for 60 minutes. The media were then removed and 70 μM KA was applied to the slices for 2 h in the presence of vehicle AM6701 or AM6702. The slices were washed once in serum-free media and incubated with vehicle AM6701 or AM6702 for 24 h before the tissues were harvested.
Immunoblot Analysis
Groups of 6 to 8 cultured hippocampal slices were gently removed from the membrane inserts with a soft brush in ice-cold buffer containing 0.32 M sucrose, 5 mM HEPES (pH 7.5), 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, and the protease inhibitors antipain, 4-(2-aminoethyl) benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin (2 μg/ml each). Samples were then centrifuged for 5 minutes at 5,000 rpm at 4°C. The tissue pellets were sonicated in cold lysis buffer of 6 mM Tris (pH 8.1), 0.2 mM ethylenediaminetetraacetic acid, 0.2 mM ethylene glycol tetraacetic acid, and the listed protease inhibitors. Protein concentration was determined with the bicinchoninic acid assay (Pierce, Rockford, IL) with bovine serum albumin as a standard. Equal amounts of total protein were boiled in sodium dodecyl sulfate for 5 minutes, separated by 4 to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The blots were probed with antibodies against GluA1 (2.5 μg/ml), BDPN (10 μg/ml), the presynaptic proteins amphiphysin I (serum diluted 1:500) and synapsin II (serum diluted 1:1000), and the protein load control actin (0.5 μg/ml). Membranes were then incubated with alkaline phosphatase-conjugated anti-IgG secondary antibodies, and antigen-antibody complexes were visualized with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate colorimetric substrates. The densities of protein bands were quantified with BIOQUANT software (R&M Biometrics, Nashville, TN), and expressed as mean values ± SEM.
Histology
Cultured hippocampal slices were fixed in formalin overnight at 4°C, rinsed in phosphate buffered saline (PBS), and adhered onto glass slides (Fisher Scientific, Pittsburgh, PA). Tissues were then stained with Nissl, dehydrated in a clearing agent, and coverslipped with Permount. Hippocampal slices from each treatment group were examined for morphological integrity using a Nikon AZ100 Microscope equipped with AZ-Plan Fluor 5X objective and Q-Imaging QI Click camera (Nikon Instruments Inc., Melville, NY). Pyknotic nuclei were defined as those nuclei exhibiting marked condensation and reduction in size. The average pyknotic nuclei count across multiple CA1 view fields of 8.5 × 104 μm2 per slice was obtained for the different treatment groups.
Model of Excitotoxicity in Vivo
Postnatal day-22 Sprague–Dawley male rats were injected intraperitoneally with PBS (no insult control rats) or with 9.8 mg/kg KA in PBS. Immediately following the KA injection, animals were injected with either vehicle (60% dimethyl sulfoxide in PBS; 1.7 ml/kg) or vehicle containing AM6701 or AM6702 (5 mg/kg). The animals were returned to their cages and seizure activities were recorded for 4 h by observers blinded to the treatment groups. After behavioral assessment, the rats were anesthetized with isoflurane (Abbott Laboratories, Chicago, IL), and the brains were removed and placed in ice cold dissection buffer with protease inhibitors. Each hippocampus was then dissected out and homogenized in ice cold lysis buffer containing a protease inhibitor cocktail. Total protein concentration was determined, followed by immunoblotting with antibodies against GluA1, BDPN, amphiphysin I, synapsin II, and actin.
Seizure Scoring
Seizure behavior was rated at 15 minute intervals for a maximum of 4 h according to a well-established rating scale [14, 33]. Briefly, this scale comprised the following 7 stages: 1) stage 0, normal behavior; 2) stage 1, freezing, staring, or mouth/facial movements; 3) stage 2, rigid posture, head nodding, or isolated twitches; 4) stage 3, tail extension, unilateral–bilateral forelimb clonus, or repetitive scratching; 5) stage 4, rearing and falling with 1 or both forepaws extended; 6) stage 5, clonic seizures with loss of posture, jumping, and falling; 7) stage 6, severe tonic seizures.
Behavioral Testing
Balance and coordination were examined using behavioral paradigms 24 to 48 h after injection procedures, as previously described [13, 14]. In the rotarod test, rats were first trained on a rotating cylinder (15 rpm) for 10 consecutive trials with a maximum of 10 seconds per trial. The animals were allowed to rest, and then re-tested on the rotarod for 3 consecutive trials up to a maximum of 2 minutes. The time the animal stayed on the rod was recorded and averaged across the 3 trials. The second behavioral paradigm was the balance beam in which rats were placed in the middle of a suspended wooden bar (1.2-cm diameter) positioned 15 cm above a padded surface. During this task, animals were assessed for the time before falling from the bar, up to a maximum of 30 second per trial for each of 3 consecutive trials. The rats were also assessed for distance of exploration in an open field (106 cm × 54 cm) that was divided into 8 segments. The animals were placed in the center of the open field and monitored for the total number of segments crossed into during a 5-minute session.
Chemical Parameters and Statistical Analyses
Physicochemical parameters of compounds were determined with ChemBioDraw Ultra 12.0 (Cambridge, MA). Immunoblot, histological, and behavioral data were analyzed with unpaired t tests or one-way analysis of variance (ANOVA) followed by Tukey’s post hoc comparisons. Titration curves were determined with GraphPad Prism, version 3.00 for Windows (GraphPad Software, San Diego, CA).
Results
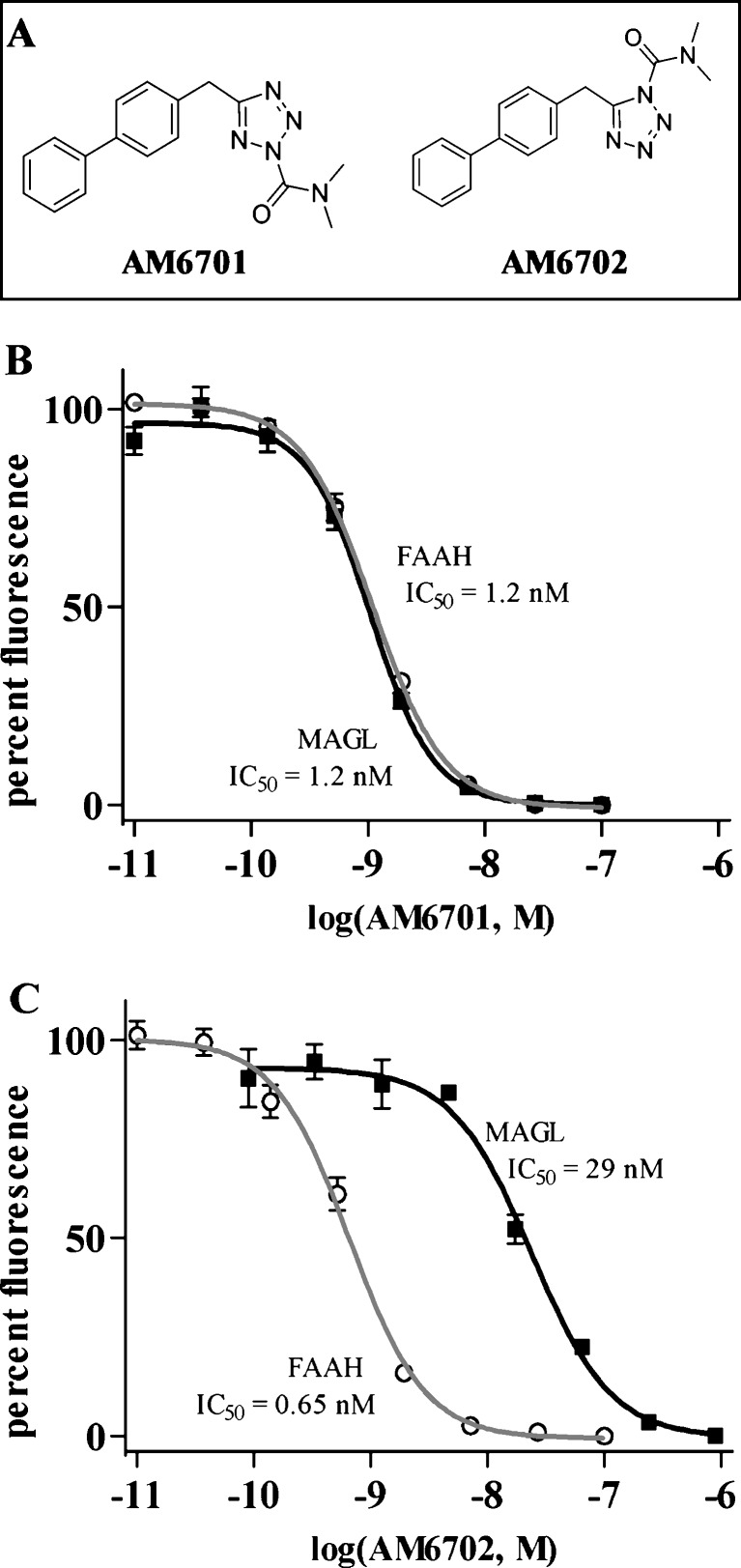
To test whether inhibition of the endocannabinoid-degrading enzymes promotes the protective nature of the endocannabinoid system, we used the new generation carbamate inhibitors AM6701 and AM6702 that are isomeric with very similar physicochemical parameters (see Fig. 1A). From calculated parameters, the 2 compounds have the expected identical molecular mass (307.35), topological polar surface area (60.63), and lipophilicity (CLogP = 4.03) (Table 1). In separate fluorogenic enzyme assays, AM6701 caused a concentration-dependent and equipotent inhibition of FAAH (IC50 = 1.2 ± 0.13 nM) and MAGL (IC50 = 1.2 ± 0.35 nM) (Fig. 1B). AM6702, however, more potently inhibited FAAH over MAGL with a 44-fold selectivity for FAAH (IC50 = 0.65 ± 0.007 nM) compared to MAGL (IC50 = 29 ± 5.9 nM) (Fig. 1C). The fact that AM6701 is equipotent for both FAAH and MAGL, whereas AM6702 is more selective for FAAH, allows us to test whether such equipotency influences neuroprotection.
