Abstract
In an earlier paper we showed that in fully developed barley (Hordeum vulgare L.) root epidermal cells a decrease in cytosolic K+ was associated with an acidification of the cytosol (D.J. Walker, R.A. Leigh, A.J. Miller [1996] Proc Natl Acad Sci USA 93: 10510–10514). To show that these changes in cytosolic ion concentrations contributed to the decreased growth of K+-starved roots, we first measured whether similar changes occurred in cells of the growing zone. Triple-barreled ion-selective microelectrodes were used to measure cytosolic K+ activity and pH in cells 0.5 to 1.0 mm from the root tip. In plants growing from 7 to 21 d after germination under K+-replete conditions, the mean values did not change significantly, with values ranging from 80 to 84 mm for K+ and 7.3 to 7.4 for pH. However, in K+-starved plants (external [K+], 2 μm), the mean cytosolic K+ activity and pH had declined to 44 mm and 7.0, respectively, after 14 d. For whole roots, sap osmolality was always lower in K+-starved than in K+-replete plants, whereas elongation rate and dry matter accumulation were significantly decreased after 14 and 16 d of K+ starvation. The rate of protein synthesis in root tips did not change for K+-replete plants but declined significantly with age in K+-starved plants. Butyrate treatment decreased cytosolic pH and diminished the rate of protein synthesis in K+-replete roots. Procaine treatment of K+-starved roots gave an alkalinization of the cytosol and increased protein synthesis rate. These results show that changes in both cytosolic pH and K+ can be significant factors in inhibiting protein synthesis and root growth during K+ deficiency.
The functions of K+ in plant cells can be divided into those that are biophysical, such as osmoregulation, and those that are biochemical, such as protein synthesis and enzyme activation (Leigh and Wyn Jones, 1984). Although there has been work demonstrating the inhibitory effect of K+ deprivation on root growth (Asher and Ozanne, 1967; Siddiqi and Glass, 1983; White, 1993), the underlying biophysical and/or biochemical mechanisms have not been explained. Leigh and Wyn Jones (1984) proposed that a decline in cytosolic [K+] below the optimum level for protein synthesis (100–150 mm; Wyn Jones et al., 1979) was the initial cause of growth reduction under K+ deprivation. “Critical” root-tissue sap [K+] has been defined as the tissue K+ concentration at which growth declines below 90% of the maximum (Ulrich and Hills, 1967). This has been measured to be between 20 and 25 mm (Spear et al., 1978; White, 1993), providing support for the hypothesis of Leigh and Wyn Jones (1984) that growth begins to decrease when vacuolar [K+] reaches a “minimum” value of 10 to 20 mm. Presumably, at this concentration vacuolar K+ can no longer be used to maintain cytosolic K+. Declining vacuolar K+ could inhibit root growth, since K+ is an important osmoticum and its accumulation in newly formed vacuoles drives cell expansion (Cram, 1976). Although the beneficial effect of increased K+ supply on wall extensibility has been demonstrated (Métraux and Taiz, 1977), the presence of K+ salts in the nutrient solution can inhibit root-cell elongation by reducing cell wall plasticity in the root-expansion zone (Pritchard et al., 1987). The effect of K+ on root elongation seems to be species specific (Pritchard, 1994), cultivar specific (Hackett, 1968), and root-type specific (Hackett, 1968; Triboulot et al., 1997).
The aim of this work was to relate measurements of pHc and K+ activity to the growth of barley (Hordeum vulgare L.) roots. We previously used triple-barreled microelectrodes to measure aK, pH, and Em simultaneously in mature cortical and epidermal cells 10 to 20 mm from the tip of seminal roots of barley (Walker et al., 1995, 1996). The cytosolic aK and pH of epidermal, but not cortical, cells declined during K+ deprivation (Walker et al., 1996). However, it is the meristematic and rapidly expanding cells within 2 mm of the root tip where the rates of protein synthesis are highest in roots (Wareing and Phillips, 1981; Curl and Truelove, 1986). A decrease in cytosolic aK in expanding cells would be expected to diminish rates of protein synthesis, leading to a decrease in the rates of root elongation and dry matter production (Leigh and Wyn Jones, 1984; White, 1993). Changes in pHc have been shown to alter the rate of protein synthesis in both animal (Grandin and Charbonneau, 1989) and plant cells (Webster et al., 1991). To test this hypothesis, pHc and protein synthesis were measured in root tips treated with butyrate or procaine to alter pHc in seedlings growing under K+-starved or K+-replete conditions. The effects of K+ starvation on pHc and cytosolic aK in expanding cells of seminal roots of barley and on the rates of protein synthesis in root tips were measured; the results indicate that decreases in pHc under K+ starvation may be the mechanism for growth inhibition.
