Abstract
Recent studies have shown that selective attention is of considerable importance for encoding task-relevant items into visual short-term memory (VSTM) according to our behavioural goals. However, it is not known whether top-down attentional biases can continue to operate during the maintenance period of VSTM. We used event-related potentials (ERPs) to investigate this question across two experiments. Specifically, we tested whether orienting attention to a given spatial location within a VSTM representation resulted in modulation of the contralateral delay activity (CDA), a lateralized ERP marker of VSTM maintenance generated when participants selectively encode memory items from one hemifield. In both experiments, retrospective cues during the maintenance period could predict a specific item (spatial retro-cue) or multiple items (neutral retro-cue) that would be probed at the end of the memory delay. Our results revealed that VSTM performance is significantly improved by orienting attention to the location of a task-relevant item. The behavioural benefit was accompanied by modulation of neural activity involved in VSTM maintenance. Spatial retro-cues reduced the magnitude of the CDA, consistent with a reduction in memory load. Our results provide direct evidence that top-down control modulates neural activity associated with maintenance in VSTM, biasing competition in favour of the task-relevant information.
Keywords: attention, top-down control, visual short-term memory, event-related potentials (ERPs), contralateral delay activity (CDA)
INTRODUCTION
Visual short-term memory (VSTM) is the function by which potentially relevant visual information is maintained in the form of internal representations that persist beyond the original sensory input in order to guide subsequent behaviour. The capacity of VSTM is limited: only a small number of items can be retained in VSTM at any given time. Recent studies have shown that selective attention is of considerable importance for gating the encoding of task-relevant items into VSTM according to behavioural goals and expectations (Gazzaley, 2011; Murray, Nobre, & Stokes, 2011; Rutman, Clapp, Chadick, & Gazzaley, 2010; Schmidt, Vogel, Woodman, & Luck, 2002; Zanto & Gazzaley, 2009). In this study, we investigate whether and how attention can also shape VSTM during maintenance, according to changing task goals.
Accumulating neural evidence has revealed that the maintenance of information in VSTM may involve stimulus-related perceptual codes in sensory brain areas (Chelazzi, Miller, Duncan, & Desimone, 1993; Druzgal & D’Esposito, 2001, 2003; Ester, Serences, & Awh, 2009; Harrison & Tong, 2009; Munneke, Heslenfeld, & Theeuwes, 2010; Pasternak & Greenlee, 2005; Postle, Druzgal, & D’Esposito, 2003; Serences, Ester, Vogel, & Awh, 2009; Stokes, Thompson, Cusack, & Duncan, 2009). According to this view, VSTM maintenance is at least partly mediated by persistent activity of neural populations that represent the perceptual characteristics of the mnemonic information. Activation in these posterior visual areas is thought to be maintained and coordinated by top-down signals from multisensory executive brain regions, such as prefrontal cortex (Awh & Jonides, 2001; Curtis & D’Esposito, 2003; Miller, Li, & Desimone, 1993). Single-unit recordings in non-human primates demonstrate sustained firing for neurons that are selective for the specific memoranda throughout the maintenance period in a delayed-match-to-sample task (Chelazzi, Duncan, Miller, & Desimone, 1998; Miller, Li, & Desimone, 1993; Super, Spekreijse, & Lamme, 2001). Electrophysiological studies in humans reveal an event-related potential (ERP) marker of sustained mnemonic activity, known as “contralateral delay activity” (CDA) or “sustained posterior contralateral negativity” (SPCN) (Drew & Vogel, 2008; Eimer & Kiss, 2010; Fukuda & Vogel, 2009; Ikkai, McCollough, & Vogel, 2010; Jolicœur, Brisson, & Robitaille, 2008; McCollough, Machizawa, & Vogel, 2007; Robitaille & Jolicoeur, 2006; Vogel & Machizawa, 2004; Vogel, McCollough, & Machizawa, 2005). This is a sustained lateralised negativity over posterior electrodes, which persists throughout the mnemonic delay period and scales with the number of items being maintained (Anderson, Vogel, & Awh, 2011; Bor & Owen, 2006; Vogel & Machizawa, 2004; Vogel, McCollough, & Machizawa, 2005). It is thought to reflect delay activity underlying VSTM maintenance in visual extrastriate, and possibly parietal areas, though the neural sources of the effect still require further substantiation. Importantly, mnemonic delay activity reflects maintenance for task-relevant items. Prior to the presentation of the memory array, participants are cued to encode stimuli from only one visual hemifield. During the subsequent maintenance interval, neural activity is greater in the posterior region that is contralateral to the location of items in VSTM, relative to the ipsilateral region, reflecting selectivity for the task-relevant items held in VSTM.
If task goals change dynamically after the initial encoding of the events, stimuli maintained in VSTM may gain or lose relevance; therefore, a mechanism for dynamic modulation of specific memoranda would be highly advantageous in optimising and controlling the limited contents of VSTM. Recent behavioural evidence demonstrates that attention can be directed to specific items held in VSTM (Griffin & Nobre, 2003; Landman, Spekreijse, & Lamme, 2003; Lepsien & Nobre, 2007; Makovski & Jiang, 2007; Makovski, Shim, & Jiang, 2006; Makovski, Sussman, & Jiang, 2008; Matsukura, Luck, & Vecera, 2007; Vandenbroucke, Sligte, & Lamme, 2011; Yeh, Kuo, & Liu, 2007). Presenting a spatial cue during the delay period to indicate which item in VSTM is likely to be probed dramatically improves VSTM performance for cued relative to uncued items. Critically, these retroactive cues (retro-cues) are presented long after iconic memory decay, and therefore do not influence initial encoding into VSTM. However, the mechanisms by which selective attention can bias maintenance in VSTM remain largely unknown.
