Abstract
In cardiovascular surgery, reduced organ perfusion and oxygen delivery contribute to increased postoperative morbidity and prolonged intensive care unit stay. Goal-directed therapy (GDT), a perioperative haemodynamic strategy aiming to increase cardiac output, is helpful in preventing postoperative complications, but studies in the context of cardiovascular surgery have produced conflicting results. The purpose of the present meta-analysis is to determine the effects of perioperative haemodynamic goal-directed therapy on mortality and morbidity in cardiac and vascular surgery. MEDLINE, EMBASE, The Cochrane Library and the DARE databases were searched until July 2011. Randomized controlled trials reporting on adult cardiac or vascular surgical patients managed with perioperative GDT or according to routine haemodynamic practice were included. Primary outcome measures were mortality and morbidity. Data synthesis was obtained by using odds ratio (OR) with 95% confidence interval (CI) by a random effects model. An OR <1 favoured GDT. Statistical heterogeneity was assessed by Q and I2 statistics. Eleven articles (five cardiac surgery and six vascular procedures), enrolling a total sample of 1179 patients, were included in the analysis. As compared with routine haemodynamic practice, perioperative GDT did not reduce mortality in either cardiac or vascular surgery (pooled OR 0.87; 95% CI 0.37–2.02; statistical power 64%). GDT significantly reduced the number of cardiac patients with complications (OR 0.34; 95% CI 0.18–0.63; P = 0.0006), but no effect was observed in vascular patients (OR, 0.84; 95% CI 0.45–1.56; P = 0.58). Perioperative GDT prevents postoperative complications in cardiac surgery patients, while it has no effect in vascular surgery. The different characteristics and comorbidities of the population enrolled could explain these conflicting results. More trials conforming to the characteristics of low-risk-of-bias studies and enrolling a larger and well-defined population of patients are needed to better clarify the effect of GDT in the specific setting of cardiovascular surgery.
Keywords: Perioperative, Haemodynamic, Goal-directed, Vascular, Cardiac, Surgery
INTRODUCTION
During surgery, organ perfusion and tissue oxygen delivery are often impaired as a consequence of the inability of the patient to face surgery-induced alterations in the cardiorespiratory and metabolic demands [1]. This particularly applies to cardiac surgery, in which episodes of reduction of gut oxygenation, related to intraoperative hypovolemia and hypotension, are frequent [2–4], leading to postoperative complications [5–9]. Inadequate oxygen delivery and higher oxygen extraction in the first 24 h after surgery [10] have been shown to be independent predictors of prolonged intensive care unit (ICU) stay [11]. The frequent coexistence of heart disease, hypertension, diabetes, and stroke in patients undergoing vascular surgery poses these patients at risk. Diminished cardiopulmonary reserve, limiting their ability to prevent oxygen delivery/demand mismatch [12], appears to be the most important factor affecting the outcome of surgery in these aged people [12].
It has been proposed that goal-directed therapy (GDT), a perioperative haemodynamic strategy aiming to increase cardiac output, could be helpful in preventing or treating tissue hypoxia and some evidence supports the benefit of this approach in septic [13] and surgical patients [14, 15]. Studies on this issue in the context of cardiovascular surgery have produced conflicting results [16, 17].
Therefore, we performed a systematic review including a meta-analysis on the effects of perioperative GDT on postoperative complications. We reviewed randomized controlled trials (RCTs) to assess the efficacy of GDT compared with standard haemodynamic management against postoperative complications in adult surgical patients undergoing cardiac and vascular procedures.
METHODS
Eligibility criteria
Studies were searched according to the following eligibility criteria [18]:
Types of participants
Adult (age ≥18 years) patients undergoing cardiac or vascular surgery were considered. Studies involving other kinds of surgeries and mixed populations of critically ill or non-surgical patients were excluded.
Type of intervention
GDT was defined as perioperative monitoring and manipulation of haemodynamic parameters to reach normal or supranormal values by fluid alone or in combination with inotropic therapy. Studies including late haemodynamic optimization treatment were excluded.
Type of comparison
Trials comparing the beneficial and harmful effects of GDT and standard haemodynamic therapy were considered. Standard haemodynamic management was defined as anaesthesiologists' routine administration of fluids and/or inotropic drugs to achieve haemodynamic stability, but not aimed to reach physiological flow-related end points and not guided by appropriate monitoring. RCTs with no description or no difference in optimization strategy between groups, RCTs with therapy titrated to the same goal in both groups or not titrated to predefined end points were excluded.
Type of outcome measures
Primary outcome measures were mortality and morbidity, evaluated as the number of patients with complications and the number of postoperative complications in cardiac and vascular surgery. Postoperative complications were classified as cardiac (e.g. acute myocardial infarction based on ECG and/or enzyme alterations, arrhythmias that required treatment, pulmonary oedema, congestive heart failure, low cardiac output syndromes, etc.) and non-cardiac (e.g. acute renal failure, liver insufficiency, gastrointestinal complications, cerebrovascular accidents, etc.). Secondary outcome was the incidence of intraoperative cardiac adverse events including hypotension, ST alterations, arrhytmias, and heart rate alterations (see Supplementary material S1 for complete list and definition of postoperative and intraoperative complications).
Types of studies
RCTs studying perioperative GDT. No language, publication date or publication status restrictions were imposed.
Information sources
Different search strategies (last update July 2011) were performed to retrieve relevant studies by using MEDLINE, The Cochrane Library and EMBASE databases. No date restriction was applied for MEDLINE and The Cochrane Library databases, while the search was limited to 2006–2011 for EMBASE database [19]. Additional RCTs were searched in The Cochrane Library and the DARE databases, and the reference lists of previously published reviews and retrieved articles, and other data sources were hand-searched in the annual proceedings (2003–2011) of the Society of Critical Care Medicine, the European Society of Intensive Care Medicine, the Society of Cardiovascular Anesthesiologists, the Royal College of Anaesthetists and the American Society of Anesthesiologists.