MATERIALS AND METHODS
Plant Growth Conditions, K+ Content, and Measurements of Root Growth
Barley (Hordeum vulgare L. cv Klaxon) plants were grown hydroponically in FNS at different K+ concentrations, as described previously (Walker et al., 1996). The dry weights of entire seminal root systems were recorded following drying at 80°C for 3 d. Extension growth was measured by marking seminal roots 10 mm from their tips and measuring the distance of the mark from the root tip again after 5 to 6 h at the same time of the day (Cohen and Lepper, 1977). The total K+ content of soaked barley seeds and growing seedlings was determined as described previously (Walker et al., 1996) using 50 seeds or 50 plants at each growth stage.
Tissue Sap Osmolality
Whole seminal root sap was extracted by the freeze-thaw method (Tomos et al., 1984) and its osmolality was measured using a vapor pressure osmometer (model 5500, Wescor, Logan, UT).
Rates of Protein Synthesis
The rate of protein synthesis in the apical 10- to 15-mm lengths of seminal roots was estimated by measuring the incorporation of 14C-labeled Leu into protein at 20°C. Any possible bacterial contamination was first removed by a 60-min incubation in FNS containing 6 mg L−1 tetracycline (Memon and Glass, 1987). This antibiotic pretreatment was important, because a comparison between roots treated with the protein synthesis inhibitor cycloheximide and nontreated roots showed that 14C-labeled Leu uptake by microorganisms accounted for almost all of the apparent 14C-labeled Leu incorporation into roots (data not shown). Plants were then transferred to 15 mL of FNS labeled with 74 kBq of l-Leu [U-14C] (specific activity 11.84 GBq mmol−1; ICN) and 0.2 mm unlabeled l-Leu. After a 60-min incubation (20°C), free-space 14C-labeled Leu was desorbed for 10 min in FNS containing 1.0 mm unlabeled l-Leu (4°C). The roots were then gently blotted dry, the root cap (0.5 mm) was excised and discarded, and the remaining apical 10 to 15 mm of the root was excised, weighed, and frozen in liquid nitrogen. Root tips were defrosted and homogenized in 0.3 mL of ice-cold extraction buffer (Davies et al., 1996) using a microhomogenizer (Biomedix Ltd., Pinner, Middlesex, UK). The homogenate was centrifuged at 3,000g for 10 min (4°C). A sample of the resulting supernatant (“total 14C uptake”) was mixed with an equal volume of 10% (w/v) SDS. An aliquot of this mixture was transferred to a scintillation vial, to which 4 mL of liquid-scintillation cocktail (Ultima Gold; Packard, Pangbourne, UK) was added, and 14C dpm were counted with a liquid-scintillation analyzer (model 2500 TR, Packard). A 170-μL sample of the remaining supernatant was diluted to 1.0 mL with extraction buffer and centrifuged at 150,000g for 30 min (4°C). The resulting supernatant (“soluble fraction”) was removed, mixed with 200 μL of 47% (w/v) TCA, and left for 60 min (4°C). This mixture was then centrifuged at 12,000g for 10 min (4°C), to pellet the precipitated protein, which (after removal of the supernatant) was solubilized in 100 μL of solubilization buffer (5% SDS in 50 mm Tris-HCl, pH 6.8; 20°C). A sample of this preparation was added to a scintillation vial for counting. The 150,000g pellet (“particulate fraction”) was resuspended in 150 μL of solubilization buffer (20°C) and a sample was added to a scintillation vial for counting. The dpm values for these two fractions were added together to give the total protein value.