As mentioned above, current evidence suggests that VSTM is mediated by sustained activation of memoranda-specific perceptual representations in posterior brain areas. A perceptual basis for VSTM could provide a natural mechanism for dynamic biasing even after initial encoding into VSTM. In particular, similar attentional mechanisms that bias competitive processing during perception may operate upon activity in these posterior areas when they are supporting VSTM representations (Dell’Acqua, Sessa, Toffanin, Luria, & Jolicoeur, 2010; Eimer & Kiss, 2010; Kuo, Rao, Lepsien, & Nobre, 2009). For example, shifts of attention during VSTM maintenance could filter out items as they become task irrelevant, thereby reducing memory load and inter-item competition (Edin et al., 2009), and consequently increasing the probability of recall for the relevant cued items.
In this study, we tested whether top-down attentional signals can continue to modulate persistent delay activity underlying VSTM across two experiments. We exploited the CDA as an index of VSTM maintenance to test whether the load of items being actively maintained can be adjusted dynamically as new information becomes available, signalling which memoranda are most relevant for task performance. As common practice in experiments using the CDA measure, at the beginning of each trial participants viewed an arrow cue, instructing them to only encode items in either the left or right visual hemifield. Next, a memory array of multiple colored squares was presented within each visual hemifield. After a retention interval, a test stimulus was presented to probe the contents of VSTM. During this retention interval, attentional orienting was manipulated by providing spatially informative (spatial) or non-informative (neutral) cues after the memory array, yet before the test probe. These retro-cues indicated the location of the single item that would be required to perform the subsequent comparison to the probe stimulus, thus effectively reducing the task-relevant load from multiple items to just one item. Importantly, because this predictive information is presented long after the offset of the memory array, retro-cues cannot be used to bias initial processing of the visual stimulus for selective access to VSTM, but may influence information that is already being maintained in VSTM.
Consistent with previous studies (Griffin & Nobre, 2003; Nobre, Griffin, & Rao, 2008), we found that recall was faster and more accurate for cued items, relative to uncued items. More importantly, we found that load-dependent neural activity reflecting VSTM maintenance could be modulated by spatial cues that effectively reduced the task-relevant memoranda to one item across two experiments. Our results provide direct evidence that top-down control directly modulates neural activity associated with maintenance in VSTM.
METHODS
Experiment 1
Participants
All participants in this study were right-handed, according to the Edinburgh handedness inventory (Oldfield, 1971). Twenty-five participants were recruited. They had normal or corrected-to-normal visual acuity, provided informed written consent, and were financially reimbursed for their time. Data from 7 participants were excluded, due to poor performance on the task (< 60% correct trials) or too few trials remaining after EEG artifact rejection (< 25 trials). The behavioral and ERP analyses were performed on the remaining 18 participants (9 females, age range 21-33 years, mean age = 27). All experimental methods had ethical approval from the Central University Research Ethics Committee of the University of Oxford.
Behavioral Task
The task of the first experiment is illustrated in Figure 1a. Retro-cue type (spatial, neutral) and VSTM load (2-item, 4-item) were manipulated within participants, in a two-way repeated-measures factorial design.
Figure 1.
Schematics of experimental trials: VSTM task (top panel) used in (a) Experiment 1 and (b) Experiment 2. Sensitivity measures (d’), Pashler-Cowan K measures (K), and mean response times (RT, ms) were estimated (bottom panel). Error bars represent standard errors of the means.
Stimuli were presented on a CRT screen using Presentation software (Neurobehavioral Systems, Inc., CA). Each trial began with the onset of a centrally displayed asterisk (500-1000 ms duration, randomised), which signaled the onset of the trial. Next, an arrow pointing to either the left or right visual hemifield was presented at the centre of the screen for 200 ms, followed, at a variable inter-stimulus interval (500-1000 ms duration), by the memory array consisting of two or four colored squares in each hemifield. Participants were instructed to remember as many items as possible that were presented within the cued hemifield. There were eight positions for the presentation of a memory array (four positions in each hemifield), each arranged according to two imaginary concentric circles with radii of approximately 3.06° and 5.44° of visual angle.
When 800ms of the delay period had elapsed, a retro-cue appeared for 200 ms at the centre of the screen [spatial retro-cue (50%), neutral retro-cue (50%)]. In spatial-cue trials, the cue was presented by highlighting two adjacent sides of the central cross, thereby pointing to one of four quadrants within the relevant hemifield, thereby indicating one specific location within the array being maintained. In neutral trials, the cue consisted of a uniform brightening of the cross, therefore providing no specific spatial information. After a randomized variable interval following the retro-cue (600-1000 ms duration for post-cue interval), a colored square was presented at central fixation for 200 ms. Participants decided whether the test probe was the same as the cued item from the previous memory array, responding match (50%) or non-match (50%) with the left or right mouse button respectively. The interval between trials, which included a 1000-ms response period, varied randomly between 2000 ms and 2500 ms.
All trial types were equiprobable and randomized within 16 blocks of 32 trials, yielding 512 trials in total (64 target-present and 64 target-absent trials in each retro-cue type and VSTM-load condition). Participants first completed one practice block of 32 trials, and they were encouraged to rest between blocks during the main experiment. Participants were comfortably seated in a dimly illuminated, electrically shielded room, facing a computer monitor placed 100 cm in front of them. They were instructed to maintain fixation on a small fixation marker at the centre of the monitor during the experimental trials, and to respond as quickly and accurately as possible. Participants responded using their right hand by pressing mouse button. Participants were also asked to minimize blinking and moving their eyes whilst performing each trial throughout the experiment.