Search
We used the following search terms to search all trials: RCTs, controlled clinical trial, cardiac surgery, vascular surgery, cardiovascular surgery, goal directed, goal oriented, goal target, cardiac output, cardiac index, oxygen delivery, oxygen consumption, cardiac volume, stroke volume, fluid therapy, fluid loading, fluid administration, optimization, supranormal. The search strategies used for MEDLINE, The Cochrane Library and EMBASE databases are showed in Supplementary material S2.
Study selection
Two investigators first examined each title and abstract to exclude clearly irrelevant studies and to identify potentially relevant articles. Two other investigators independently determined the eligibility of the full-text articles retrieved. At this stage, the names of the authors, institution, journal of publication and results were not known to the two investigators.
Data collection process
Data were independently collected by two investigators with any discrepancy resolved by reinspection of the original article. To avoid errors in transcribing, the data were entered into the statistical software and rechecked by other investigators.
Data items
Data abstraction included patients characteristics (age and sex) and risk factors (based on physiological and operative severity—POSSUM—score [20], age >60 years and preoperative morbidity), type of surgery (i.e. elective or emergent, cardiac or vascular), type of haemodynamic GDT (monitoring tools, haemodynamic end points and therapeutic intervention), incidence (number of patients and/or number of episodes) and type and rate of intraoperative adverse events and postoperative complications.
Risk of bias in individual studies
The Scottish Intercollegiate Guidelines Network (SIGN) checklist for RCTs [21] was used to evaluate the methodological quality of RCTs. The SIGN checklist was independently filled by two investigators and whenever different, the study was further assessed to reach consensus. A double plus (++) denotes studies very unlikely to have bias, a single plus (+) denotes studies in which bias is unlikely, and a minus (−), studies with high risk of bias. A double plus was assigned to studies that adequately described all the criteria of randomization, concealment, blinding, intention-to-treat analysis and predefined outcomes, whereas a single plus was given to studies meeting only four of the five criteria. The adequacy of these five criteria is strongly associated with bias reduction [22, 23]. Regarding blinding, those studies in which the outcome was explicitly predefined, the outcome assessment was blinded or both were considered adequately masked [24].
Summary measures and planned method of analysis
Meta-analytic techniques (analysis software RevMan, version 5.1.6 Cochrane Collaboration, Oxford, UK) were used to combine studies using odds ratio (OR) and 95% confidence intervals (CI). The statistical method used for combining the results was to weight studies by the inverse variances of their effect estimates. Smaller studies, that were subject to greater sampling variation and hence were less precise, as reflected in the wider CI around the intervention effect estimate, received less weight while larger studies that gave more precise results (narrower CI s) were given more weight [24]. A statistical difference between groups was considered to occur if the pooled 95% CI did not include 1 for the OR. An OR <1 favoured GDT when compared with the control group. Two-sided P values were calculated. A random effects model was chosen for all analyses. Statistical heterogeneity and inconsistency were assessed by using the Q and I2 tests, respectively [25, 26]. When the P value of the Q-test was <0.10 and/or the I2 was >40%, heterogeneity and inconsistency were considered significant [27]. Statistical power (i.e. the probability of correctly rejecting the null hypothesis that the OR equals 1 given n treatment patients, m control patients per treatment patient, P0 the probability of the outcome for a control patient, P1 the probability of the outcome in an experimental subject and a Type I error probability α of 0.05) was calculated for each analysis and was considered adequate if ≥80%.
RESULTS
Study selection
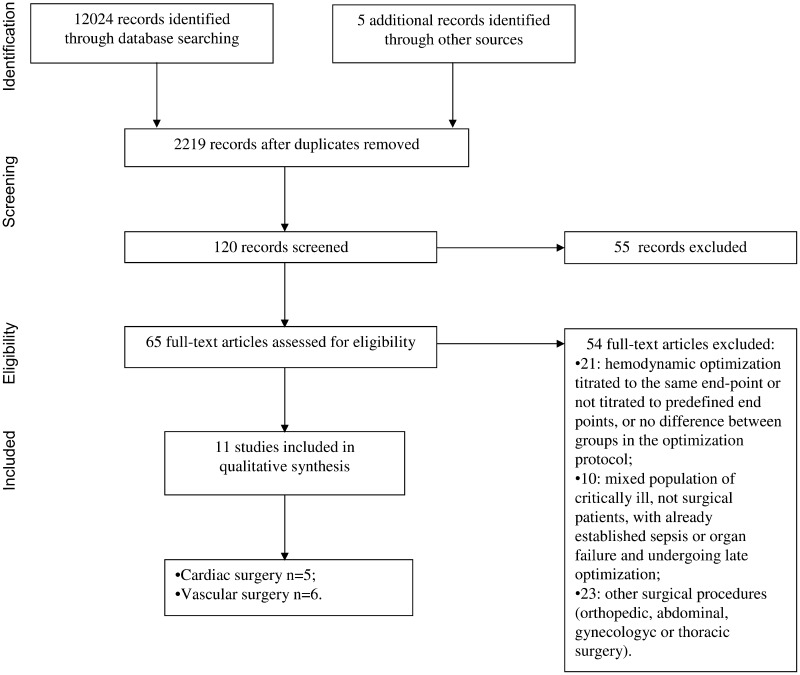
The search strategies identified 2685 (MEDLINE), 8543 (Cochrane Library) and 795 (EMBASE) articles. After initial screening and subsequent more detailed selection, a pool of 65 potentially relevant RCTs was identified. The eligibility process (see Fig. 1) excluded 54 articles and, therefore, 11 articles [4, 16, 17, 28–35], enrolling a total sample of 1179 patients, were included in the analysis.
Figure 1:
Outline of studies selection process. Flow chart summarizing the procedure of studies selection for the meta-analysis.