To study the effect of cytosolic acidification on protein synthesis, 7-d plants growing in FNS containing 0.5 mm K+ were incubated in FNS containing 10 mm sodium butyrate for 90 min before transfer to FNS containing 14C-labeled Leu (plus butyrate) for a further 60 min (Sanders et al., 1981). The effect of procaine on protein synthesis in the root tips was studied by incubating the plants for 10 min in 2 μm K+ FNS containing 0.2 mm procaine (pH 8.95) prior to a 60-min incubation in the same solution to which 14C-labeled Leu was added. The percentage incorporation of 14C-labeled Leu into the protein was calculated as:
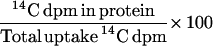 |
Measuring the rate of protein synthesis as the rate of incorporation of 14C-labeled Leu into proteins removes any effect of different treatments on the uptake of Leu into cells.
Electrophysiology
Simultaneous measurements of intracellular aK, pH, and Em in expanding cells 0.5 to 1.0 mm from the tips of barley seminal roots (Jeschke and Stelter, 1976; Huang and van Steveninck, 1989) were performed using triple-barreled microelectrodes (Walker et al., 1995, 1996). The impaled cells were those destined to become mature cortical cells, being the second, third, and fourth layers of cells encountered by the microelectrode tips as they penetrated into the root (Huang and van Steveninck, 1989). These electrode impalements may have produced tissue damage, but stable membrane potential recordings were obtained from the cells, and the value of these was similar in magnitude to those obtained from mature cells growing under the same conditions (Walker et al., 1996). Measurements were also made in fully vacuolate epidermal and cortical cells 10 to 20 mm from the tips of adventitious roots of 21-d plants. Butyrate-treated plants (grown for 7 d in FNS containing 0.5 mm KCl) were transferred for 2 h to the same solution (but containing 10 mm sodium butyrate, pH 5.8) prior to microelectrode measurements. Procaine-treated plants (21 d, K+ starved) were incubated for 20 to 50 min, before the electrode impalements, in a nutrient solution containing (in mm) 0.5 NaH2PO4, 0.5 NaNO3, 1.0 MgSO4, 0.5 Ca(NO3)2, 0.025 NaFeEDTA, and 0.2 procaine (Felle and Bertl, 1986), pH 8.95, and with added micronutrients (Hoagland and Arnon, 1950). The output from the pH-selective barrel changed slightly after the procaine treatment (data not shown). Therefore, 0.2 mm procaine was included in the pH-calibration solutions used to calibrate microelectrodes before and after measurements in procaine-treated roots.
Results are shown as mean values ± se (n), except for aK and [H+], which are shown as means with confidence limits obtained following conversion from the corresponding −log(aK) and pH values (Fry et al., 1990). Analysis of variance was performed using Microsoft Excel and linear regression analysis was performed using Sigmaplot (Jandel Scientific, Erkrath, Germany).
Thermodynamic Calculations
Uptake of K+ at the plasma membrane involves two different mechanisms (for review, see Maathuis and Sanders, 1996). For K+-starved plants growing in 2 μm K+ solution, only the high-affinity plasma membrane uptake system in root cells can retrieve K+. The mechanism for this is cotransport with either Na+ or H+ (Rubio et al., 1995). The free-energy relationship for high-affinity K+ uptake in millivolts across the plasma membrane via H+ or Na+ symport was calculated as:
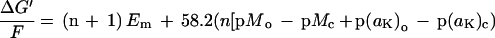 |
1 |
where n is the stoichiometry of H+ or Na+ to K+ transported by a high-affinity K+ transporter, M is the activity of H+ or Na+, and the subscripts o and c refer to the external medium and cytosol, respectively. The mean values of Em, aK, and pHc were measured with ion-selective microelectrodes for seedlings growing in FNS at 2 μm [K+]o.