Fig. 1.
Assessment of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) inhibition. The structures of the carbamoyl tetrazole compounds used in this study are shown (a): 5-([1,1'-biphenyl]-4-ylmethyl)-N,N-dimethyl-2H-tetrazole-2-carboxamide (AM6701) and 5-([1,1'-biphenyl]-4-ylmethyl)-N,N-dimethyl-1H-tetrazole-1-carboxamide (AM6702). Recombinant FAAH and MAGL were treated with increasing concentrations of AM6701 (b) and AM6702 (c) in triplicate in independent experiments, and the inhibitory activities of the compounds were determined. Enzyme activity data were normalized to 100% fluorescence generation in the absence of drugs and shown as means ± standard deviation. In the FAAH and MAGL assays, AM6701 caused a concentration-dependent inhibition of both enzymes and demonstrated equal potency for FAAH (IC50 = 1.2 ± 0.13 nM) and MAGL (IC50 = 1.2 ± 0.35 nM). AM6702 caused a concentration-dependent inhibition of both activities, but more potently inhibited FAAH in comparison to MAGL. AM6702 demonstrated a 44-fold selectivity for FAAH (IC50 = 0.65 ± 0.007 nM) compared to MAGL (IC50 = 29 ± 5.9 nM)
Table 1.
Physico-chemical parameters of the carbamate inhibitor compounds AM6701 and AM6702*
| Compound | Molecular Mass (Mr) | Topo Polar Surface Area (tPSA) | Lipophilicity (CLogP) |
|---|---|---|---|
| AM6701 | 307.35 | 60.63 | 4.03 |
| AM6702 | 307.35 | 60.63 | 4.03 |
*The parameters were determined with ChemBioDraw Ultra 12.0
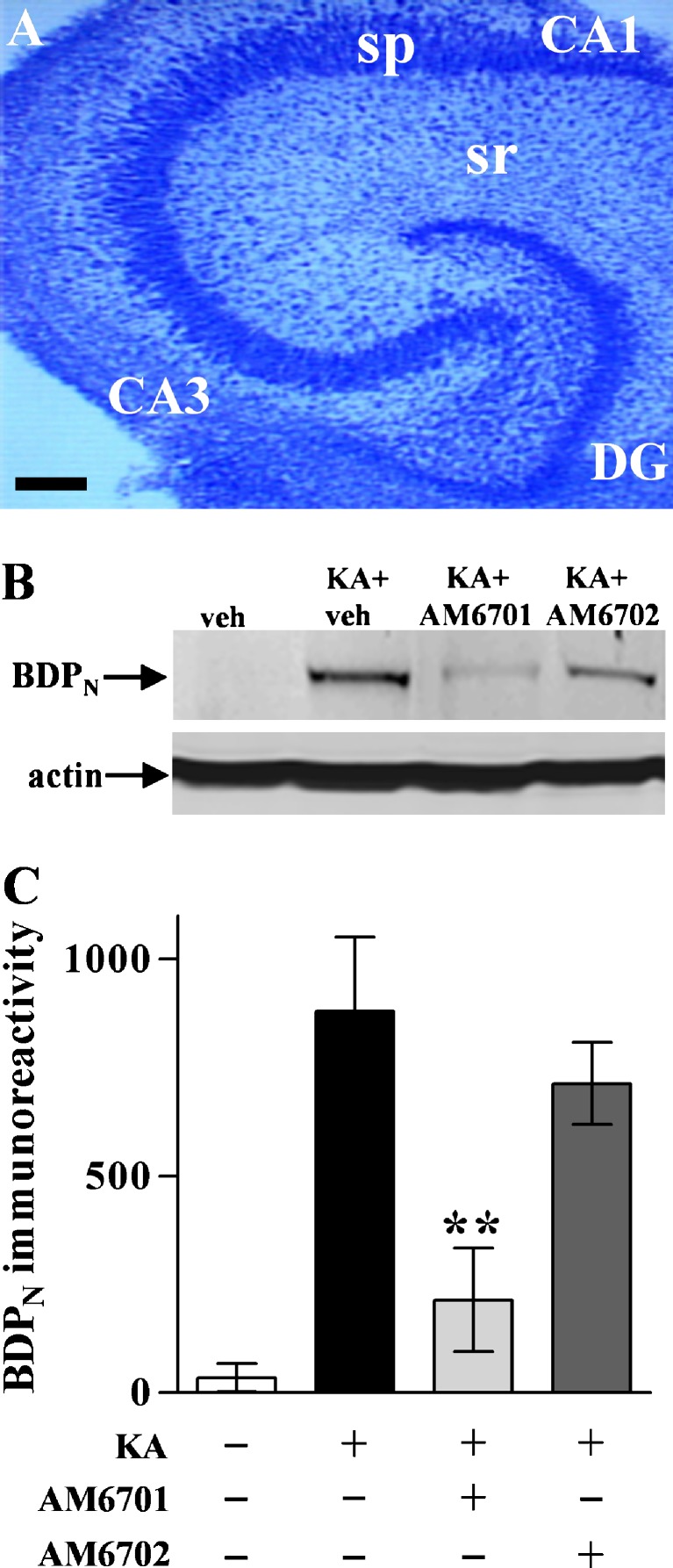
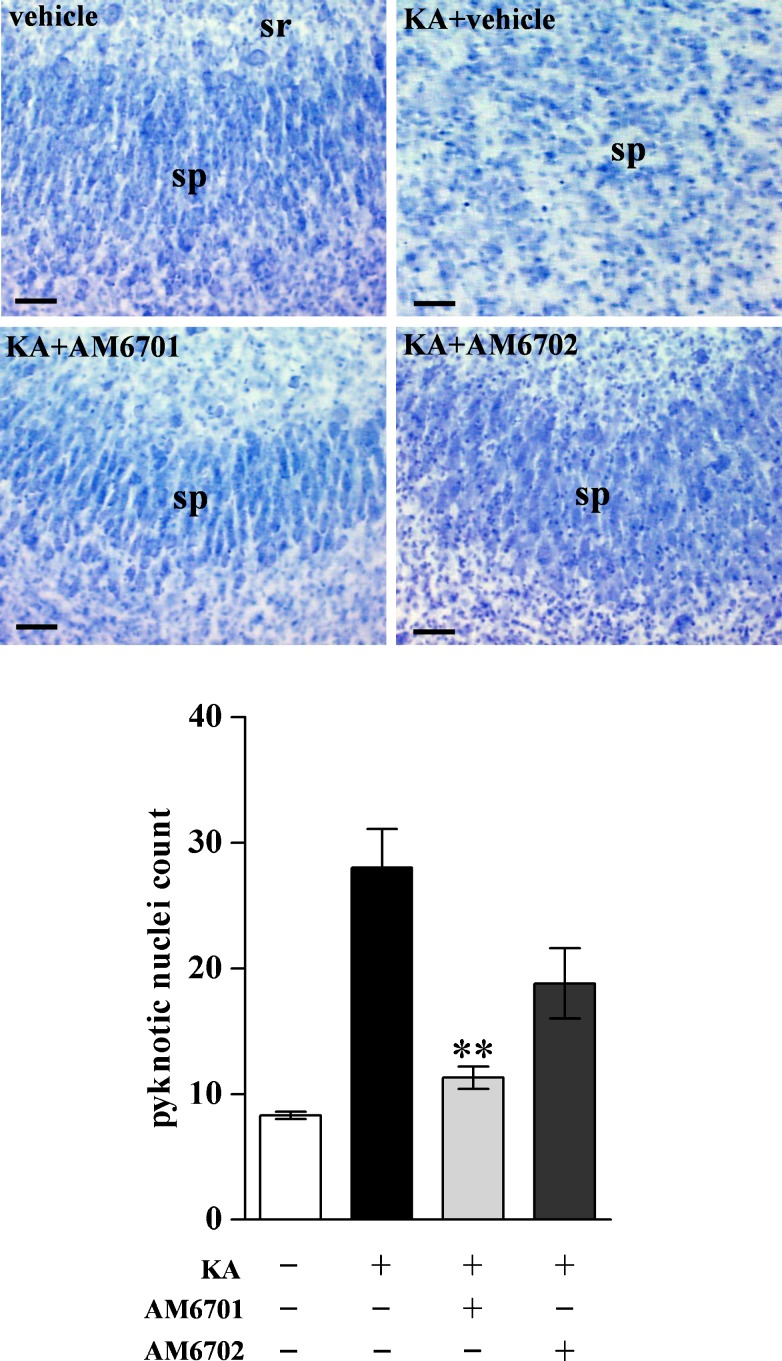
We examined the neuroprotective abilities of AM6701 and AM6702 against molecular and cellular events in hippocampal slice cultures treated with KA, an initiator of seizure activity. The organotypic model exhibits native cytoarchitecture and subfields of pyramidal neurons and granule cells of the stratum granulosum, all well maintained after weeks in culture as found in Nissl-stained control slices (Fig. 2A). KA-induced excitotoxic degeneration was monitored in the hippocampal slices by immunoblotting for BDPN, an indicator of cytoskeletal breakdown, and early cellular degeneration [38]. The KA insult caused a dramatic increase in the calpain-mediated spectrin fragment BDPN, whereas the housekeeping protein actin was unchanged (Fig. 2B). Treatment with 1 μM AM6701 before, during, and after the KA insult did not allow KA to produce the characteristic increase in BDPN as compared to the no-insult control slices (t test, p = 0.33; not significant [NS]), whereas the typical KA effect on cytoskeletal breakdown was evident during treatment with 1 μM AM6702 (p < 0.01). In Fig. 2C, the different protective influences of the carbamate inhibitors are evident across the 4 treatment groups (ANOVA, p = 0.020), indicating better neuroprotection by the equipotent FAAH/MAGL inhibitor AM6701. As expected from the excitotoxic production of the BDPN marker of cellular compromise, KA also caused an increase in pyknotic nuclei (p = 0.01) and a dramatic loss of neuronal density evident in the CA1 pyramidal layer (Fig. 3). Corresponding with its cytoskeletal protection, AM6701 reduced the KA-induced pyknotic nuclei count (post hoc test, p < 0.01) and produced evident maintenance of neuronal density and morphology. Although the dual inhibitor’s neuroprotective potential is most evident, it should be noted that the reduced pyknosis by AM6702 approached statistical significance (t test: p = 0.0607; not significant).