Study characteristics
All selected articles were RCTs evaluating the effects of GDT on postoperative mortality and morbidity, as primary or secondary outcome, and had a population sample of adult patients undergoing cardiac or vascular surgical procedures. Of 11 studies, five included cardiac surgery [4, 17, 31–33] and six vascular procedures [16, 28–30, 34, 35]. Six studies [4, 17, 30, 32–34] were performed in Europe, four [16, 28, 29, 35] in the USA, and one [31] in India, from 1991 to 2010.
All studies provided data about mortality and morbidity with the incidence of single postoperative complications. Intraoperative adverse events were clearly defined and reported in all except one [34] of the six vascular surgery studies; no cardiac study reported the incidence of intraoperative adverse events. Eight studies [4, 16, 28–32, 35] clearly classified and reported postoperative complications, according to the definitions provided in the Supplementary material S1. Data concerning RCTs quality assessment, type of surgery, timing, goals, monitoring, and modality of perioperative GDT are presented in Table 1. The methodological evaluation, according to the SIGN score, showed that only 4 of 11 studies were considered to be high-quality studies.
Table 1.
Quality assessment, sample characteristics and intervention details of the included studies
| Author (year), country [reference] | SIGN score | SIGN comment | Risk | Surgery | Timing | Tools and goals | Modality of optimization |
|---|---|---|---|---|---|---|---|
| Berlauk et al. (1991), USA [28] | − | Concealment and trial flow not described | Elective peripheral vascular | Preop | PAC; CI ≥ 2.8 l min−1 m−2, 8 ≤ Pcwp ≤ 14 mmHg, SVR ≤ 1100 dyne s cm−5 |
Fluids and inotropes | |
| Bender et al. (1997), USA [29] | − | Randomization and concealment not clear | Elective aortic and vascular | Preop | PAC; CI ≥ 2.8 l min−1 m−2, 8 ≤ Pcwp ≤ 14 mmHg, SVR ≤ 1100 dyne s cm−5 |
Fluids and inotropes | |
| Bonazzi et al. (2002), Italy [30] | − | concealment and trial flow not described | Elective vascular | Preop | PAC; CI ≥ 3 l min−1 m−2, 10 ≤ Pcwp ≤ 18 mmHg, SVR ≤ 1450 dyne s cm−5 |
Fluids and inotropes | |
| Kapoor et al. (2008), India [31] | + | Randomization not clear | Moderate to high risk | Elective cardiac (on-pump) | Postop | FloTrac™; CI ≥ 2.5 l min−1 m−2, SVV ≤ 10%, ScVO2 >70% | Fluids and inotropes |
| McKendry et al. (2004), England [17] | + | Complication not defined | Elective cardiac (on-pump) | Postop | Oesophageal Doppler; SI > 35 ml/m2 |
Fluids | |
| Mythen et al. (1995), England [4] | − | Randomization not clear, flow of patient not described | High risk | Elective cardiac (off-pump) | Intraop | Oesophageal Doppler; SV optimization and rise in CVP < 3 mmHg |
Fluids |
| Polonen et al. (2000), Europe [32] | + | Randomization not clear | Elective cardiac (on-pump) | Postop | PAC; SvO2 > 70%, lactate ≤ 2.0 mmol/L | Fluids and inotropes | |
| Smetkin et al. (2009), Russia [33] | − | Randomization not adequate, not blinded, concealment not described | ASA II and III | Elective cardiac (off-pump) | Intraop | PiCCO plus; ITBV 850–1000 ml m−1, ScvO2 > 60% |
Fluids and inotropes |
| Valentine et al. (1998), USA [16] | − | Randomization not clear, not blinded | Elective aortic | Preop | PAC; CI ≥ 2.8 l min−1 m−2 8 ≤ Pcwp ≤ 15 mmHg, SVR ≤ 1100 dyne s cm−5 |
Fluids and inotropes | |
| Van der Linden et al. (2010), Belgium [34] | ++ | ASA II and III | Elective peripheral vascular | Intraop | FloTrac™; CI ≥2 l min−1 m−2 CVP ≤ 15 mmHg | Fluids and inotropes | |
| Ziegler et al. (1997), USA [35] | − | Randomization and concealment not clear, flow of patient not described | Elective vascular (aortic and limb salvage) | Preop | PAC; SvO2 ≥ 65%, Hb ≥ 10 g/dl, Pcwp ≥ 12 mmHg | Fluids and inotropes |
ASA: American Society of Anesthesiologists; Preop: preoperatively; intraop: intraoperatively; postop: postoperatively; CI: cardiac index; Pcwp: pulmonary capillary wedge pressure; SV: stroke volume; SVV: stroke volume variation; CVP: central venous pressure; SI: stroke index; PAC: pulmonary artery catheter; SVR: systemic vascular resistance; ITBVI: intrathoracic blood volume index; ScvO2: central venous oxygen saturation; Hb: haemoglobin; SvO2: mixed venous oxygen saturation; SIGN: Scottish Intercollegiate Guidelines Network checklist for RCTs.
‘++’ describes studies with very unlikely bias, ‘+’ describes studies with unlikely bias and ‘−’ describes studies with high risk of bias. See text for risk definition.
In five of six studies concerning vascular surgery [16, 28–30, 35], haemodynamic monitoring and management started before surgery, and in one [34], it was performed intraoperatively. In one study [29], treatment was started either 12 h or 3 h before surgery; both groups were pooled together for the purpose of the analysis. In two cardiac studies [4, 33], GDT was started during surgery, while in the remaining three GDT [17, 31, 32] was started postoperatively (Table 1).
In six studies (five of them concerning vascular surgery) [16, 28–30, 32, 35], haemodynamic monitoring was performed with pulmonary artery catheter (PAC), with oxygen delivery, cardiac output, mixed venous oxygen saturation and lactate as goal parameters. In one study [33], the PICCO plus (single transpulmonary dilution Cardiac Output) system was used to measure cardiac output. In two studies [31, 34], a non-invasive cardiac output measurement (FloTrac), based on the analysis of arterial waveform, was performed, associated with central venous oxygen saturation (ScvO2). In two studies [4, 17], an oesophageal Doppler was used and stroke volume guided haemodynamic optimization. In all studies, except two [4, 17], the treatment group received both fluids (crystalloids and/or colloids and/or blood) and inotropes (dopamine, dobutamine, dopexamine or epinephrine) with vasodilators (Table 1).