RESULTS
Root Dry Matter Production, Elongation Rate, and Osmolality
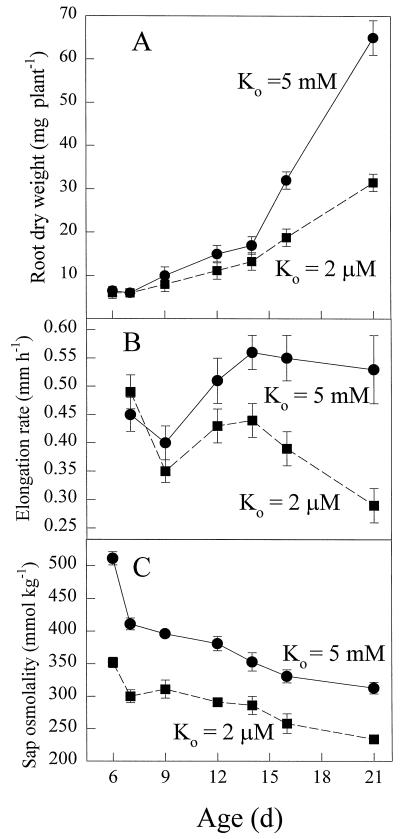
Figure 1A shows the changes in the root dry weight of barley seedlings growing in either 2 μm or 5 mm K+ FNS for 6 to 21 d after germination. Whole-root dry weight for K+-starved plants was not significantly lower than that for K+-replete plants until d 16 (Fig. 1A). Shoot dry weight for the same plants showed a similar relationship (data not shown). The dry weights of barley seedlings growing in 0.5 mm K+ FNS were not significantly different from those of roots growing in 5 mm K+ (data not shown), although root-elongation rates were significantly lower in K+-starved plants from 14 d on (Fig. 1B). This change in growth and elongation rates at 14 to 16 d may occur at the developmental stage when seed K+ reserves have been consumed and K+-starved and K+-replete plants both begin to develop adventitious roots (Russell, 1970). Figure 1C shows the osmolality values of extracts from both K+-starved and K+-replete plants. In contrast to the effects on growth shown in Figure 1, A and B, the osmolality of K+-replete plants was always significantly higher than that of K+-starved plants, but in both cases there was a general decline with time (Fig. 1C). Because the differences in sap osmolality occurred from 6 d on, before significant differences in growth were detected, this result suggests that there is no relationship between this parameter and the growth inhibition that occurs in the K+-starved roots.
Figure 1.
The effect of K+ supply on the growth and sap osmolality of barley roots growing from d 5 in FNS containing either 2 μm (▪) or 5 mm (•) K+. A, Seminal root dry weights; B, root-elongation rate; and C, osmolality of whole seminal root sap.
To prove that K+ uptake from the external solution continued throughout the 21-d experimental period for plants growing in 2 μm K+, the total K+ of the seed and whole plants was measured throughout the experiment. These measurements showed that the mean total quantity of K+ in the seed allowed to soak was 4.1 μmol per seed but had increased to 6.8 μmol per plant at 6 d and to 12.2 μmol per plant by 21 d.
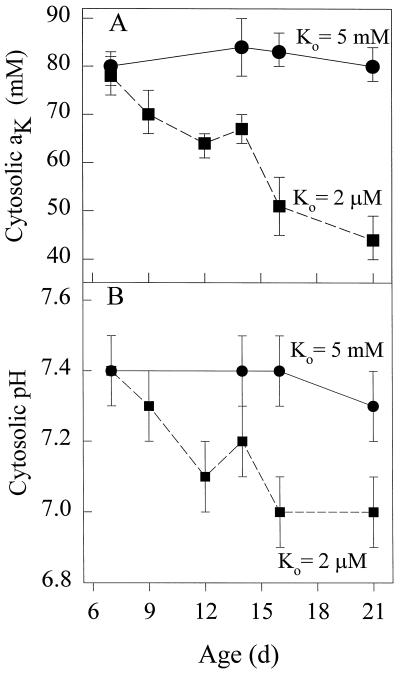
The Effect of K+ Supply on Cytosolic aK and pHc
Continued growth of barley seedlings at a [K+]o of 2 μm resulted in a decline in the cytosolic aK of expanding cells of seminal roots from 78 mm at 7 d to 44 mm at 21 d (Fig. 2A). For K+-replete plants growing at a [K+]o of 0.5 or 5 mm, the mean cytosolic aK values of expanding cells did not change significantly between 6 to 21 d. The mean Em values for plants growing in 2 μm K+ decreased from −141 ± 4 mV at 7 d to −124 ± 8 mV at 21 d, whereas for plants growing in 5 mm K+, the mean Em values were in the range −80 to −89 mV. Figure 2B shows the effect of changing the K+ supply on the pHc of expanding cells. The mean pHc was 7.4 ± 0.1 in the expanding cells of seminal roots of K+-replete plants until d 16 and was still 7.3 ± 0.1 at 21 d (Fig. 2B). The decrease in mean pHc, from 7.4 ± 0.1 on 7 d to 7.0 ± 0.1 by 16 d, for K+-starved plants occurred in parallel with a decrease in cytosolic aK (Fig. 2A); the latter value is similar to that obtained for fully vacuolate K+-starved epidermal cells (Walker et al., 1996).