Fig. 2.
Testing AM6701 and AM6702 for cytoskeletal protection in rat hippocampal slice cultures after KA-induced excitotoxicity. A low-power photomicrograph of the hippocampal slice preparation, after 3 weeks in culture, exhibits preservation of the native cellular organization and morphology (a). DG = dentate gyrus; sp = stratum pyramidale; sr = stratum radiatum. Scale bar: 400 μm. Slice cultures were pre-treated with vehicle (veh), 1 μM AM6701, or 1 μM AM6702 for 1 h before a 2-h kainic acid (KA) exposure that was conducted with continuation of the pre-treatment condition. Following a brief washout step, the slices were again incubated with vehicle (n = 6 groups of 6–8 slices each), AM6701, or AM6702 (n = 6), in parallel with untreated control slices (n = 9). The slices were harvested 24-h post-insult and assessed by immunoblotting for calpain-mediated spectrin breakdown product BDPN and the protein load control actin (b). Integrated optical densities for BDPN across treatment groups are shown as means ± SEM. (c). Unpaired t test compared to KA only group: **p = 0.019
Fig. 3.
Effects of AM6701 and AM6702 on cellular integrity in the excitotoxic slice model. From the Fig. 2 treatment groups, slice cultures were treated with vehicle AM6701 or AM6702 for 1 h, and subsequently during the 2-h kainic acid (KA) exposure, as per the described protocol. The slices were fixed 24-h post-insult, then sectioned and stained with cresyl violet. Photomicrographs of field CA1 are shown and pyknotic nuclei are especially evident in the KA + vehicle group. DG = dentate gyrus; sp = stratum pyramidale; sr = stratum radiatum. Scale bar: 45 μm. Pyknotic nuclei were counted across multiple CA1 view fields of 8.5 × 104 μm2 per cultured slice, and mean counts ± SEM are shown in the lower graph (analysis of variance, p = 0.001). Post hoc test compared to KA only group: **p < 0.01
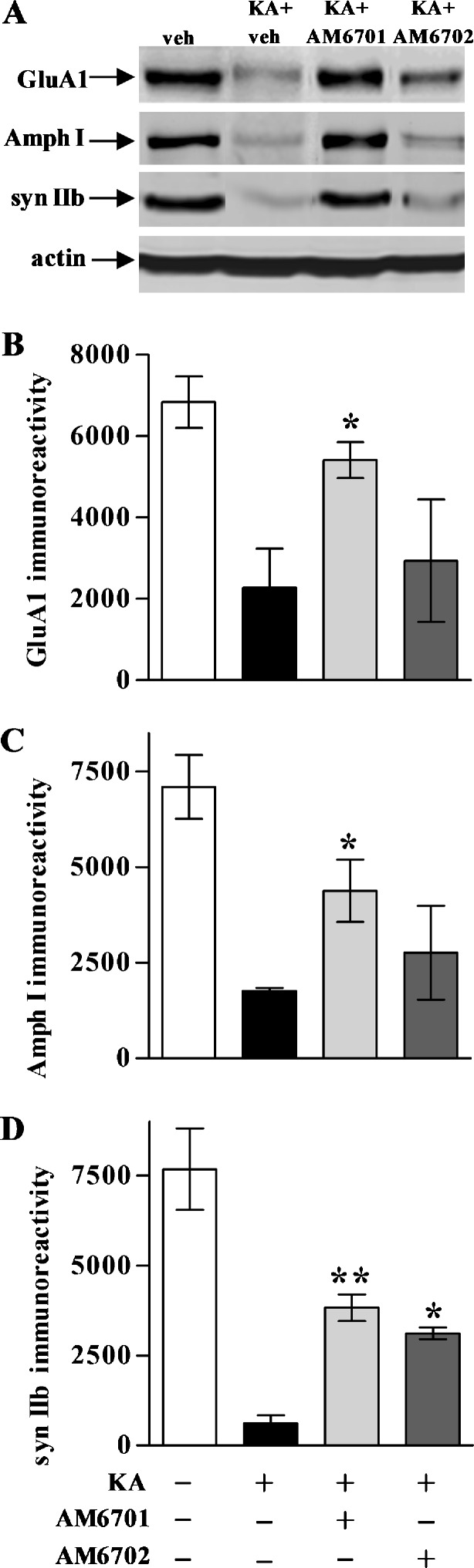
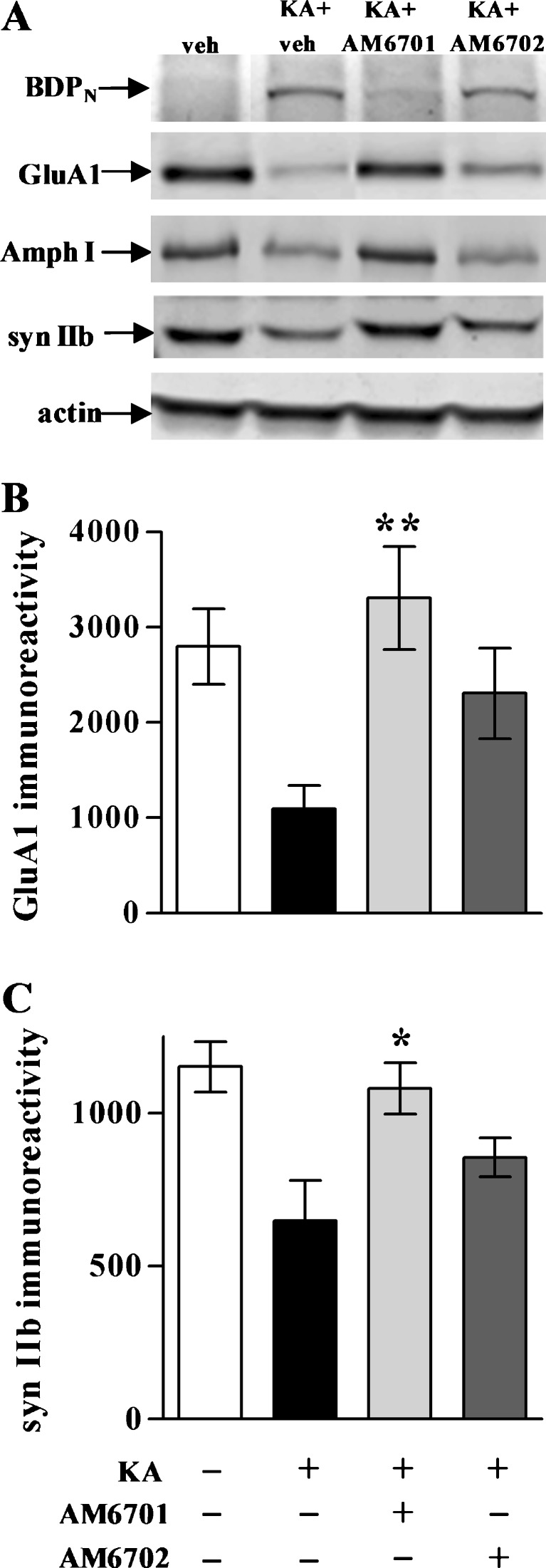
In addition to cytoskeletal breakdown, KA-induced excitotoxicity included the loss of synaptic markers. Accordingly, measures of distinct synaptic components were used to further monitor neuroprotection by the carbamate inhibitors in the hippocampal slice model (Fig. 4A). The KA insult caused significant reductions in the postsynaptic AMPA receptor subunit GluA1 (t test, p = 0.012; Fig. 4B) and in the synaptic vesicle-associated proteins amphiphysin I (p < 0.01; Fig. 4C) and synapsin IIb (p < 0.01; Fig. 4D). The choice of antibodies for immunoblots was indicated by previous studies that linked cytoskeletal and synaptic compromise to events of excitotoxic brain damage [13, 33, 38, 44]. The better protective influence of AM6701 vs. AM6702 is evident across the 3 synaptic proteins (see post hoc tests of Fig. 4B-D). In addition, note that AM6701 treatment did not allow KA-elicited declines in GluA1 or amphiphysin I as compared to no-insult slices (p = 0.16-0.34; NS), whereas the KA-induced deficits in the 2 synaptic markers were produced during AM6702 treatment (p = 0.015 and p = 0.021, respectively). Regarding synapsin IIb, both carbamate inhibitors were protective, with the FAAH/MAGL inhibitor AM6701 remaining more influential than AM6702 (Fig. 4D).