In six [16, 28, 29, 30, 31, 33] studies, control groups were not advanced monitored and no flow-related data are available.
Only two studies [17, 33] reported a potential conflict of interest.
Quantitative data synthesis
Mortality
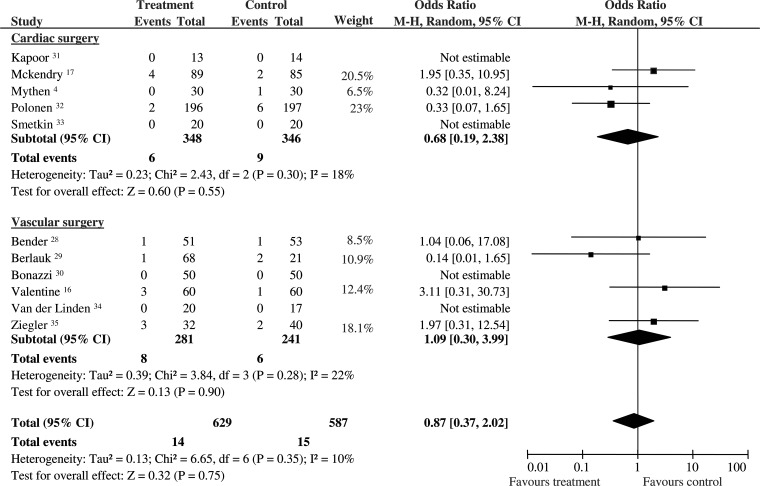
Twenty-nine patients died: 15/587 (2.5%) had been randomized to control and 14/629 (2.2%) to the perioperative GDT group. Pooled OR was 0.87, and 95% CI was 0.37–2.02 (11 RCTs). No statistical heterogeneity and inconsistency were detected. The statistical power was 64%. Sub-group analysis confirmed that GDT did not reduce mortality in cardiac (OR 0.68; 95% CI 0.19–2.38; 694 pts; 5 RCTs; P = 0.55) and vascular patients (OR 1.09; 95% CI 0.30–3.99; 522 pts; 6 RCTs; P = 0.90). No statistical heterogeneity and inconsistency were observed in the two sub-groups (Fig. 2).
Figure 2:
Mortality. Rates of postoperative mortality for each of the studies with ORs and 95% Cl. The studies were divided into two sub-groups defined as cardiac and vascular surgery. The pooled OR and 95% CI are shown as the total. The size of the box at the point estimate of the OR gives a visual representation of the ‘weighting’ of the study. The diamond represents the point estimate of the pooled OR and the length of the diamond is proportional to the CI. OR: odds ratios; 95% CI: 95% confidence intervals.
Patients with complications
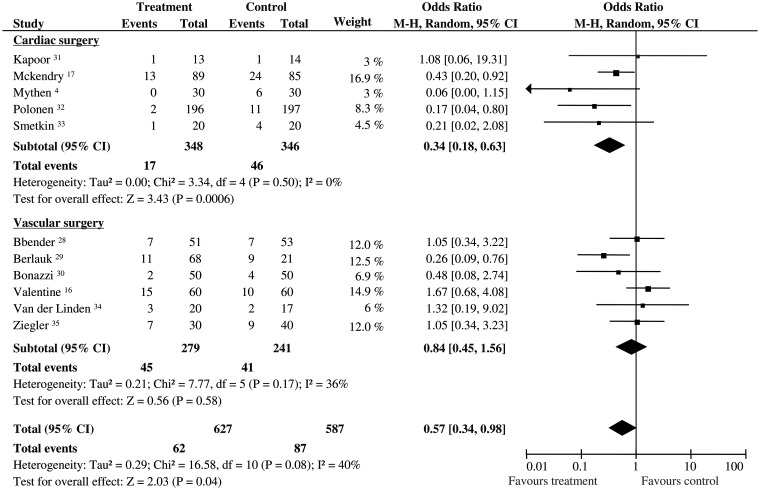
About 149 patients developed postoperative complications: 87/587 (14.8%) had been randomized to control group and 62/627 (9.8%) to GDT. Pooled OR was 0.57, and 95% CI was 0.34–0.98 (11 RCTs). The statistical power was 74.5%. Significant statistical heterogeneity and inconsistency were detected (Q statistic P = 0.08; I2 = 40%). Sub-group analyses demonstrated that GDT significantly reduced the number of cardiac patients with complications (OR 0.34; 95% CI 0.18–0.63; 694 pts; 5 RCTs; P = 0.0006, statistical power 97%), but no effect was observed in vascular patients (OR 0.84; 95% CI 0.45–1.56; 520 pts; 6 RCTs; P = 0.58, statistical power 59%). No statistical heterogeneity and inconsistency were observed in the two sub-groups (Fig. 3).
Figure 3:
Patients with postoperative complications. Rates of patients with postoperative complications for each of the studies with ORs and 95% Cl. The studies were divided into two sub-groups defined as cardiac and vascular surgery. The pooled OR and 95% CI are shown as the total. The size of the box at the point estimate of the OR gives a visual representation of the ‘weighting’ of the study. The diamond represents the point estimate of the pooled OR and the length of the diamond is proportional to the CI. OR: odds ratios; 95% CI: 95% confidence intervals.