Figure 2.
The effect of K+ supply on cytosolic aK and pHc of expanding barley root cells. Triple-barreled microelectrodes were used to measure cytosolic aK (A) and pHc of seminal root cells (B) of plants growing in either 2 μm (▪) or 5 mm (•) K+.
In contrast, the mean values for cytosolic aK (70 mm) and pHc (7.0) in the epidermal cells of adventitious roots (Table I) were higher than those of analogous cells in the seminal roots of 21-d K+-starved plants (39 mm and 6.7, respectively; Walker et al., 1996). The growth of adventitious roots was decreased in seedlings growing in 2 μm K+ when compared with plants grown in 5 mm K+ (data not shown).
Table I.
pHc and cytosolic aK and Em of epidermal cells 10 to 20 mm from the tips of adventitious roots of 21-d barley plants growing in FNS containing 2 μm K+
Em and pH values are means ± se, and aK values are means with confidence limits in parentheses and the number of measurements in the final parentheses.
| pH | aK | Em |
|---|---|---|
| mm | mV | |
| 7.0 ± 0.1 (8) | 70 (66, 75) (8) | −134 ± 7 (8) |
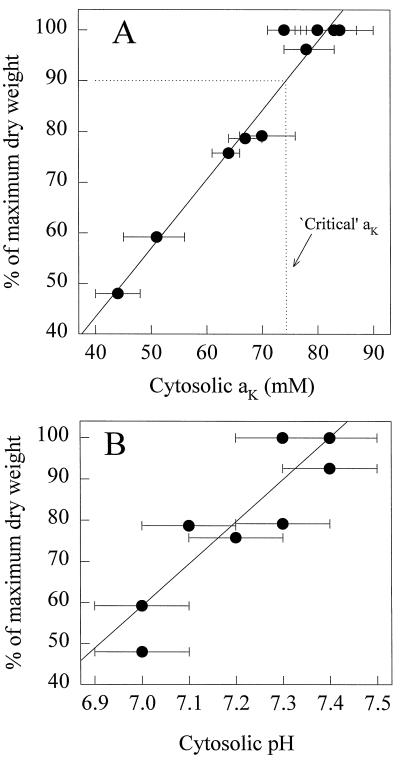
For 7-d plants growing in 0.5 mm K+, a 2-h treatment with butyrate had no effect on cytosolic aK or Em but decreased the mean pHc from 7.3 ± 0.1 to 6.8 ± 0.1 (Table II). For 21-d K+-starved plants, procaine treatment increased the mean pHc of expanding cells from 7.0 ± 0.1 to 7.3 ± 0.1 and altered the mean Em by 16 mV but had no significant effect on cytosolic aK (Table II). In Figure 3A, growth, shown as the percentage of maximum dry weight, is plotted against cytosolic aK; the results show that for expanding cells there was a correlation between the two parameters (r = 0.997). Furthermore, the ”critical” cytosolic aK for growth was 73 mm, equivalent to a [K+] of almost 100 mm (Robinson and Stokes, 1970), which is similar to the predicted value (Leigh and Wyn Jones, 1984). Figure 3B shows the relationship between growth and pHc in expanding root-tip cells and the acidification of the cytosol; like the decline in cytosolic aK, the change in pHc may also cause decreased growth.
Table II.