Fig. 4.
Synaptic protection in hippocampal slice cultures after kainic acid (KA)-induced excitotoxicity. Using the slice samples from Fig. 2, the slice cultures were treated with vehicle (veh) AM6701 or AM6702 for 1 h, and subsequently during the KA insult. Slices were harvested 24-h post-insult in groups of 6 to 8 each, and assessed by immunoblotting for the postsynaptic marker GluA1, the presynaptic markers amphiphysin I (Amph I) and synapsin IIb (syn IIb), and actin (a). Integrated optical densities for GluA1 (b) (analysis of variance, p = 0.019), Amph I (c) (p = 0.015), and syn IIb (d) (p < 0.01) are shown as means ± SEM across the treatment groups. Post hoc tests compared to KA only group: *p < 0.05; **p < 0.01
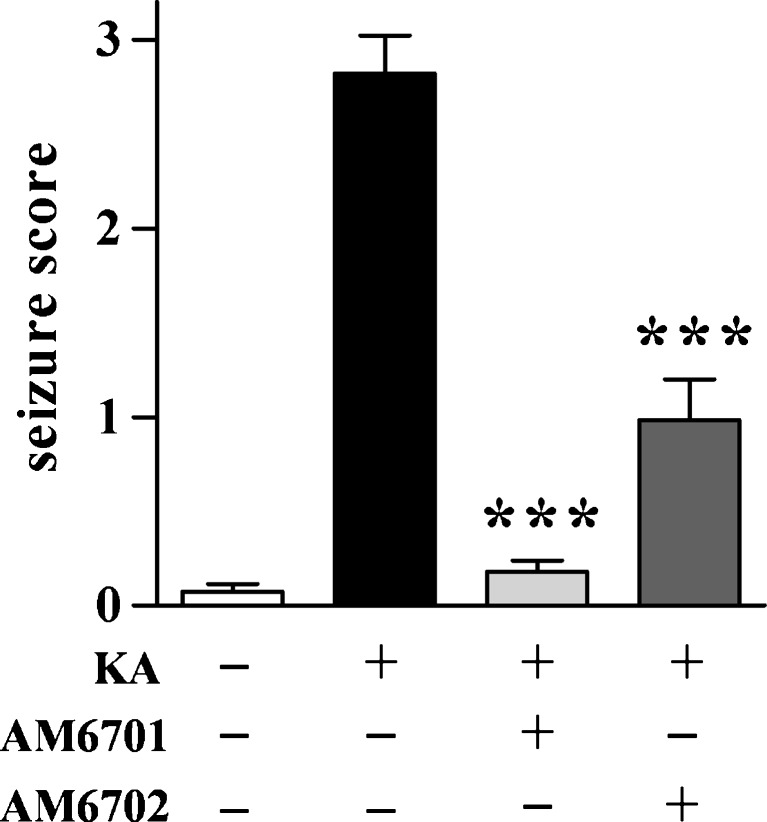
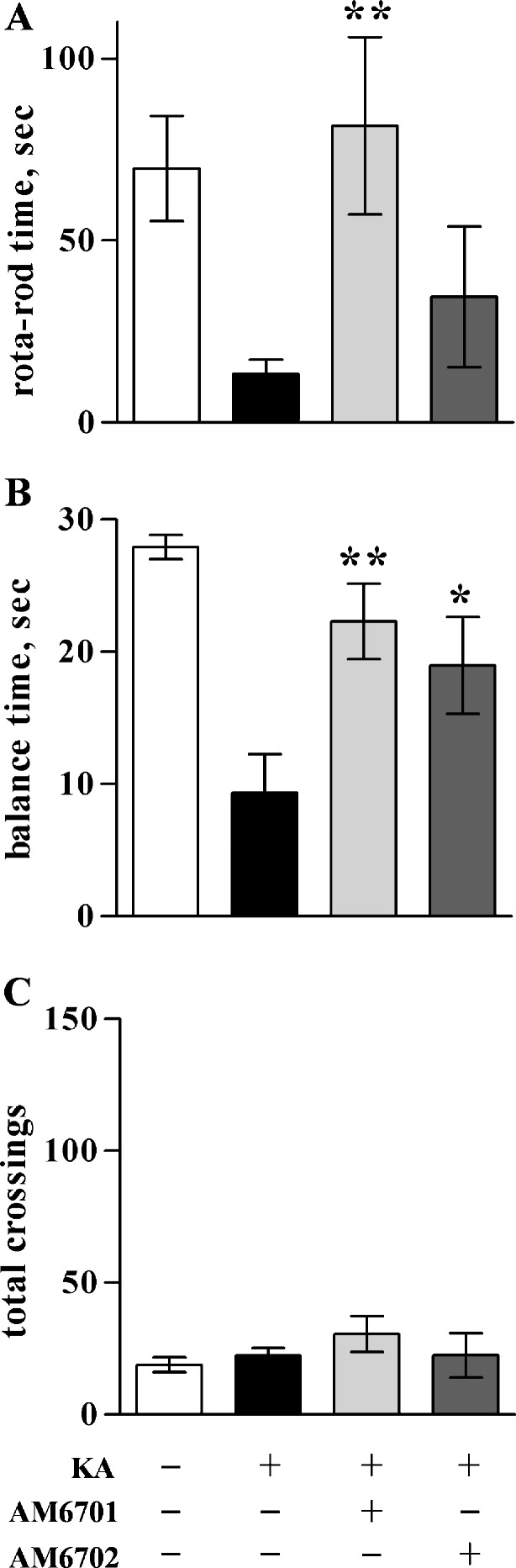
The cultured hippocampal slice data suggest that the dual inhibitor AM6701 is a more effective neuroprotectant than the selective FAAH inhibitor AM6702. To test whether such is the case in an in vivo model of excitotoxicity, KA was injected intraperitoneally into rats to initiate excitotoxic seizures. Immediately following the KA administration, animals were injected with either 5 mg/kg AM6701 or AM6702 and seizure activity was scored for 4 h (Fig. 5). AM6701 markedly reduced seizure severity by 93% compared to animals that only received vehicle after the excitotoxin injection (ANOVA; p < 0.0001). Although AM6702 also reduced seizure scores, it produced less seizure protection than AM6701. Behavioral testing was also used to compare functional protection elicited by the 2 carbamate inhibitors. Rats in the different treatment groups were assessed for balance and coordination in which KA caused an 80% reduction in performance on the rotarod paradigm (Fig. 6A; p = 0.0016) and reduced balance beam performance by 67% (Fig. 6B; p < 0.0001). KA rats that received AM6701 exhibited improved performance on the 2 paradigms, from 70% to complete recovery of function. AM6702, on the other hand, produced no evident recovery of function on the rotarod test (Fig. 6A) and a smaller 52% improvement on the balance beam (Fig. 6B). In line with the previously described results, the equipotent FAAH/MAGL inhibitor AM6701 produced better behavioral improvement than AM6702. Note that the KA-induced changes in balance and coordination were not due to gross impairment in motor ability because there was no change across treatment groups in explorative distance when placed in a novel open field (Fig. 6C). Also, the carbamate inhibitors did not express psychoactive issues, because no indication of catalepsy was seen in the 3 behavioral tests with animals that received the compounds.