Cardiac and non-cardiac intraoperative and postoperative complications in vascular surgery
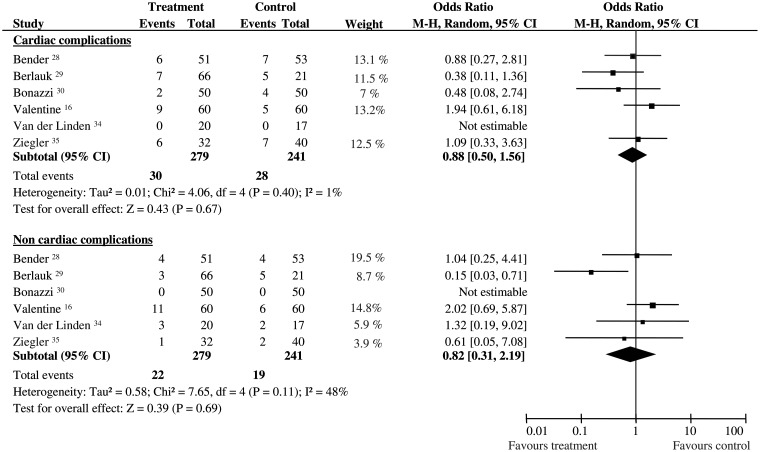
In vascular surgery, no difference was observed between groups in cardiac intraoperative adverse events (OR 1.10; 95% CI 0.66–1.83; 520 pts; P = 0.72, Q-test: P = 0.002; I2 = 77%). With regard to postoperative complications, no differences were found in cardiac (OR 0.88; 95% CI 0.50–1.56; 520 pts; P = 0.67, no statistical heterogeneity and inconsistency, statistical power 63%) and non-cardiac (OR 0.82; 95% CI 0.31–2.19; 520 pts; P = 0.69, Q-test: P = 0.11, I2 = 48%, statistical power 50%) complications (Fig. 4).
Figure 4:
Postoperative complications in vascular surgery. Rates of postoperative complications for each of the studies involving vascular surgery with ORs and 95% Cl. The studies were divided into two sub-groups defined as cardiac and non-cardiac complications (see text for details). The pooled OR and 95% CI are shown as the total. The size of the box at the point estimate of the OR gives a visual representation of the ‘weighting’ of the study. The diamond represents the point estimate of the pooled OR and the length of the diamond is proportional to the CI. OR: odds ratios; 95% CI: 95% confidence intervals.
Cardiac and non-cardiac postoperative complications in cardiac surgery
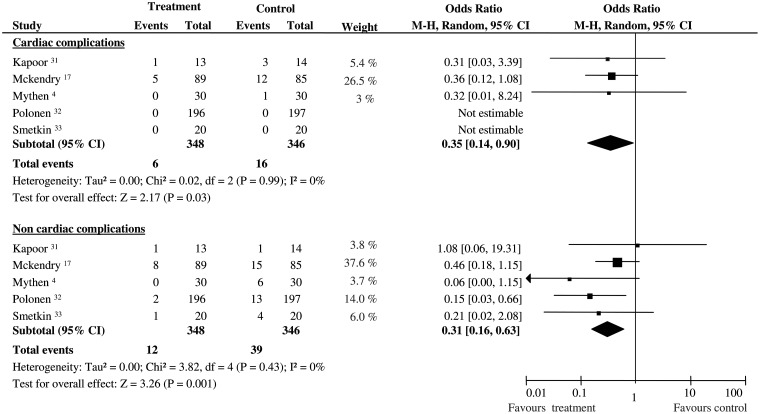
The number of postoperative cardiac complications was significantly different between GDT and control group (OR 0.35; 95% CI 0.14–0.90; 694 pts; P = 0.03, statistical power 58.8%), as well as non-cardiac postoperative complications (OR 0.31; 95% CI 0.16–0.63; 694 pts; P = 0.001, statistical power 97.8%). No statistical heterogeneity and inconsistency were observed in the two sub-groups (Fig. 5).
Figure 5:
Postoperative complications in cardiac surgery. Rates of postoperative complications for each of the studies involving cardiac surgery with ORs and 95% Cl. The studies were divided into two sub-groups defined as cardiac and non-cardiac complications (see text for details). The pooled OR and 95% CI are shown as the total. The size of the box at the point estimate of the OR gives a visual representation of the ‘weighting’ of the study. The diamond represents the point estimate of the pooled OR and the length of the diamond is proportional to the CI. OR: odds ratios; 95% CI: 95% confidence intervals.
DISCUSSION
The results of the present meta-analysis show that perioperative GDT is effective in reducing postoperative complications in cardiac surgical patients, while it cannot decrease postoperative complications in vascular surgery. No difference between GDT and standard haemodynamic treatment could be detected in mortality in both cardiac and vascular surgery.
Traditionally, in cardiac surgery, postoperative complications have been related to the impairment between oxygen delivery and uptake [3]. During cardiopulmonary by-pass, high lactate levels, as an index of inadequate oxygen delivery, have been observed [36–38]. In off-pump procedures, intraoperative hypoperfusion, as expressed by gastric mucosal acidosis, is common and has been associated with an increased risk for postoperative complications [9]. In the postoperative period, higher oxygen extraction and low SvO2 values, implying an unmatched oxygen demand, have been associated with prolonged ICU stay and increased morbidity [39, 40]. Preventing or paying back soon after tissue oxygen debt has been shown to reduce postoperative complications [13]. This may be accomplished by the strategy of GDT, referring to the monitoring and manipulation of oxygen transport parameters by means of fluids, red blood cells and/or inotropic drugs. In major surgery, GDT reduces postoperative dysfunction of organs particularly at risk of hypoperfusion and hypoxia [41, 42]. This meta-analysis, pooling patients undergoing both intra- (off-pump procedures) and postoperative (on-pump) GDT, demonstrated that, in cardiac surgical patients, the number of patients with postoperative complications may be significantly reduced. Interestingly, there was a significant reduction in both specific cardiac and non-cardiac complications. This result fits well with previous analyses in which a significant reduction in kidney (4220 patients with 3 of 20 studies enrolling cardiac patients) and gastrointestinal (major surgery excluding cardiac surgery) dysfunction was observed, and emphasizes the role of specific organ perfusion during surgical stress [41, 42].