All visual stimuli were presented against a black background, and a central fixation point was present throughout the experiment. The colour of each memory item was randomly selected from a set of eight possible colors (red, green, yellow, blue, grey, pink, purple and brown), and the size and position of each item was computed according to the human cortical magnification factor (Cowey & Rolls, 1974). Memory items presented at 3.06° and 5.44° of visual angle from central fixation subtended 0.77° and 1.36° of visual angle respectively. The test item presented at fixation subtended 0.5° of visual angle in size.
Behavioral analyses
Behavioural measures, including sensitivity score for match/no-match discrimination (d’ score) (Green & Swets, 1966), memory-capacity measurement (Pashler-Cowan K measure) (Cowan, 2001; Pashler, 1988), and reaction times (RTs) were each analyzed by a repeated-measures analysis of variance (ANOVA) with two factors: retro-cue type (spatial and neutral) and VSTM load (2-items and 4-items). The d’ score was calculated using the following equation: d’ = Z (hit rate) – Z (false alarm rate). The K measure was calculated using the following equation: K = S (set size of the initial array) × (hit rate – false alarm rate). For d’ score and K measure, hit rate was defined as the conditional probability that the participants responded “target-present” given that the target was presented, and the false-alarm rate was defined as the conditional probability that the participants responded “target-present” when the target was absent. Only correct responses were included for RT analyses.
EEG recording
The EEG was recorded continuously using NuAmp amplifiers (Neuroscan, Inc.) from 34 silver/silver-chloride electrodes placed on the scalp with an elasticated cap, positioned according to the 10-20 international system (AEEGS, 1991). The montage included six midline sites (FZ, FCZ, CZ, CPZ, PZ, and OZ) and fourteen sites over each hemisphere (FP1/FP2, F3/F4, F7/F8, FC3/FC4, FT7/FT8, C3/C4, T7/T8, CP3/CP4, TP7/TP8, P3/P4, P7/P8, PO3/PO4, PO7/PO8, and O1/O2). Vertical eye movements were recorded by electrodes placed on the supraorbital and infraorbital ridges of the right eye [vertical electro-oculogram (VEOG)], and horizontal eye movements by electrodes placed on the outer canthi of the right and left eyes [horizontal electro-oculogram (HEOG)]. Additional electrodes were used as ground and reference sites. Electrodes were referenced to the right mastoid site (A2) during recording. The electrode between FPZ and FZ (AFZ) on the midline served as the ground electrode. Electrode impedances were kept below 5kΩ. The ongoing brain activity at each electrode site was sampled every 1 ms (1000-Hz analogue-to-digital sampling rate). Activity was filtered with a low-pass filter of 300 Hz, and no high-pass filter was used.
EEG processing
The EEG was re-referenced offline to the algebraic average of the right and left mastoids. Bipolar EOG signals were derived by computing the difference between the voltages at electrodes placed to the side of each eye (HEOG) and above and below the left eye (VEOG). The re-referenced and transformed continuous data were then low-pass filtered (40 Hz) to exclude high-frequency noise.
The continuous EEG was then segmented into epochs, time-locked at the onset of the memory array. Epochs were 2000 ms long, beginning 200 ms before the onset of the memory array, and ending after 1800 ms, which corresponded to the minimal time point before the presentation of the probe stimulus. The pre-stimulus baseline of 200 ms was used for all analyses. Data quality was initially inspected using automated algorithms. Epochs containing excessive noise or drift (+/-100uV) at any electrode were excluded, and epochs with eye-movement artifacts (blinks or saccades) were also rejected. Blinks were identified as large deflections (+/-50uV) in the HEOG or VEOG electrodes. Visual inspection was also carried out to confirm appropriate removal of artifacts and to identify residual saccades in the individual HEOG traces. Trials with incorrect behavioral responses were also discarded from the final analyses.
Epochs were then averaged according to retro-cue type, VSTM load and visual hemifield. ERPs were derived from both target-present and target-absent trials. ERPs from trials containing targets located in the right and left hemifield were combined by an averaging procedure that preserves the electrode location relative to the target side (contralateral or ipsilateral). To maintain an acceptable signal-to-noise ratio, a lower limit of 25 artifact-free trials per subject per condition was set.
EEG analysis
The aim of this study was to test whether the information carried by spatial retro-cues modulates persistent delay activity, namely CDA. The mean amplitudes of the delay activity were computed between 500 and 800 ms during pre-cue interval (before appearance of retro-cue) and between 1500 and 1800 ms during post-cue interval (500-800 ms after appearance of retro-cue) at the parietal-occipital electrodes (PO7/8) contralateral and ipsilateral to the side of the target (McCollough, Machizawa, & Vogel, 2007; Vogel & Machizawa, 2004; Vogel, McCollough, & Machizawa, 2005). A three-way repeated-measures ANOVA was computed on the mean amplitudes of the delay activity, testing the effects of retro-cue type (spatial and neutral), VSTM load (2-item and 4-item) and visual hemifield (contralateral and ipsilateral to target). Of main interest was the three-way interaction among retro-cue type, VSTM load and visual hemifield; which would indicate a change in the size of the lateralized CDA after a spatial retro-cue. The Greenhouse-Geisser epsilon correction for non-sphericity was applied to all ERP analyses where appropriate (Jennings & Wood, 1976), and only corrected probability values and degrees of freedom were reported.