Fig. 5.
Reduction of kainic acid (KA)-induced seizure severity by AM6701 and AM6702. Seizures were initiated in rats with an intraperitoneal injection of 9.8 mg/kg KA. Immediately following the KA administration, animals were injected with vehicle (n = 16) or 5 mg/kg AM6701 or AM6702 (n = 6–8). No-insult control rats received 2 vehicle injections (n = 13). Seizures scores were tabulated by blinded raters for a 4-h period following the injections, and the mean scores ± SEM are shown. Analysis of variance, p < 0.0001; post hoc test compared to KA only group: ***p < 0.001
Fig. 6.
Improved balance and coordination in the KA rat model. Seizures were initiated by KA injections (9.8 mg/kg), followed by immediate injections of vehicle (n = 10–16) or 5 mg/kg AM6701 or AM6702 (n = 6–8). No-insult control rats received 2 vehicle injections (n = 12–13). At 24-h post-injection, the animals were evaluated with a rotarod paradigm to assess their coordination, as determined by the mean time ± SEM maintaining coordinated movement on a rod rotating at 15 rpm (a). At 48-h post-injection, the different treatment groups were assessed for their balance time (mean ± SEM) when placed on a narrow, suspended bar (b). The rats were also monitored for locomotor activity in a novel open field, determining mean number ± SEM of area segments crossed into during the exploring period (c). Analysis of variance, p < 0.01 (a), p < 0.0001 (b); post hoc tests compared to KA only group: *p < 0.05; **p < 0.01
After the behavioral testing, hippocampi were removed from the rats and tissue homogenates assessed by immunoblot for cytoskeletal and synaptic markers. As shown in Fig. 7A, the KA-exposed animals exhibited a pronounced increase in calpain-mediated spectrin breakdown (BDPN) and a loss of synaptic proteins, including GluA1 (p = 0.001), synapsin IIb (p = 0.013), and amphiphysin I. Previous studies found associations between cytoskeletal breakdown, synaptic decline, and behavioral deficits, and enhancement of endocannabinoid responses protected against such pathogenic manifestations (13, 14, 32, 33). Amelioration of cytoskeletal damage (demonstrated by a decrease in KA-induced BDPN immunoreactivity) was observed in AM6701-treated rats, but not in those treated with AM6702 (Fig. 7A). As in the slice model, in addition to protective effects on cytoskeletal integrity, AM6701 preserved the levels of GluA1 (Fig. 7B), synapsin IIb (Fig. 7C), and amphiphysin I (Fig. 7A) in the excitotoxic animal model. This pattern of cytoskeletal and synaptic protection in the hippocampus was also observed in cortical samples from the AM6701 rats (data not shown). Note that AM6702 did not provide protection for the hippocampal synaptic markers as indicated by post hoc tests. However, as evident in Fig. 7A, marginal protection of GluA1 was found in the AM6702-treated animals (t test, compared to KA only rats: p < 0.05), and the synapsin IIb protection approached significance (p = 0.081; NS).
Fig. 7.
Synaptic and cytoskeletal protection in the kainic acid (KA) rat model. No-insult control rats from Figs. 5 and 6, along with the KA groups treated with or without carbamate inhibitor, were subsequently assessed by immunoblot for spectrin breakdown product (BDPN), GluA1, amphiphysin I (Amph I), synapsin IIb (syn IIb), and actin in dissected hippocampal tissue (a). Integrated optical densities for GluA1 (b) (analysis of variance, p < 0.01; n = 7–9 per group) and Amph I (C) (p < 0.01) are shown as means ± SEM, indicating that AM6701 is more protective than AM6702 against excitotoxin-induced synaptic marker decline. Post hoc tests compared to KA only group: *p < 0.05, **p < 0.01
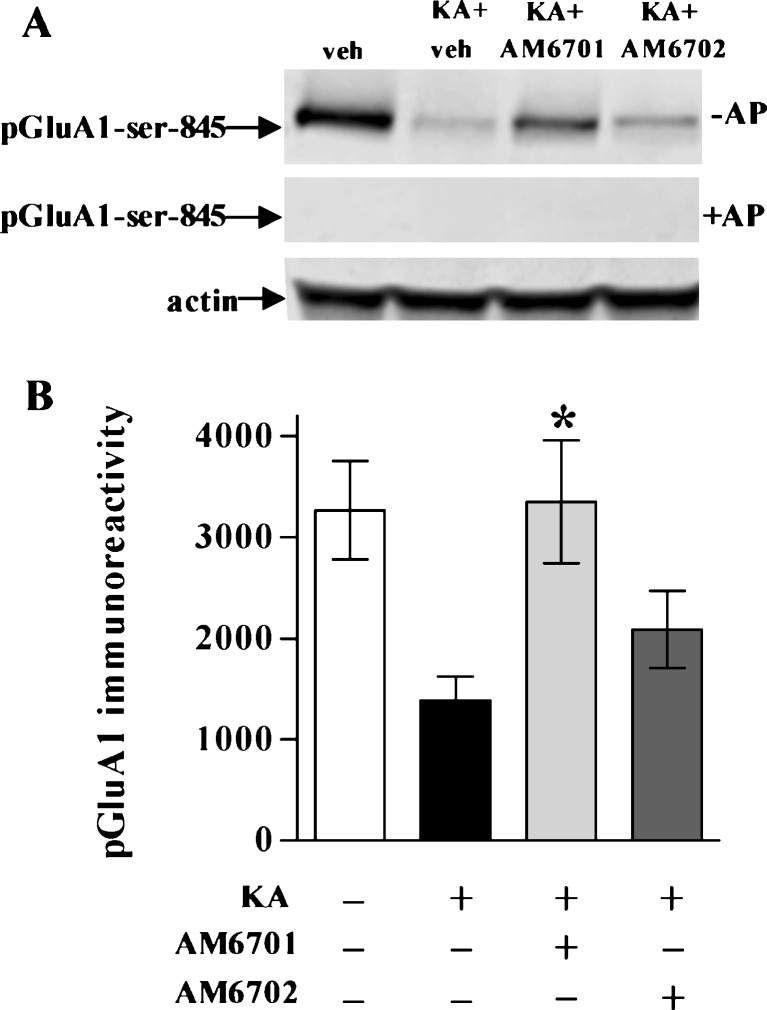
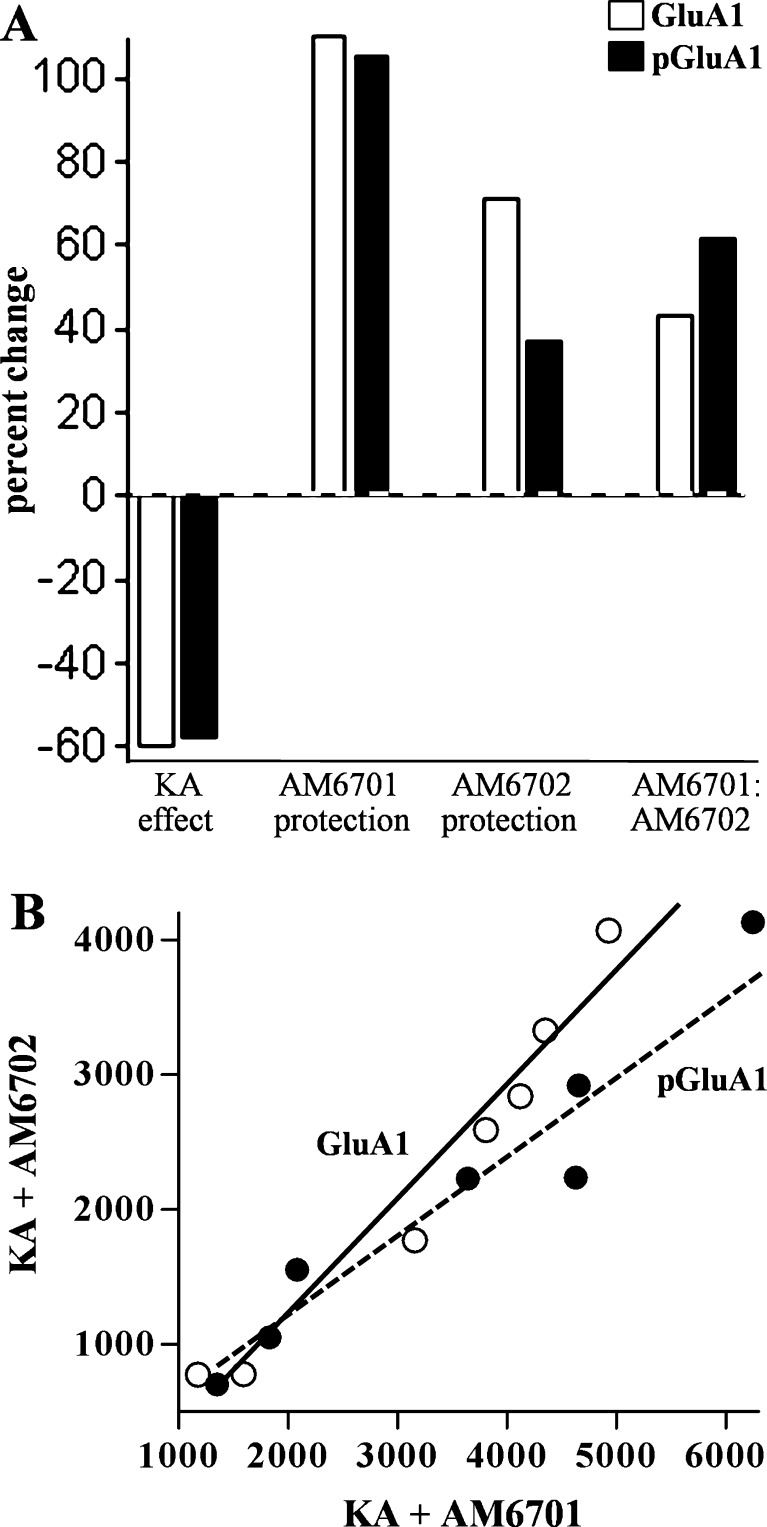
Similar to the KA-elicited 60.6% decline in the AMPA receptor subunit GluA1, KA also caused a 58.0% decrease in the phosphorylation form of the subunit, pGluA1-Ser-845 (Fig. 8; p = 0.001). Phosphorylation-specific antibody recognition was confirmed by pre-treating nitrocellulose-bound immunoblot samples for 2 h with alkaline phosphatase (Fig. 8A). AM6701 preserved the phosphorylation state of GluA1 more effectively than AM6702 (Fig. 8B). As with the comparable percent changes in GluA1 and the pGluA1 epitopes mediated by KA, AM6701 produced similar percent protection of the 2 species (Fig. 9A). AM6702, on the other hand, increased pGluA1-Ser-845 levels by only half the influence it had on GluA1, a small effect that approached significance (p = 0.066; NS). As a result, the AM6701 effect as compared to the immunoreactivity levels of the AM6702 group was larger for pGluA1 versus GluA1. This difference was also exhibited by the 44% slope difference among correlation plots for the 2 species (Fig. 9B).
Fig. 8.
Assessment of pGluA1 in the kainic acid (KA) rat model. No-insult control rats from the Fig. 6 experiment, along with the KA groups treated with or without AM6701 or AM6702, were assessed for GluA1 phosphorylated at serine-845 (pGluA1-Ser-845) and actin in hippocampal homogenates (a). A nitrocellulose strip containing identical immunoblot samples was pre-treated with alkaline phosphatase (AP) at 37°C before incubating with the antibody against pGluA1-Ser-845, indicating phosphorylation-specific staining. Integrated optical densities for pGluA1-Ser-845 are shown as means ± SEM (b), indicating that AM6701 is more influential than AM6702 (analysis of variance, p < 0.01; n = 7–9). Post hoc tests compared to KA only group: *p < 0.05