In contrast to cardiac surgery, vascular surgical patients do not seem to benefit from this approach. The patients undergoing surgery for vascular disease tend to be elderly, diabetic, and with a reduced cardiopulmonary reserve [12]. These particular characteristics would theoretically take advantage of a strategy of haemodynamic tune up for surgery through PAC or other haemodynamic monitoring. However, although in most vascular studies, GDT was started preoperatively and was continued till the postoperative phase, no improvement in the number of complications could be observed. A hypothesis to explain this different result is that, in this particular setting of patients, the expected advantage of preventing tissue hypoxia could have been counterbalanced by an increase in the number of adverse events. An aggressive use of fluids (actually in all studies GDT group received more fluids) and catecholamines might have carried potential complications such as arrhythmias, or a mismatch between myocardial oxygen supply and requirements with the risk of myocardial ischaemia [43]. However, analysing both intraoperative adverse events (including hypotension, arrythmias, ST depression or pulmonary oedema) or postoperative cardiac complications (including congestive heart failure, acute myocardial infarction, arrythmias and pulmonary oedema), no difference was detected between groups. An alternative explanation might have been the inclusion criteria. Most vascular studies excluded severe cardiopathic patients, and by preselecting patients who are at lower risk for adverse cardiac complications, any beneficial role for GDT would expectedly be more difficult to estimate. A recent review [44] substantiates this concept, demonstrating that higher the patient risk, higher the benefit of GDT. Therefore, while it is intellectually appealing to use a PAC in a population with a known incidence of cardiac abnormalities and postoperative complications, maybe its real utility is limited to a restricted, well-defined group of high-risk patients.
In patients undergoing both cardiac and vascular surgery, GDT did not reduce mortality. In patients undergoing major surgery, the occurrence of any major complications in the 30-day postoperative period is more important than preoperative risk and intraoperative factors in determining short- and long-term survivals [45]. Therefore, while in vascular surgery the absence of any reduction in mortality may be explained by the absence of benefit in postoperative complication occurrence, in cardiac surgery the mortality result is less obvious. However, the overall low control-group mortality in cardiac surgical patients (<3%) may help in explaining this result. Shoemaker et al. [46] found that GDT was effective only in those studies when optimization treatment was performed in high-risk surgical patients (i.e. control group mortality >20%), and this result has been recently confirmed [44]. However, it is not yet clear whether the absence of mortality reduction with GDT is due to a lack of GDT efficacy (patients who are not very ill may not really benefit) or if it is due to a low statistical power of the analysis, needing a much larger numbers of patients since the event rate is low in the control group. Moreover, no events were observed in both groups in 4 of the 11 included studies, further reducing the overall sample size of this analysis.
Limitations
The main limitations of all meta-analyses include reporting bias, quality assessment, end point definition and methodological heterogeneity of the included studies.
Publication bias refers to the propensity of trials with positive results to be published as full text and of trials with negative results not to be published or published only in abstract form. In order to reduce this bias, an attempt was made to include all grey and published reports [47] that met the inclusion criteria, and to retrieve unpublished data by contacting the authors of the studies. No abstract and no unpublished data were retrieved. Available statistical tests are not accurate enough to detect publication bias [48] and asymmetry in funnel plots is not an accurate predictor of publication bias [48].
Biased effect estimates may be produced by sub-optimal quality of RCTs, since less rigorous studies are biased toward overestimating an intervention's effectiveness and result in ‘false positive’ conclusions. Quality assessment was evaluated by the SIGN checklist that gives particular weight to the domains most relevant to the control of bias (randomization, concealment, blinding, intention-to-treat analysis and predefined outcomes). In the present meta-analysis, most RCTs presented not clear randomization and concealment, and blinding was not always feasible. However, some studies take other measures to reduce the risk of bias, such as adopting objective, well-defined outcomes, that are less prone to risk of bias than subjective ones. Eight trials of the present meta-analysis clearly and uniformly defined postoperative complications, while in three studies, complications were not adequately defined. Moreover, selection bias (i.e. systematic differences between baseline characteristics of the groups that are compared) could affect the reliability of the results of RCTs, and could explain, for example, the apparently conflicting results between two similar studies [28, 29]. For all these reasons, only four studies obtained a SIGN score of at least one plus. Owing to the low number of studies, a quality-sensitive analysis including studies with low risk of bias (SIGN evaluation ++ or +) was not performed.
Two studies [17, 33] clearly reported a potential conflict of interests. In both trials, however, these potential conflicts of interest are unlikely to lead directly to a risk of bias [49], since ethical approval was obtained in both trials and a sample size calculation was performed in the one by Mckendry.
The major problem with meta-analysis is the differences between RCTs included. First, the timing of GDT differs markedly between the studies, since some studies started haemodynamic monitoring and management before surgery, some started during surgery, and some started in the postoperative period. GDT before surgery has been shown to be effective, but difficult to institute because of resource constraints. Shoemaker et al. [14] have observed that if flow and oxygen debts developing at the time of surgical stress are paid back soon after, that is within 8 h, the incidence of postoperative complications may equally decrease, but if it is never paid back cell dysfunction and death occur. Since there is increasing evidence that both intra- and postoperative optimization work well, these are alternative strategies when preoptimization is not possible, allowing the avoidance of preoperative haemodynamic monitoring, which is difficult to pursue if resources are limited or if the time prior to surgery is not sufficient. A recent meta-analysis [41] substantiates this hypothesis, demonstrating that, from a ‘renal standpoint’, haemodynamic optimization performed during or soon after surgery is a feasible alternative when preoperative optimization is difficult to pursue.
Of equal concern are the differences in the technique of haemodynamic monitoring. In some studies, the PAC was used, in one, the PICCO system was used, in two, the FloTrac system was used, and in two, an oesophageal Doppler was used. These methodologies are not necessarily equivalent or equally accurate. Furthermore, in most studies, GDT consisted of both administration of fluids and inotropes, but in two, only fluids were used as part of the ‘therapy’.
No significant difference in specific complications (except for non-cardiac complications in cardiac patients) were observed between GDT and control group: the very low statistical power of these analyses, due to the low event rate, may explain this finding and does not allow drawing any meaningful clinical conclusion, calling for further trials.