RESULTS
Behavioral results
The behavioral results are summarized in Figure 1 and Table. A significant retro-cueing effect was observed across all measures, with higher mean d’ scores [F (1, 17) = 58.70, p < .005], higher mean K measures [F (1, 17) = 76.72, p < .005] and faster RT [F (1, 17) = 252.93, p < .005], in spatial trials (2.34 ± 0.71 d’ score, 2.05 ± 0.54 K value, 528.46 ± 87.71 ms in RT) relative to neutral trials (1.83 ± 0.78 d’ score, 1.59 ± 0.42 K value, 648.20 ± 98.02 ms in RT). We also observed a significant main effect of VSTM load across all measures, showing higher mean d’ scores [F (1, 17) = 113.16, p < .005], lower K measures [F (1, 17) = 21.91, p < .005] and faster RT [F (1, 17) = 98.85, p < .005], in 2-item trials (2.66 ± 0.52 d’ score, 1.59 ± 0.17 K value, 551.41 ± 90.89 ms in RT) relative to 4-item trials (1.52 ± 0.56 d’ score, 2.06 ± 0.66 K value, 625.25 ± 116.12 ms in RT). Finally, the interaction between retro-cue type and VSTM load was significant in all measures [d’ score: F (1, 17) = 6.12, p < .05; K measure: F (1, 17) = 40.91, p < .005; RT: F (1, 17) = 9.16, p < .05], owing to a reduction of the load effect associated with spatial retro-cues [increased d’ score: t (17) = 2.46, p < .05; enhanced K measure: t (17) = 6.44, p < .005; faster RT: t (17) = 3.03, p < .05]. These behavioural results confirmed that participants were able to orient their attention toward the item held in VSTM, resulting in benefits of VSTM performance, and consequently attenuating the load effect.
Table.
Mean d’ scores, K measures, and reaction times (RTs in ms) and standard deviations of the means of correct responses in each condition.
| Experiment 1 | |||
| Neutral cue | Spatial cue | ||
|
| |||
| d’ score | 2-item | 2.48 ± 0.44 | 2.84 ± 0.56 |
| 4-item | 1.19 ± 0.46 | 1.85 ± 0.47 | |
| K measure | 2-item | 1.53 ± 0.16 | 1.65 ± 0.16 |
| 4-item | 1.66 ± 0.58 | 2.45 ± 0.51 | |
| RT (ms) | 2-item | 603.38 ± 84.22 | 499.44 ± 68.40 |
| 4-item | 693.01 ± 94.86 | 557.48 ± 99.15 | |
|
| |||
| Experiment 2 | |||
| No cue | Neutral cue | Spatial cue | |
|
| |||
| d’ score | 1.11 ± 0.43 | 1.22 ± 0.31 | 1.84 ± 0.57 |
| K measure | 1.59 ± 0.45 | 1.74 ± 0.38 | 2.45 ± 0.64 |
| RT (ms) | 745.63 ± 64.11 | 695.27 ± 71.14 | 538.86 ± 71.83 |
ERP results
The ERP results are plotted in Figure 2. Firstly, we tested for the presence of CDA during the pre-cue interval (500-800 ms), and found a significant main effect of visual hemifield with a greater mean negative amplitude on the contralateral side relative to ipsilateral sides (F (1, 17) = 37.73, p < .005). As predicted, we found the CDA to be load dependent. We observed a significant interaction of VSTM load and visual hemifield [F (1, 17) = 20.56, p < .005], owing to a greater increase in voltage negativity over posterior electrodes that were contralateral to the memorized hemifield relative to the ipsilateral hemifield within increasing VSTM load (2-item: 0.97 ± 0.97 μV, 4-item: 1.70 ± 0.99 μV) [t (17) = 4.54, p < .005]. There was also a significant main effect of VSTM load, attributable to a greater negative amplitude for 4-item (−2.46 ± 2.58 μV), relative to 2-item trials (−1.45 ± 2.07 μV) [F (1, 17) = 15.13, p < .005].
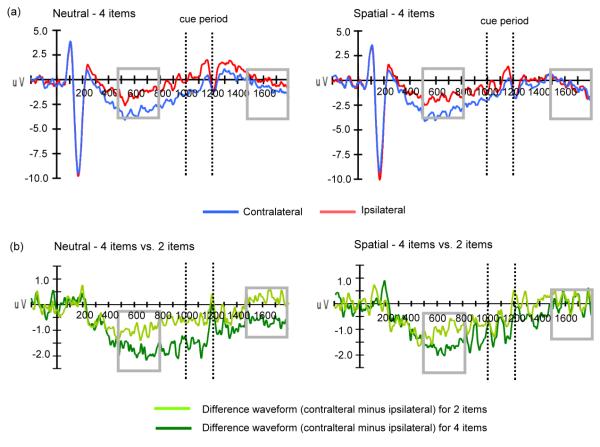
Figure 2.
The ERP results of Experiment 1. (a) ERP waveforms averaged across all participants are shown for the 4 items for neutral (left panel) and spatial retro-cue trials (right panel) over contralateral (blue lines) and ipsilateral (red lines) posterior parietal-occipital electrode pair: PO7/8. Our result shows equivalent CDA for neutral and spatial trials during the pre-cue interval (500-800 ms). The amplitude of CDA is attenuated after a spatial cue, relative to the neutral cue, during the post-cue interval (1500-1800 ms). (b) The voltage difference between contralateral versus ipsilateral side is also shown for 2 items (light green lines) and 4 items (dark green lines) in the neutral and spatial retro-cue conditions, respectively. CDA is load dependent, showing greater CDA for 4 items than 2 items during the pre-cue interval (500-800 ms). Spatial retro-cues reduced the magnitude of the CDA during the post-cue interval (1500-1800 ms). The temporal windows for CDA analyses are indicated by grey squares.