Fig. 9.
Comparison of effects on GluA1 versus pGluA1-Ser-845. Percent change in the 2 species is shown for the kainic acid (KA) insult, AM6701-mediated excitotoxic protection, the change due to AM6702, and the AM6701 effect as a percent change in the immunoreactivity levels of the AM6702 group (a). KA plus AM6701 rats were plotted against the KA plus AM6702 group in rank order of immunoblot levels for GluA1 (open circles) and pGluA1-Ser-845 (closed circles) in hippocampal homogenates (b). Linear regression was applied to the 2 species (r = 0.975 and r = 0.955, respectively; p < 0.001), and the slopes were found to be different (p = 0.02)
Discussion
An increasing body of evidence suggests that endocannabinoids are significant contributors to the repair process in response to excitotoxic events. The present study indicates that protection against seizure damage is best produced by inhibiting the endocannabinoid-inactivating enzymes FAAH and MAGL in combination. Protection by AM6701, an inhibitor equally potent for FAAH and MAGL, was evident with respect to cytoskeletal damage and synaptic decline in both a brain slice model and in vivo, and seizure scores and behavioral deficits were reduced in the KA rat model. AM6701 afforded greater protection against the excitotoxic events than AM6702, an inhibitor more potent toward FAAH than MAGL. Blocking endocannabinoid deactivation mechanisms represents an exploitable avenue for reducing excitotoxic damage through dual targeting of pharmacotherapeutic pathways [13, 45–48]. Blocking the inactivation of endocannabinoid responses is 1 such strategy to promote protective signaling after pathological injury and ameliorate cellular disturbances associated with excitotoxicity.
CB1 receptor activation by endocannabinoids is important for cell viability, and disruption of such signals has been found to prevent neuronal maintenance and to increase excitotoxic vulnerability [45]. A propensity for the receptor activation may be part of repair responses as previous studies have demonstrated elevated endocannabinoid levels in the brain of animals injected with an excitotoxin [14, 31–33]. To augment the lifetime of the on-demand cannabinergic response to brain injury, several studies have used FAAH inhibitors to reduce endocannabinoid catabolism [14, 33, 47]. Dual modulation of the endocannabinoid system produced marked excitotoxic protection when inhibitors of FAAH and AEA transport were used together [13]. Here, blocking endocannabinoid inactivation used a single compound (AM6701), which exhibits equipotency against FAAH and MAGL. Thus, AM6701 alone offers a dual approach for modulating 2 types of endocannabinoids, which are synthesized and released on demand. AM6701 was first tested for protection in convenient hippocampal slice cultures, an organotypic model that possess the cellular architecture and neuronal connectivity, as found in the intact brain [43, 49, 50]. The excitotoxic hippocampal slice model is valuable for evaluating neuropathological events, as it reproduces many of the pathogenic changes found in vivo [13, 51–53]. In the slices, KA induced a pathogenic cascade leading to calpain-mediated spectrin proteolysis, loss of synaptic proteins, and pyknotic changes among pyramidal neurons. AM6701 promoted cell survival, as well as synaptic maintenance and cytoskeletal protection, more effectively than AM6702. Regarding presynaptic composition, KA abrogated levels of synapsin II and amphiphysin I, a protein involved in the endocytosis of synaptic vesicles [54–57]. Excitotoxic stimulation of glutamate receptors leads to calcium-activated calpain cleavage of amphiphysin I and the inhibition of synaptic vesicle endocytosis [54], thus consistent with the calpain-mediated cytoskeletal damage and synaptic decline in the present results. As with most pathogenic parameters assessed in KA-treated hippocampal slice cultures, AM6701, but not AM6702, attenuated the induced loss of amphiphysin I. The better neuroprotectant quality assessed for AM6701 also translated to better seizure protection in vivo, thus indicating the valuable usefulness of the slice model.
The molecular and cellular indicators of excitotoxicity in the slice model were also observed in the KA rat model. KA is well known to evoke seizure activity leading to neurodegeneration, particularly hippocampal pathology [14, 33, 58]. Treatments with AM6701 or AM6702 were associated with decreased seizure scores in KA rats, with AM6701 producing a greater suppression of seizures and excitotoxic progression compared to AM6702. In the AM6701-treated rats exhibiting reduced seizure severity, attenuation of behavioral impairment was determined to be significant, and the functional protection was clearly more effective than the results produced by AM6702 administration. AM6701 protected against the induced deficits in motor memory, as well as in balance and coordination that were assayed by multiple trial rotarod and suspended rod tasks. These data indicate that the equipotent FAAH/MAGL inhibitor, when administered systemically, was the better compound at preventing KA-induced functional deficits.