Research agenda
Owing to the variability in the methodology and definition of postoperative complications among studies, further prospective randomized controlled studies are warranted to investigate the relationship between haemodynamic GDT and postoperative complications, conforming to the characteristics of low risk of bias studies and adopting widely accepted and clinically relevant definitions for each specific outcome. Moreover, further studies on this topic should be powered enough to determine whether in cardiac patients, GDT is really able to reduce mortality in low-risk patients also.
CONCLUSIONS
Within the limitations of existing data and the analytic approaches employed in the present meta-analysis, perioperative GDT prevents postoperative complications in cardiac surgery patients, but produces no benefit in vascular surgery.
The occurrence of any postoperative complication is the most important factor in determining patient's survival and, therefore, perioperative strategies aiming to prevent organ dysfunction are highly necessary in clinical practice. A very recent meta-analysis [50] has addressed this question, confirming that GDT is associated with an overall improvement of perioperative outcome, while no effect of GDT was found in mortality. This article, however, excluded cardiac trials and pooled together different surgical procedures (i.e., vascular and major abdominal). Therefore, with the present state of knowledge, no definite conclusion can be made.
Further research may be required to resolve the controversial issue of optimal perioperative haemodynamic strategy, controlling for specific sub-groups of surgery and adopting similar haemodynamic protocols.
SUPPLEMENTARY MATERIAL
Funding
Support was provided solely from departmental sources.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors express their sincere gratitude to Vincent Pellegrino, Senior Staff Specialist, Intensive Care Unit, the Alfred Hospital and Monash Medical Centre, Melbourne.
REFERENCES
- 1.Shoemaker WC, Appel PL, Kram HB. Haemodynamic and oxygen transport responses in survivors and nonsurvivors of high-risk surgery. Crit Care Med. 1993;21:977–90. doi: 10.1097/00003246-199307000-00010. doi:10.1097/00003246-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Okano N, Miyoshi S, Owada R, Fujita N, Kadoi Y, Saito S, et al. Impairment of hepatosplanchnic oxygenation and increase of serum hyaluronate during normothermic and mild hypothermic cardiopulmonary bypass. Anesth Analg. 2002;95:278–86. doi: 10.1097/00000539-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Jakob SM, Ruokonen E, Takala J. Assessment of the adequacy of systemic and regional perfusion after cardiac surgery. Br J Anaesth. 2000;84:571–7. doi: 10.1093/bja/84.5.571. doi:10.1093/bja/84.5.571. [DOI] [PubMed] [Google Scholar]
- 4.Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423–29. doi: 10.1001/archsurg.1995.01430040085019. doi:10.1001/archsurg.1995.01430040085019. [DOI] [PubMed] [Google Scholar]
- 5.Laffey JG, Boylan J. The systemic inflammatory response to cardiac surgery. Anestesiology. 2002;97:215–52. doi: 10.1097/00000542-200207000-00030. doi:10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Davies H. Systemic inflammatory response syndrome. Br J Surg. 1997;84:920–35. doi: 10.1002/bjs.1800840707. doi:10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- 7.Grover FL. The Society of Thoracic Surgeons National Database: current status and future directions. Ann Thorac Surg. 1999;68:367–73. doi: 10.1016/s0003-4975(99)00599-8. doi:10.1016/S0003-4975(99)00599-8. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH, Wragge T, Pasque C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest. 1995;107:1395–401. doi: 10.1378/chest.107.5.1395. doi:10.1378/chest.107.5.1395. [DOI] [PubMed] [Google Scholar]
- 9.Mythen MG, Webb AR. Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Cure Med. 1994;20:99–104. doi: 10.1007/BF01707662. doi:10.1007/BF01707662. [DOI] [PubMed] [Google Scholar]
- 10.Routsi C, Vincent J-L, Bakker J, De-Backer D, Lejeune P, d'Hollander A, et al. Relation between oxygen consumption and oxygen delivery in patients after cardiac surgery. Anesth Analg. 1993;77:1104–10. doi: 10.1213/00000539-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ryan TA, Rady MY, Bashour CA, Leventhal M, Lytle B, Starr NJ. Predictors of outcome in cardiac surgical patients with prolonged intensive care stay. Chest. 1997;112:1035–42. doi: 10.1378/chest.112.4.1035. doi:10.1378/chest.112.4.1035. [DOI] [PubMed] [Google Scholar]
- 12.Babu SC, Sharma PV, Raciti A, Mayr CH, Jr, Elrable NA, Clauss RH, et al. Monitor-guided responses. Arch Surg. 1980;115:1384–6. doi: 10.1001/archsurg.1980.01380110116018. doi:10.1001/archsurg.1980.01380110116018. [DOI] [PubMed] [Google Scholar]
- 13.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. doi:10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 14.Shoemaker WC, Appel PL, Kram HB. Prospective trial of supranormal values of survivors as therapeutic goals in high risk surgical patients. Chest. 1988;94:1176–86. doi: 10.1378/chest.94.6.1176. doi:10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 15.Boyd O, Grounds M, Bennett DE. A randomised clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270:2699–708. doi:10.1001/jama.1993.03510220055034. [PubMed] [Google Scholar]
- 16.Valentine RJ, Duke ML, Inman MH, Grayburn PA, Hagino RT, Kakish HB, et al. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg. 1998;27:203–11. doi: 10.1016/s0741-5214(98)70351-9. doi:10.1016/S0741-5214(98)70351-9. [DOI] [PubMed] [Google Scholar]
- 17.McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M. Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ. 2004;329:258. doi: 10.1136/bmj.38156.767118.7C. doi:10.1136/bmj.38156.767118.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. doi:10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefebre C, Manheimer E, Glanville J. Searching for studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6. Version 5.0.1. The Cochrane Collaboration; 2008. (updated September 2008) [Google Scholar]