Effect of treatment with either 10 mm sodium butyrate or 0.2 mm procaine on pHc and the cytosolic aK and Em of expanding cells in barley root tips
Em and pHc values are means ± se, and aK values are means with confidence limits in parentheses and the number of measurements in the final parentheses.
| Treatment | Age | [K+]o | pHc | aK | Em |
|---|---|---|---|---|---|
| d | mm | mm | mV | ||
| Control | 7 | 0.5 | 7.3 ± 0.1 (9) | 74 (71, 78) (9) | −97 ± 6 (9) |
| + Butyrate | 7 | 0.5 | 6.8 ± 0.1 (12) | 77 (71, 84) (11) | −91 ± 4 (12) |
| Control | 21 | 0.002 | 7.0 ± 0.1 (11) | 44 (40, 49) (8) | −124 ± 8 (11) |
| + Procaine | 21 | 0.002 | 7.3 ± 0.1 (13) | 49 (44, 53) (10) | −108 ± 6 (13) |
Figure 3.
Relationship between seminal root growth (expressed as a percentage of the dry weight of the seminal roots of plants growing in FNS containing 5 mm K+) and cytosolic aK (A) and pHc (B) of expanding barley root cells. In A, the fitted linear regression is y = 1.359x − 10.976 (r2 = 0.997, n = 11). Critical aK is defined as the concentration at which growth was 90% of the maximum (Ulrich and Hills, 1967). In B, the fitted linear regression is y = 102.46x − 658.08 (r2 = 0.976, n = 11). The root dry weight of plants growing in 5 mm K+ was assumed to represent maximum growth and was not significantly different from that of plants growing in 0.5 mm K+.
The decline in the mean pHc of expanding cells, from 7.3 to 6.8, caused by the 2-h butyrate treatment resembles that observed previously in the epidermal and cortical cells of the seminal roots of barley (Walker et al., 1996) and other butyrate-treated tissues (Sanders et al., 1981; Reid et al., 1985b). Although deleterious effects of prolonged butyrate treatment (>6 h) on the cell cycle have been reported (Lanzagorta et al., 1988; Tramontano et al., 1991), Reid et al. (1985a) observed no effect of a 2-h exposure to 10 mm butyrate on ATP concentrations in barley roots. In the current study the root-elongation rates at 9 d for plants growing in 5 mm K+ and exposed for 2 h to 10 mm butyrate on d 7 (0.46 ± 0.03 mm h−1, n = 9) were no different from those of control plants (0.40 ± 0.03 mm h−1, n = 44).
The thermodynamic calculations for two different high-affinity K+ uptake mechanisms at the plasma membrane are shown in Table III. Both are symport mechanisms with transport coupled to either H+ or Na+, and in the calculations of Na+ cotransport two different cytosolic Na+ concentrations were used. It has been suggested that the mechanism of high-affinity K+ uptake in wheat is a Na+:K+ symport with a 1:2 stoichiometry (Rubio et al., 1995), but such a mechanism could not have maintained the measured aK gradient across the plasma membrane of expanding barley root cells (Table III). However, assuming a cytosolic Na+ concentration of 1 mm and a 1:1 stoichiometry, this mechanism becomes feasible (Table III). A H+:K+ symport mechanism with a 1:1 stoichiometry (Schachtman and Schroeder, 1994) might also have maintained the measured trans-plasma membrane aK gradient in the expanding barley root cells (Table III).
Table III.
Free-energy values (mV) for the uptake of K+ by H+ or Na+ symport mechanisms into expanding cells of seminal roots of barley plants growing in FNS containing 2 μm K+ for various times
| Na+:K+
(1:1)
|
Na+:K+ (1:2)
|
||||
|---|---|---|---|---|---|
| Plant Age | H+:K+ (1:1) | 1 mm Na+ | 20 mm Na+ | 1 mm Na+ | 20 mm Na+ |
| d | |||||
| 7 | −102 | −14 | +61 | +118 | +194 |
| 9 | −99 | −17 | +59 | +112 | +189 |
| 14 | −84 | −8 | +68 | +125 | +201 |
| 16 | −57 | +7 | +83 | +144 | +220 |
| 21 | −59 | +5 | +81 | +140 | +216 |
The values were calculated using Equation 1 and assumed two different cytosolic Na+ concentrations of 1 and 20 mm. Negative values indicate that the mechanism could maintain the observed trans-plasma membrane K+ gradient.