The main hypothesis of interest was whether the load-dependent neural activity reflecting VSTM maintenance (i.e., CDA) could be modulated dynamically by spatial cues that effectively reduced the task-relevant memoranda to one item. Accordingly, we tested whether the amplitude of CDA was attenuated after a spatial cue, relative to the neutral cue, during the post-cue interval (1500-1800 ms). Direct comparison of the voltage difference between ipsilateral versus contralateral side indicated a significant main effect of load [F (1, 17) = 8.17, p < .05], showing greater mean amplitude for 4-item (0.28 ± 1.09 μV) relative to 2-item trials (−0.22 ± 0.90 μV) [t (17) = 2.86, p < .05]. More importantly, we observed a significant three-way interaction among retro-cue type, VSTM load and visual hemifield [F (1, 17) = 4.35, p = .05]. Follow-up analyses were conducted to clarify the pattern of interaction effect. Analysis of neutral trials still revealed a significant load effect [t (17) = 3.42, p < .005] (4-item: 0.67 ± 1.20 μV; 2-item: −0.32 ± 1.45 μV). In contrast, no difference was found in amplitude of CDA between 4-item (−0.11 ± 1.23 μV) and 2-item (−0.12 ± 1.04 μV) spatial cue trials (p > .1). Overall, the results highlighted that the difference in the CDA between two and four items was reduced after a spatial cue (0.01 ± 1.26 μV) in contrast to a neutral cue (1.0 ± 1.23 μV) [t (17) = 2.09, p = .05], revealing a greater reduction in CDA after a spatial cue than after a neutral cue.
To test for a functional link between the neural activity reflected in the CDA and VSTM performance in our task, we also examined the correlation between the increase in amplitude of CDA between two and four items and the increase in K measured on neutral trials between two and four items across participants (Vogel & Machizawa, 2004). During the pre-cue interval, there was a significant relationship between the set-size effect on CDA amplitudes and K [Pearson correlation: r(17) = 0.63, p < .05]. This finding showed that individual differences in VSTM capacity can be reflected in the measures of delay activity, replicating previous reports of correlations between CDA and VSTM capacity (Vogel & Machizawa, 2004). We also observed a significant correlation on neutral trials in the interval after the cue was presented [r(17)= 0.43, p < .05], further demonstrating that the neutral cue did not alter the relationship between the maintenance-related delay activity and capacity estimates.
Experiment 2
The goals of experiment 2 were to replicate our finding that VSTM maintenance could be dynamically modulated by spatial attention and to ensure that these effects did not simply result from a potential disruption of VSTM maintenance by the presentation of a neutral cue. In order to rule out any effect related to the mere presentation of a visual stimulus as a cue, we included an additional no-cue condition. In experiment 2, the memory array always consisted of four colored squares in each hemifield. In the no-cue control condition and in the neutral retro-cue condition, stimuli maintained in VSTM did not gain or lose relevance. As in experiment 1, retro-cue type was manipulated within participants, in a one-way repeated-measures design. All trial types were equiprobable and randomized, and presentation order was randomised within 16 blocks of 24 trials, yielding 384 trials in total (64 target-present and 64 target-absent trials in each retro-cue type).
In order to focus on the modulatory effect of spatial and neutral retro-cues in the post-cue interval more clearly, the spatial or neutral retro-cue was presented at the time we found the CDA to be around its maximal amplitude in experiment 1 – 600-800 ms after the memory array. The earlier presentation of the retro-cue enabled us to test the modulatory effect over an extended time period, and to obtain a more stable measure of the CDA during the post-cue interval (1100-1800 ms duration) (see Figure 1b for an example). The sequence of events in each trial and all other procedures for EEG recording, processing and analyses were the same as the previous experiment.
Participants
All participants in this study were right-handed, according to the Edinburgh handedness inventory (Oldfield, 1971). Sixteen participants were recruited. They had normal or corrected-to-normal visual acuity, provided informed written consent, and were financially reimbursed for their time. Data from one participant was excluded, owing to too few trials remaining after EEG artifact rejection (< 25 trials). The behavioral and ERP analyses were performed on the remaining 15 participants (8 females and 7 males, age range 21-33 years, mean age = 26.5). All experimental methods had ethical approval from the Central University Research Ethics Committee of the University of Oxford.
Behavioral analysis
Each behavioural measure (d’ score, K measure, and RT) was analyzed using a one-way repeated-measures ANOVA (retro-cue type: spatial, neutral and no-cue).
EEG analysis
The mean amplitudes of the delay activity were computed between 1100 and 1800 ms (500-1200 ms after appearance of retro-cue) during post-cue interval at PO7/8 contralateral and ipsilateral to the side of the target. A two-way repeated-measures ANOVA was computed on the mean amplitudes of the delay activity, testing the effects of retro-cue type (spatial, neutral, and no-cue) and visual hemifield (contralateral and ipsilateral to target). Of main interest was interaction between retro-cue type and visual hemifield.
RESULTS
Behavioral results
The behavioral results are summarized in Figure 1 and Table. Overall, participants had better performance in spatial trials compared to neutral and no-cue trials. We observed a significant main effect of retro-cue types across all measures [d’ scores: F (2, 14) = 19.45, p < .005; K measures: F (2, 14) = 19.70, p < .005; RT: F (2, 14) = 203.71, p < .005]. Follow-up comparisons showed higher mean d’ scores, higher K measure, and faster RT in spatial trials than in both neutral [d’ score: t(14) = 5.11, p < . 005; K value: t(14) = 4.89, p < .005, RT: t(14) = 12.00, p < .005] and no-cue trials [d’ score: t(14) = 4.80, p < .005, K value: t(14) = 5.12, p < .005, RT: t(14) = 18.00, p < .005]. Faster RT was also observed in neutral trials than in no-cue trials [t(14) = 7.92, p < .005]. No other significant effect was observed for d’ scores or K values (ps > .1). As in experiment 1, participants were capable of orienting their attention toward the item held in VSTM, resulting in benefits of VSTM performance.