- 20.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–60. doi: 10.1002/bjs.1800780327. doi:10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 21. Scottish Intercollegiate Guidelines Network (SIGN) 50: A Guideline Developer's Handbook http://www.sign.ac.uk/guidelines/fulltext/50/index.html. (12 June 2012, date last accessed)
- 22.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. doi:10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. J Am Med Assoc. 1999;282:1054–60. doi: 10.1001/jama.282.11.1054. doi:10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. Chapter 8. Version 5.0.1 (updated September 2008) www.cochrane-handbook.org. (12 June 2012, date last accessed) [Google Scholar]
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. doi:10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. doi:10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeks JJ, Higgins JPT, Altman DG. Analysing data undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. Chapter 9. Version 5.0.1 (updated September 2008) www.cochrane-handbook.org. (12 June 2012, date last accessed) [Google Scholar]
- 28.Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery: a prospective, randomized clinical trial. Ann Surg. 1991;214:289–99. doi: 10.1097/00000658-199109000-00011. doi:10.1097/00000658-199109000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender JS, Smith-Meek MA, Jones CE. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg. 1997;226:229–36. doi: 10.1097/00000658-199709000-00002. doi:10.1097/00000658-199709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonazzi M, Gentile F, Biasi GM, Migliavacca S, Esposti D, Cipolla M, et al. Impact of perioperative haemodynamic monitoring on cardiac morbidity after major vascular surgery in low risk patients. A randomised pilot trial. Eur J Vasc Endovasc Surg. 2002;23:445–51. doi: 10.1053/ejvs.2002.1617. doi:10.1053/ejvs.2002.1617. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor PM, Kakani M, Chowdhury U, Choudhury M, Lakshmy R, Kiran U. Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Ann Card Anaesth. 2008;11:27–34. doi: 10.4103/0971-9784.38446. doi:10.4103/0971-9784.38446. [DOI] [PubMed] [Google Scholar]
- 32.Polonen P, Ruokonen E, Hippelainen M, Pöyhönen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg. 2000;90:1052–9. doi: 10.1097/00000539-200005000-00010. doi:10.1097/00000539-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Smetkin AA, Kirov MY, Kuzkov VV, Lenkin AI, Eremeev AV, Slastilin VY, et al. Single transpulmonary thermodilution and continuous monitoring of central venous oxygen saturation during off-pump coronary surgery. Acta Anaesthesiol Scand. 2009;53:505–14. doi: 10.1111/j.1399-6576.2008.01855.x. doi:10.1111/j.1399-6576.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 34.Van der Linden PJ, Dierick A, Wilmin S, Bellens B, De Hert SG. A randomized controlled trial comparing an intraoperative goal-directed strategy with routine clinical practice in patients undergoing peripheral arterial surgery. Eur J Anaesthesiol. 2010;27:788–93. doi: 10.1097/EJA.0b013e32833cb2dd. doi:10.1097/EJA.0b013e32833cb2dd. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler DW, Wright JG, Coban PS, Flancbaum L. A prospective randomized trial of preoperative ‘optimization’ of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery. 1997;122:584–92. doi: 10.1016/s0039-6060(97)90132-x. doi:10.1016/S0039-6060(97)90132-X. [DOI] [PubMed] [Google Scholar]
- 36.Ranucci M, Romitti F, Isgrò G, Cotza M, Brozzi S, Boncilli A, et al. Oxygen delivery during cardiopulmonary by-pass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–20. doi: 10.1016/j.athoracsur.2005.05.069. doi:10.1016/j.athoracsur.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 37.Chioléro RL, Revelly JP, Leverve X, Gersbach P, Cayeux MC, Berger MM, et al. Effect of cardiogenic shock on lactate and glucose metabolism after heart surgery. Crit Car Med. 2000;28:3784–91. doi: 10.1097/00003246-200012000-00002. doi:10.1097/00003246-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Demers P, Elkouri S, Martineau R, Couturier A, Cartier R. Outcome with high blood lactate levels during cardiopulmonary by-pass in adult cardiac operation. Ann Thorac Surg. 2000;70:2882–6. doi: 10.1016/s0003-4975(00)02160-3. [DOI] [PubMed] [Google Scholar]
- 39.Svedjeholm R, HAkansson E, Vanhanen I. The short-term prognostic value of SvOz measurement on admission to ICU after CABG surgery. Br J Anesth. 1995;74(Suppl 2):A40. [Google Scholar]
- 40.Routsi C, Vincent JL, Bakker J, De Backer D, Lejeune P, d'Hollander A, et al. Relation between oxygen consumption and oxygen delivery in patients after cardiac surgery. Anesth Analg. 1993;77:1104–10. doi: 10.1213/00000539-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–90. doi: 10.1097/CCM.0b013e3181a00a43. doi:10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- 42.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–46. doi: 10.1093/bja/aep279. doi:10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 43.Harten J, Kinsella J. Perioperative optimisation. Scott Med J. 2003;49:6–9. doi: 10.1177/003693300404900102. [DOI] [PubMed] [Google Scholar]
- 44.Gurgel ST, do Nascimento P. Maintaining tissue perfusion in high-risk surgical patients: a systematic review of randomized clinical trials. Anesth Analg. 2011;112:1384–91. doi: 10.1213/ANE.0b013e3182055384. doi:10.1213/ANE.0b013e3182055384. [DOI] [PubMed] [Google Scholar]
- 45.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–43. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patient. Crit Care Med. 2002;30:1686–92. doi: 10.1097/00003246-200208000-00002. doi:10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 47.McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–31. doi: 10.1016/S0140-6736(00)02786-0. doi:10.1016/S0140-6736(00)02786-0. [DOI] [PubMed] [Google Scholar]
- 48.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. doi:10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higgins JPT, Deeks JJ. Selecting studies and collecting data. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Chapter 7. Version 5.1.0 (updated March 2011) www.cochrane-handbook.org. (12 June 2012, date last accessed) [Google Scholar]
- 50.Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012;114:640–51. doi: 10.1213/ANE.0b013e318240d6eb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.