Protein Synthesis
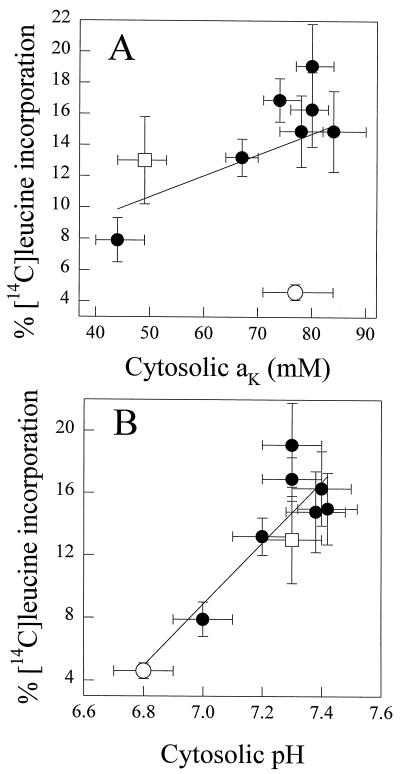
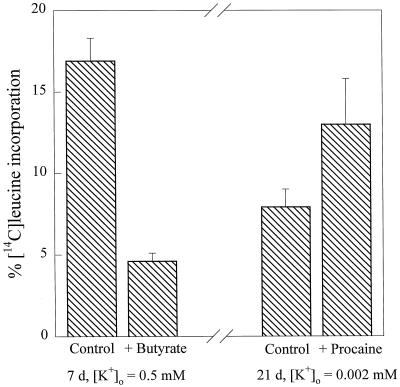
The rate of protein synthesis, measured as the percentage incorporation of 14C-labeled Leu into protein in the apical regions of roots within a 1-h period, was independent of seedling age for K+-replete plants (data not shown). However, for K+-starved plants, the incorporation of radiolabeled Leu decreased as the starvation increased. Figure 4 shows the relationships between the percentage of 14C-labeled Leu incorporation into protein and cytosolic aK and pH; different symbols are used to identify the butyrate or procaine treatments. In both data sets, the rate of protein synthesis decreased with cytosolic aK and pHc, but Figure 4A shows that the butyrate treatment gave a value that does not comply with the relationship provided by the other points. Figure 5 shows the effect of butyrate or procaine treatment on radiolabeled Leu incorporation into protein in barley root tips relative to control tips. Cytosolic acidification following treatment with butyrate (Table II) decreased 14C-labeled Leu incorporation, from 16.9 ± 1.4% to 4.6 ± 0.5% (Fig. 5), whereas alkalinization of the cytosol of expanding cells of 21-d K+-starved plants using procaine (Table II) almost doubled 14C-labeled Leu incorporation (Fig. 5). The decrease in the mean pHc of expanding cells treated with butyrate (to 6.8) was more pronounced than the effect of 21 d of K+ starvation (decrease to 7.0; Table II compared with Fig. 5). Furthermore, 14C-labeled Leu incorporation was decreased to a greater extent in butyrate-treated roots than in K+-starved roots (Table II). Protein synthesis was also partially restored by procaine treatment in K+-starved root tips, whereas butyrate treatment of K+-replete cells inhibited protein synthesis (Table II). These results show that cytosolic acidification inhibits protein synthesis to a greater extent than depletion of cytosolic aK.
Figure 4.
Relationship between the rate of protein synthesis and cytosolic aK (A) and pHc (B) of expanding cells of barley root tips. The fitted linear regressions are: A, y = 0.135x + 3.90 (r2 = 0.183, n = 9); B, y = 19.59x − 128.1 (r2 = 0.790, n = 9). Protein synthesis was measured as the percentage incorporation of 14C-labeled Leu. The roots were treated with butyrate (○) or procaine (□). The remaining symbols represent seedlings supplied with different K+ concentrations (Walker et al., 1996).
Figure 5.
Histogram showing the effects on protein synthesis of treating K+-replete seedlings with butyrate or K+-starved seedlings with procaine. Protein synthesis was measured as the incorporation of 14C-labeled Leu into protein. Seedlings were growing from d 5 in FNS containing 0.5 mm K+ and treated with butyrate (10 mm) and 21-d barley plants were growing from d 5 in FNS containing 2 μm K+ treated with procaine (0.2 mm).