ERP results
We tested whether the magnitude of CDA could be influenced by a neutral cue as well as a spatial cue, relative to the no-cue condition. The ERP results are plotted in Figure 3. We firstly demonstrated a significant main effect of visual hemifield [F (1, 14) = 40.91, p < .005], and, more importantly, a significant interaction between retro-cue type and visual hemifield [F (1, 14) = 16.45, p < .005]. Follow-up analyses showed that the difference in voltage for the ipsilateral side in contrast to contralateral side was significantly larger in both neutral [t (14) = 3.32, p = .005] (0.75 ± 0.48 μV) and no-cue trials (1.46 ± 0.90 μV) [t (14) = 4.67, p < .005] than in the spatial trials (−0.42 ± 0.99 μV). We also observed larger CDA for no-cue trials in contrast to neutral cue trials [t (14) = 3.43, p < .005], although both were significantly greater than zero [neutral: t (14) = 6.04, p < .005; no-cue: t (14) = 6.27, p < .005].
Figure 3.
The ERP results of Experiment 2. ERP waveforms averaged across all participants are shown for no-cue (left), neutral (middle), and spatial (right) cue conditions over contralateral (waveforms: blue lines; topographies: right side) and ipsilateral (waveforms: red lines; topographies: left side) posterior parietal-occipital electrode pair: PO7/8. The amplitude of CDA is attenuated after a spatial cue, relative to the neutral cue and no-cue condition, during the post-cue interval (1100-1800 ms). The voltage difference between contralateral versus ipsilateral side is also shown (waveforms: green lines). The temporal windows for CDA analyses are indicated by grey squares. The topographic maps isolated the lateralized differences in voltage between contralateral (CO) and ipsilateral (IP) sites. These maps show the symmetrical relative differences in voltage, which is more negative over the contralateral scalp and more positive over the ipsilateral scalp. The voltage distributions are shown from posterior perspective. The color scale shows the range of possible voltage values. Blue indicates negative voltage and red indicates positive voltage.
DISCUSSION
It is now well established that maintenance of information in VSTM is associated with persistent activity in neural ensembles in posterior brain regions that represent the perceptual characteristics of mnemonic information (see Pasternak & Greenlee, 2005 for a review). In this study, we tested whether top-down attention can directly modulate the maintenance of VSTM representations across two ERP experiments. By manipulating the spatially predictive information of an attentional retro-cue, we showed that maintenance-related activity is modulated by changes in task-relevance of particular items in VSTM, as indicated by spatially informative retro-cues. Specifically, spatial retro-cues reduced the magnitude of persistent delay activity, consistent with a reduction in the effective memory load. This neural modulation was also consistent with behavioural benefits of spatial retro-cueing. Presumably, reducing the number of task-irrelevant items stored in VSTM increases the probability of recall for the cued item.
The pattern of behavioural results replicates and extends previous studies, confirming that VSTM performance can be regulated by orienting attention to the internal representation of the task-relevant item (Griffin & Nobre, 2003; Landman, Spekreijse, & Lamme, 2003). Spatial retro-cues were accompanied by faster RT, higher d’ scores, and K measures relative to neutral retro-cue and no-cue conditions. This behavioral facilitation associated with spatial retro-cues was more pronounced as VSTM load increased (Lepsien & Nobre, 2006; Nobre, Griffin, & Rao, 2008), consistent with the hypothesis that attentional selection during VSTM maintenance effectively reduces the number of items that need to be retained. These behavioural results are in accordance with previous studies that have demonstrated that attentional retro-cues presented beyond the phase of VSTM encoding can still shape internal representations (Nobre, Griffin, & Rao, 2008), providing further evidence that VSTM representations can be modulated dynamically to accommodate changing task-goals.
Our result is also in line with previous evidence that CDA reflects VSTM maintenance and its magnitude correlates with VSTM capacity (Vogel & Machizawa, 2004). The strength of persistent activity varied as a function of VSTM load before appearance of retro-cues. Moreover, the correlation between the CDA and capacity estimates was still evident after appearance of the neutral cue, demonstrating that the CDA continuous to provide a valid index of VSTM maintenance, even after the presentation of a potentially distracting visual stimulus.
More importantly, our ERP data revealed that the magnitude of persistent activity was sharply attenuated by appearance of spatial retro-cues. This finding supports the hypothesis that top-down attention in VSTM may share properties with attentional mechanisms that modulate perceptual analysis to bias competition in favour of the task-relevant information. Previously, Lepsien and Nobre (2007) used fMRI to test for modulation of maintenance-related activity in an object-based VSTM task. They showed that retro-cues signalling the relevance of the face or scene in the previous two-item array for performance of a subsequent probe-match comparison modulated activity in perceptual areas preferentially processing faces (posterior fusiform gyrus) or scenes (parahippocampal gyrus). However, these results were ambiguous. Their task design made it unclear whether the cue-related modulations within these areas reflected changes to maintenance-related activity or anticipation of a specific category of probe stimulus (face or scene). Indeed, in a recent follow-up study, Lepsien et al. (2011) provides further evidence that anticipatory attention to relevant probe items can influence activity in visual areas. We were careful, therefore, to design our task in a way that would preclude any effect of anticipatory spatial attention to the cue or probe arrays.