The systemic injections of AM6701 also prevented synaptic decline and cytoskeletal damage in the hippocampus of the KA-insulted rats, in part by reducing the extent of excitotoxic calpain action on synaptic components, as well as spectrin molecules perhaps located within or near synaptic structures. Protection against the induced synaptic deterioration entailed the restoration and/or preservation of GluA1, synapsin IIb, and amphiphysin I levels. Interestingly, in addition to protecting the AMPA receptor GluA1 subunit, the equipotent endocannabinoid modulator protected the ability of GluA1 to be phosphorylated by cAMP-dependent protein kinase at residue serine-845, moreso than the AM6702 treatment. This post-translational modification of AMPA channels has been found to increase their open probability [59], to regulate their cell surface expression [60], and to promote long-term potentiation and retention of spatial memory [61, 62]. KA-induced disruption of the GluA1 phosphorylated state may contribute to the seizure-mediated decline in brain function. Our study shows that AM6701 is particularly better than AM6702 in regard to the preservation of pGluA1-Ser-845, and/or the extent of its formation. The results point to the possibility that the neuroprotective effects of AM6701 may be due to the promotion of GluA1 phosphorylation and AMPA channel responses. Note that survival signaling and excitotoxic protection has been linked to glutamatergic transmission mediated by AMPA receptors [63]. A bifunctional effect is perhaps occurring in which basal AMPA signals are enhanced through kinase activity, whereas excitotoxic activation of glutamate receptors is reduced through the positive modulation of cannabinergic signaling. An alternative bifunctional mechanism for the modulation of glutamatergic transmission has been proposed, mediated by the inhibition of FAAH [46]. The protective nature of AM6701 that influences the synapse is key, in which preservation of presynaptic and postsynaptic integrity likely explains the corresponding protection at the behavioral level in the KA rat model. Together, the findings suggest that enhancement of endocannabinoid tone preserves the native composition of central synapses and their control of important brain functions.
The equipotency of AM6701 for blocking FAAH and MAGL may explain the comparative protection over AM6702. AM6701 is a regioisomer of AM6702, a compound that does not exhibit synergistic action on the 2 enzymes. In fact, AM6702 was found to have near equipotency for the inhibition of FAAH and AEA transport [64], suggesting the compound has a focused effect on AEA tone. On the other hand, the dual modulation of AM6701 of AEA and 2-AG tone by targeting both FAAH and MAGL may point to a reparative synergy. AM6701 efficiently inhibits the endocannabinoid-deactivating enzymes, AM6701 being 24-fold more potent than AM6702 regarding its action on MAGL. Others have found AM6701 to be even more potent than AM6702 regarding its action on 2-AG catabolism [42, 64]. Potent inhibition of MAGL by AM6701 is thought to be due to the covalent carbamylation of serine-122 in MAGLs catalytic triad characteristic of serine hydrolases [42]. In regard to FAAH, proteomic analyses indicate carbamylation of its serine-241 to cause inhibition of enzymatic activity [37]. Bowman and Makriyannis [65] suggest that the steric geometries of FAAH and MAGL are important considerations in regard to the inhibitory potential of drugs that target them. MAGL is thought to have a single access binding channel, whereas models indicate that the catalytic triad of FAAH lies between 2 channels. It is possible that the properties of AM6701 allow interactions with the environment surrounding the distinct serine residues in the enzymes, thus accounting for the equal blocking of AEA and 2-AG inactivation.
The vulnerability of neural circuitry to epileptic events indicates the need for effective, novel, and safe therapeutic strategies against seizure damage. It is proposed that AM6701 exerts a synergistic action on the endocannabinoid system by acting on AEA and 2-AG responses to protect against seizures. According to Long et al. [66], dual augmentation of the AEA and 2-AG signaling pathways allows these 2 lipid transmitters to engage in extensive crosstalk. A critical balance between AEA and 2-AG levels, controlled by endocannabinoid synthesizing and metabolizing enzymes, may therefore be important for normal physiological processes, as well as to promote repair after pathological events [1, 45, 48, 67]. Dual FAAH/MAGL inhibitors may thus be more viable as neuroprotectants against excitotoxic brain injury. A single drug targeting more than 1 protective pathway, such as AM6701, provides a unique approach related to the use of combinatorial treatments for different neurodegenerative diseases. For example, the use of inhibitor cocktails in studies of Parkinson’s disease [68, 69], Alzheimer’s disease [70–72], and stroke [73, 74] appears effective for therapeutic intervention. The additive or synergistic efficacy of a compound such as AM6701 may also help alleviate adverse drug effects by reducing the number of administered agents to target different pathways for precise fine-tuning of cannabinergic signaling. Understanding the effects of enhancing endocannabinoid responses in the nervous system remains a critical issue, and safety assessments for dual FAAH/MAGL inhibitors need to be further explored to be able to fully exploit their therapeutic usefulness.
Electronic supplementary material
(PDF 210 kb)
Acknowledgments
The authors thank Ana Charalambides and Tyler Loehr for their laboratory assistance, and Alyson Bahr for editing services. The work was supported by the National Institutes of Health (grants DA07215, R44 DA023737, and R25 GM077634), the Oliver Smithies Grant from the North Carolina Biotechnology Center (Research Triangle Park, NC), and by funds supporting the William C. Friday Chair. The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Conflict of interest
B.A.B. and A.M. are consultants for a company developing novel fatty acid amide hydrolase (FAAH) inhibitors.
References
- 1.Hwang J, Adamson C, Butler D, Janero DR, Makriyannis A, Bahr BA. Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: a neuroprotective therapeutic modality. Life Sci. 2010;86:615–623. doi: 10.1016/j.lfs.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanettini C, Panlilio LV, Alicki M, et al. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janero DR, Vadivel SK, Makriyannis A. Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies. Int Rev Psychiatry. 2009;21:122–133. doi: 10.1080/09540260902782778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neuroscience. 2011;178:159–168. doi: 10.1016/j.neuroscience.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito C, Nunez E, Tolon RM, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maccarrone M, Gubellini P, Bari M, et al. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem. 2003;85:1018–1025. doi: 10.1046/j.1471-4159.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- 8.Pisani V, Madeo G, Tassone A, et al. Homeostatic changes of the endocannabinoid system in Parkinson's disease. Mov Disord. 2011;26:216–222. doi: 10.1002/mds.23457. [DOI] [PubMed] [Google Scholar]
- 9.Mievis S, Blum D, Ledent C. Worsening of Huntington disease phenotype in CB1 receptor knockout mice. Neurobiol Dis. 2011;42:524–529. doi: 10.1016/j.nbd.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Micale V, Mazzola C, Drago F. Endocannabinoids and neurodegenerative diseases. Pharmacol Res. 2007;56:382–392. doi: 10.1016/j.phrs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Ludanyi A, Eross L, Czirjak S, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmentier-Batteur S, Jin K, Mao XO, et al. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–9775. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci. 2005;25:7813–7820. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karanian DA, Karim SL, Wood JT, et al. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J Pharmacol Exp Ther. 2007;322:1059–1066. doi: 10.1124/jpet.107.120147. [DOI] [PubMed] [Google Scholar]
- 15.Hajos N, Katona I, Naiem SS, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 16.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/S0896-6273(01)00246-X. [DOI] [PubMed] [Google Scholar]
- 17.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/S0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/S0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 19.Deadwyler SA, Hampson RE, Mu J, et al. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- 20.Gomez del Pulgar T, Velasco G, Guzman M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina-Holgado F, Pinteaux E, Heenan L, et al. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Neurosci. 2005;28:189–194. doi: 10.1016/j.mcn.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Galve-Roperh I, Rueda D, Gomez del Pulgar T, et al. Mechanism of extracellular signal-regulated kinase activation by the CB1 cannabinoid receptor. Mol Pharmacol. 2002;62:1385–1392. doi: 10.1124/mol.62.6.1385. [DOI] [PubMed] [Google Scholar]