In our task, the changes in the magnitude of lateralised neural activity in this task can only reflect changes in spatiotopic VSTM maintenance during the retention period. Spatial and neutral retro-cues were presented centrally, and therefore should not result in changes in the lateralisation of neural activity. The continued correlation between CDA amplitude and capacity measures after neutral cues confirms this to be the case. Probe stimuli also appeared centrally, and did not differ across the conditions of interest. A single colour probe stimulus was presented at fixation, and the participant had to decide whether it was one of the items in the initial memory array. There was no basis, therefore, for the formation of any anticipatory spatial bias that could interfere with our CDA measure. Though lateralised markers of VSTM markers and spatial attention can often co-occur in many experimental designs (for a discussion see Stokes, 2011), these were de-coupled within the present task design.
Our results also revealed some attenuation of the CDA in neutral retro-cue trials, relative to no-cue trials in experiment 2. We speculate that the CDA may reflect neural activity correlated to tonic firing in extrastriate visual areas during the delay, which is known to be sensitive to perceptual interference (Miller, Li, & Desimone, 1993). Interestingly, this CDA decrement was not accompanied by a change in accuracy or sensitivity.
The nature of VSTM is still not fully understood. However, recent studies have suggested that VSTM may involve similar neural codes to those that mediate perceptual information (Astle, Scerif, Kuo, & Nobre, 2009; Dell’Acqua, Sessa, Toffanin, Luria, & Jolicoeur, 2010; Gratton, 1998; Jiang, Olson, & Chun, 2000; Kuo, Rao, Lepsien, & Nobre, 2009). We suggest that the shared neural organization for perceptual and VSTM representations provides a common framework for top-down attentional modulations that optimize task-relevant processing during multiple domains of processing – perception, VSTM, and possibly beyond. Top-down attentional signals during VSTM maintenance can bias internal representations based on the original spatial configuration of the perceptual inputs.
In conclusion, evidence that retro-cues can modulate VSTM maintenance through top-down attentional orienting has important implications for views about the nature of VSTM. In particular, our findings provide further support for the view that VSTM representations are flexible, and can be modulated dynamically according to changing goals and expectations (Kuo, Rao, Lepsien, & Nobre, 2009; Kuo, Yeh, Chen, & D’Esposito, 2011; Lepsien & Nobre, 2006, 2007). Top-down attentional orienting can modulate the maintenance of the short-lived representations within VSTM and bias competition in a favour of the most task-relevant information. We suggest that dynamic modulation of maintenance-related activity is likely to operate in conjunction with other optimising mechanisms, such as attention-dependent encoding into VSTM (Murray, Nobre, & Stokes, 2011; Schmidt, Vogel, Woodman, & Luck, 2002), and selective biasing of search/retrieval processes (Nobre, Griffin, & Rao, 2008). As in perception, we argue that goal-dependent biases do not operate at a single bottleneck, but at multiple stages between stimulus and response depending on how task-goals and expectations are determined, and how they unfold over time.
Acknowledgements
This study was supported by a project grant from the Wellcome Trust (082791/Z/07/Z) to ACN; MS is supported by St John’s College, Oxford. We thank Alexandra Murray for her comments and suggestions on an early version of the manuscript.
REFERENCE
- AEEGS American Electroencephalographic Society guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1991;8(2):200–202. [PubMed] [Google Scholar]
- Anderson DE, Vogel EK, Awh E. Precision in visual working memory reaches a stable plateau when individual item limits are exceeded. Journal of Neuroscience. 2011;31(3):1128–1138. doi: 10.1523/JNEUROSCI.4125-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Astle DE, Scerif G, Kuo B-C, Nobre AC. Spatial selection of features within perceived and remembered objects. Frontiers in Human Neuroscience. 2009;3:1–9. doi: 10.3389/neuro.09.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences. 2001;5(3):119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Bor D, Owen AM. Working memory: linking capacity with selectivity. Current Biology. 2006;16(4):R136–R138. doi: 10.1016/j.cub.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. Journal of Neurophysiology. 1998;80(6):2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363(6427):345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24(1):87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowey A, Rolls ET. Human cortical magnification factor and its relation to visual acuity. Experimental Brain Research. 1974;21:447–454. doi: 10.1007/BF00237163. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Dell’Acqua R, Sessa P, Toffanin P, Luria R, Jolicoeur P. Orienting attention to objects in visual short-term memory. Neuropsychologia. 2010;48(2):419–428. doi: 10.1016/j.neuropsychologia.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Drew T, Vogel EK. Neural measures of individual differences in selecting and tracking multiple moving objects. Journal of Neuroscience. 2008;28(16):4183–4191. doi: 10.1523/JNEUROSCI.0556-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. Activity in fusiform face area modulated as a function of working memory load. Cognitive Brain Research. 2001;10(3):355–364. doi: 10.1016/s0926-6410(00)00056-2. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. Journal of Cognitive Neuroscience. 2003;15(6):771–784. doi: 10.1162/089892903322370708. [DOI] [PubMed] [Google Scholar]
- Edin F, Klingberg T, Johansson P, McNab F, Tegnér J, Compte A. Mechanism for top-down control of working memory capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Kiss M. An electrophysiological measure of access to representations in visual working memory. Psychophysiology. 2010;47(1):197–200. doi: 10.1111/j.1469-8986.2009.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ester EF, Serences JT, Awh E. Spatially global representations in human primary visual cortex during working memory maintenance. Journal of Neuroscience. 2009;29(48):15258–15265. doi: 10.1523/JNEUROSCI.4388-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. Human variation in overriding attentional capture. Journal of Neuroscience. 2009;29(27):8726–8733. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2010.12.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G. The contralateral organization of visual memory: a theoretical concept and a research tool. Psychophysiology. 1998;35(6):638–647. [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Robert E. Krieger Publishing Company; Huntington: New York: 1966. [Google Scholar]
- Griffin IC, Nobre AC. Orienting attention to locations in internal representations. Journal of Cognitive Neuroscience. 2003;15(8):1176–1194. doi: 10.1162/089892903322598139. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458(7238):632–635. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai A, McCollough AW, Vogel EK. Contralateral delay activity provides a neural measure of the number of representations in visual working memory. Journal of Neurophysiology. 2010;103(4):1963–1968. doi: 10.1152/jn.00978.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Wood CC. Letter: the epsilon-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13(3):277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Jiang YV, Olson IR, Chun MM. Organization of visual-short term memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26:683–702. doi: 10.1037//0278-7393.26.3.683. [DOI] [PubMed] [Google Scholar]
- Jolicœur P, Brisson B, Robitaille N. Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain Research. 2008;1215(18):160–172. doi: 10.1016/j.brainres.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Kuo B-C, Rao A, Lepsien J, Nobre AC. Searching for targets within the spatial layout of visual short-term memory. Journal of Neuroscience. 2009;29(25):8032–8038. doi: 10.1523/JNEUROSCI.0952-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo B-C, Yeh Y-Y, Chen AJW, D’Esposito M. Functional connectivity during top-down modulation of visual short-term memory representations. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2010.12.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman R, Spekreijse H, Lamme VAF. Large capacity storage of integrated objects before change blindness. Vision Research. 2003;43(2):149–164. doi: 10.1016/s0042-6989(02)00402-9. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC. Cognitive control of attention in the human brain: Insights from orienting attention to mental representations. Brain Research. 2006;1105(1):20–31. doi: 10.1016/j.brainres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC. Attentional modulation of object representations in working memory. Cerebral Cortex. 2007;17(9):2072–2083. doi: 10.1093/cercor/bhl116. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Thornton I, Nobre AC. Modulation of working-memory maintenance by directed attention. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.03.011. in press. [DOI] [PubMed] [Google Scholar]
- Makovski T, Jiang YV. Distributing versus focusing attention in visual short-term memory. Psychonomic Bulletin & Review. 2007;14(6):1072–1078. doi: 10.3758/bf03193093. [DOI] [PubMed] [Google Scholar]
- Makovski T, Shim WM, Jiang YV. Interference from filled delays on visual change detection. Journal of Vision. 2006;6(12):1459–1470. doi: 10.1167/6.12.11. [DOI] [PubMed] [Google Scholar]
- Makovski T, Sussman R, Jiang YV. Orienting attention in visual working memory reduces interference from memory probes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(2):369–380. doi: 10.1037/0278-7393.34.2.369. [DOI] [PubMed] [Google Scholar]
- Matsukura M, Luck SJ, Vecera SP. Attention effects during visual short-term memory maintenance: protection or prioritization? Perception & Psychophysics. 2007;69(8):1422–1434. doi: 10.3758/bf03192957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollough AW, Machizawa MG, Vogel EK. Electrophysiological measures of maintaining representations in visual working memory. Cortex. 2007;43(1):77–94. doi: 10.1016/s0010-9452(08)70447-7. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. Journal of Neuroscience. 1993;13(4):1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munneke J, Heslenfeld DJ, Theeuwes J. Spatial working memory effects in early visual cortex. Brain and Cognition. 2010;72(3):368–377. doi: 10.1016/j.bandc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Murray AM, Nobre AC, Stokes MG. Markers of preparatory attention predict visual short term memory performance. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.02.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Griffin IC, Rao A. Spatial attention can bias search in visual short-term memory. Frontiers in Human Neuroscience. 2008;1:1–9. doi: 10.3389/neuro.09.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Perception & Psychophysics. 1988;44(4):369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nature Reviews Neuroscience. 2005;6(2):97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D’Esposito M. Seeking the neural substrates of visual working memory storage. Cortex. 2003;39(4-5):927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- Robitaille N, Jolicoeur P. Fundamental properties of the N2pc as an index of spatial attention: effects of masking. Canadian Journal of Experimental Psychology. 2006;60(2):101–111. doi: 10.1037/cjep2006011. [DOI] [PubMed] [Google Scholar]
- Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience. 2010;22(6):1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BK, Vogel EK, Woodman GF, Luck SJ. Voluntary and automatic attentional control of visual working memory. Perception & Psychophysics. 2002;64(5):754–763. doi: 10.3758/bf03194742. [DOI] [PubMed] [Google Scholar]
- Serences JT, Ester EF, Vogel EK, Awh E. Stimulus-specific delay activity in human primary visual cortex. Psychological Science. 2009;20(2):207–214. doi: 10.1111/j.1467-9280.2009.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG. Top-down visual activity underlying VSTM and preparatory attention. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.02.004. in press. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Thompson R, Cusack R, Duncan J. Top-down activation of shape-specific population codes in visual cortex during mental imagery. Journal of Neuroscience. 2009;29(5):1565–1572. doi: 10.1523/JNEUROSCI.4657-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VAF. A neural correlate of working memory in the monkey primary visual cortex. Science. 2001;293(5527):120–124. doi: 10.1126/science.1060496. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke ARE, Sligte IG, Lamme VAF. Manipulations of attention dissociate fragile visual short-term memory from visual working memory. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2010.12.044. in press. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6894):748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438(7067):500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Yeh Y-Y, Kuo B-C, Liu HL. The neural correlates of attention orienting in visuospatial working memory for detecting feature and conjunction changes. Brain Research. 2007;1130(26):146–157. doi: 10.1016/j.brainres.2006.10.065. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience. 2009;29(10):3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]