- 23.Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237–246. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandevoorde S, Lambert DM. The multiple pathways of endocannabinoid metabolism: a zoom out. Chem Biodivers. 2007;4:1858–1881. doi: 10.1002/cbdv.200790156. [DOI] [PubMed] [Google Scholar]
- 25.Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 26.Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and CB1 cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/S0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 28.Egertova M, Giang DK, Cravatt BF, et al. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Biol Sci. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulyas AI, Cravatt BF, Bracey MH, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 30.Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen HH, Schmid PC, Bittigau P, et al. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- 32.Marsicano G, Goodenough S, Monory K, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 33.Naidoo V, Nikas SP, Karanian DA, et al. A new generation fatty acid amide hydrolase inhibitor protects against kainate-induced excitotoxicity. J Mol Neurosci. 2011;43:493–502. doi: 10.1007/s12031-010-9472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampson RE, Miller F, Palchik G, Deadwyler SA. Cannabinoid receptor activation modifies NMDA receptor mediated release of intracellular calcium: implications for endocannabinoid control of hippocampal neural plasticity. Neuropharmacology. 2011;60:944–952. doi: 10.1016/j.neuropharm.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onaivi ES. Cannabinoid receptors in brain: pharmacogenetics, neuropharmacology, neurotoxicology, and potential therapeutic applications. Int Rev Neurobiol. 2009;88:335–369. doi: 10.1016/S0074-7742(09)88012-4. [DOI] [PubMed] [Google Scholar]
- 36.Araujo BH, Torres LB, Cossa AC, et al. Hippocampal expression and distribution of CB1 receptors in the Amazonian rodent Proechimys: an animal model of resistance to epilepsy. Brain Res. 2010;1335:35–40. doi: 10.1016/j.brainres.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Alexander JP, Cravatt BF. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem Biol. 2005;12:1179–1187. doi: 10.1016/j.chembiol.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderklish PW, Bahr BA. The pathogenic activation of calpain: A marker and mediator of cellular toxicity and disease states. Internatl J Exp Pathol. 2000;81:323–339. doi: 10.1046/j.1365-2613.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahr BA, Tiriveedhi S, Park GY, Lynch G. Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther. 1995;273:902–908. [PubMed] [Google Scholar]
- 40.Ramarao MK, Murphy EA, Shen MW, et al. A fluorescence-based assay for fatty acid amide hydrolase compatible with high-throughput screening. Anal Biochem. 2005;343:143–151. doi: 10.1016/j.ab.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 41.Patricelli MP, Lashuel HA, Giang DK, et al. Comparative characterization of a wild type and transmembrane domain-deleted fatty acid amide hydrolase: identification of the transmembrane domain as a site for oligomerization. Biochemistry. 1998;37:15177–15187. doi: 10.1021/bi981733n. [DOI] [PubMed] [Google Scholar]
- 42.Zvonok N, Pandarinathan L, Williams J, et al. Covalent inhibitors of human monoacylglycerol lipase: ligand-assisted characterization of the catalytic site by mass spectrometry and mutational analysis. Chem Biol. 2008;15:854–862. doi: 10.1016/j.chembiol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathologic processes. J Neurosci Res. 1995;42:294–305. doi: 10.1002/jnr.490420303. [DOI] [PubMed] [Google Scholar]
- 44.Araújo IM, Gil JM, Carreira BP, et al. Calpain activation is involved in early caspase-independent neurodegeneration in the hippocampus following status epilepticus. J Neurochem. 2008;105:666–676. doi: 10.1111/j.1471-4159.2007.05181.x. [DOI] [PubMed] [Google Scholar]
- 45.Bahr BA, Karanian DA, Makanji SS, Makriyannis A. Targeting the endocannabinoid system in treating brain disorders. Expert Opin Investig Drugs. 2006;15:351–365. doi: 10.1517/13543784.15.4.351. [DOI] [PubMed] [Google Scholar]
- 46.Kawahara H, Drew GM, Christie MJ, Vaughan CW. Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey. Br J Pharmacol. 2011;163:1214–1222. doi: 10.1111/j.1476-5381.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coomber B, O'Donoghue MF, Mason R. Inhibition of endocannabinoid metabolism attenuates enhanced hippocampal neuronal activity induced by kainic acid. Synapse. 2008;62:746–755. doi: 10.1002/syn.20547. [DOI] [PubMed] [Google Scholar]
- 48.Busquets-Garcia A, Puighermanal E, Pastor A, Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Paola V, Arber S, Caroni P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci. 2003;6:491–500. doi: 10.1038/nn1046. [DOI] [PubMed] [Google Scholar]
- 50.Gogolla N, Galimberti I, DePaola V, et al. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc. 2006;1:1165–1171. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- 51.Caba E, Bahr BA. Biphasic NF-κB activation in the excitotoxic hippocampus. Acta Neuropathol. 2004;108:173–182. doi: 10.1007/s00401-004-0876-5. [DOI] [PubMed] [Google Scholar]
- 52.Ryzhikov S, Bahr BA. Gephyrin alterations due to protein accumulation stress are reduced by the lysosomal modulator Z-Phe-Ala-diazomethylketone. J Mol Neurosci. 2008;34:131–139. doi: 10.1007/s12031-007-9009-7. [DOI] [PubMed] [Google Scholar]
- 53.Jourdi H, Hamo L, Oka T, Seegan A, Baudry M. BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP + −induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology. 2009;56:876–885. doi: 10.1016/j.neuropharm.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Liang S, Oda Y, et al. Truncations of amphiphysin I by calpain inhibit vesicle endocytosis during neural hyperexcitation. EMBO J. 2007;26:2981–2990. doi: 10.1038/sj.emboj.7601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.David C, McPherson PS, Mundigl O, et al. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takei K, Slepnev VI, Haucke V, et al. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 57.Slepnev VI, Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 58.Akaike K, Tanaka S, Tojo H, et al. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res. 2001;900:65–71. doi: 10.1016/S0006-8993(01)02252-1. [DOI] [PubMed] [Google Scholar]
- 59.Banke TG, Bowie D, Lee H, et al. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/S0896-6273(00)00129-X. [DOI] [PubMed] [Google Scholar]
- 61.Lee HK, Takamiya K, Han JS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/S0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 62.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- 63.Bahr BA, Bendiske J, Brown QB, et al. Survival signaling and selective neuroprotection through glutamatergic transmission. Exp Neurol. 2002;174:37–47. doi: 10.1006/exnr.2001.7852. [DOI] [PubMed] [Google Scholar]
- 64.Ortar G, Cascio MG, Moriello AS, et al. Carbamoyl tetrazoles as inhibitors of endocannabinoid inactivation: a critical revisitation. Eur J Med Chem. 2008;43:62–72. doi: 10.1016/j.ejmech.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 65.Bowman AL, Makriyannis A. Refined homology model of monoacylglycerol lipase: toward a selective inhibitor. J Comput Aided Mol Des. 2009;23:799–806. doi: 10.1007/s10822-009-9289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Long JZ, Nomura DK, Vann RE, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alexander SP, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol. 2007;152:602–623. doi: 10.1038/sj.bjp.0707456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petzer JP, Castagnoli N, Jr, Schwarzschild MA, et al. Dual-target-directed drugs that block monoamine oxidase B and adenosine A(2A) receptors for Parkinson's disease. Neurotherapeutics. 2009;6:141–151. doi: 10.1016/j.nurt.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pretorius J, Malan SF, Castagnoli N, Jr, et al. Dual inhibition of monoamine oxidase B and antagonism of the adenosine A(2A) receptor by (E, E)-8-(4-phenylbutadien-1-yl)caffeine analogues. Bioorg Med Chem. 2008;16:8676–8684. doi: 10.1016/j.bmc.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 70.Venneri A, McGeown WJ, Shanks MF. Empirical evidence of neuroprotection by dual cholinesterase inhibition in Alzheimer's disease. Neuroreport. 2005;16:107–110. doi: 10.1097/00001756-200502080-00006. [DOI] [PubMed] [Google Scholar]
- 71.Ballard CG. Advances in the treatment of Alzheimer's disease: benefits of dual cholinesterase inhibition. Eur Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- 72.Bartorelli L, Giraldi C, Saccardo M, et al. Effects of switching from an AChE inhibitor to a dual AChE-BuChE inhibitor in patients with Alzheimer's disease. Curr Med Res Opin. 2005;21:1809–1818. doi: 10.1185/030079905X65655. [DOI] [PubMed] [Google Scholar]
- 73.Zausinger S, Scholler K, Plesnila N, et al. Combination drug therapy and mild hypothermia after transient focal cerebral ischemia in rats. Stroke. 2003;34:2246–2251. doi: 10.1161/01.STR.0000083622.65684.21. [DOI] [PubMed] [Google Scholar]
- 74.Schmid-Elsaesser R, Hungerhuber E, Zausinger S, et al. Combination drug therapy and mild hypothermia: a promising treatment strategy for reversible, focal cerebral ischemia. Stroke. 1999;30:1891–1899. doi: 10.1161/01.STR.30.9.1891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 210 kb)