DISCUSSION
For many plants the [K+]o required to give optimal growth is small; for example, the concentration needed for maximal growth in 14 different species was only 30 μm K+ (Asher and Ozanne, 1967). In barley seedlings supplied with an external concentration of only 2 μm, the growth of seminal roots continued but at a rate that at 21 d was 50% of that measured for K+-replete plants (Fig. 1A). Growing plants with a suboptimal K+ supply has allowed the relationship among cytosolic aK, pHc, and growth to be determined.
Root Growth and K+ Supply
The growing root tip is generally described as a sink for K+ (Silk et al., 1986; Moritsugu et al., 1993). There are three possible sources of K+ to support the growth of the root tips in K+-starved barley seedlings: seed reserve, remobilization of K+ from other tissues, and uptake from the external solution.
During the time course of these measurements, the seed reserve of K+ was being depleted and the growth differences between K+-starved and -replete roots started to become significant at 14 to 16 d. This observation suggests that the seed reserve of K+ may have been consumed, although adventitious roots also began to appear at this time, which would have provided a new sink for K+. The appearance of adventitious roots coincided with a significant slowing of seminal root growth in K+-starved barley plants (Fig. 1). To sustain continued growth of adventitious roots, withdrawal of K+ from the shoot or the seminal roots may have occurred. The different responses to K+ starvation shown by fully developed epidermal cells of seminal roots (Walker et al., 1996) and analogous cells of adventitious roots (Table I) may be an example of this redistribution process.
Total K+ content increased with age in the K+-starved seedlings, showing that K+ uptake occurred throughout the 21-d measurement period. This K+ uptake may have been limited to the cortical cells, because in wheat roots the high-affinity plasma membrane K+ transporter (HKT1) was mainly expressed in this tissue (Schachtman and Schroeder, 1994). Furthermore, the expanding cells of seminal roots were unable to maintain optimal growth rates by uptake of K+ from an external concentration of 2 μm. The high-affinity plasma membrane K+-uptake system may be located only in the growing tip cells of adventitious roots and not in seminal roots; although uptake of the radiolabeled K+ analog, 86Rb+, by the apical 10 mm of excised barley seminal roots could be measured when the combined [Rb+]o and [K+]o was 23 μm (Moritsugu et al., 1993).
It has been proposed that K+ uptake by HKT1 is coupled to either H+ or Na+ gradients across the plasma membrane (Rubio et al., 1995), and a related gene has been identified in barley (Santa-María et al., 1997), which also mediates low-affinity Na+ uptake. However, no direct evidence for the coupling of K+ and Na+ uptake by barley roots has been found (Maathuis et al., 1996). The microelectrode measurements in the barley cells allow the energetics of these various high-affinity K+ uptake mechanisms to be determined. These calculations suggest a 1:1 H+ symport as the most likely mechanism (Table III), but this calculation depends on knowledge of the cytosolic Na+ concentration, so intracellular measurements of this are needed.
The Cellular Mechanism of Growth Inhibition during K+ Starvation
The cytosolic acidification associated with cytosolic K+ depletion in expanding cells was similar to that reported for cells of barley root epidermis (Walker et al., 1996) and the fungus Neurospora (Blatt and Slayman, 1987). For one variety of barley, Compana, the rate of protein synthesis ([3H]Leu incorporation) increased 0.5- to 2.7-fold when [K+]o was increased from 10 to 100 μm (Memon and Glass, 1987). However, whole-root compartmental efflux analysis showed that cytosolic [K+] increased only from 133 to 140 mm. In another barley variety, Betzes, the root protein synthesis increased by 7-fold, whereas cytosolic [K+] increased from 127 to 187 mm as [K+]o was increased from 10 to 100 μm (Memon and Glass, 1987). However, this averaging technique for measuring cytosolic aK may have hidden tissue differences and the observed effect on protein synthesis may have resulted partly from an increase in pHc. It was found using cytosolic extracts of Xenopus oocytes that the rates of in vitro translation at pH 7.0 and 6.8 were reduced by approximately 15% and 65%, respectively, relative to pH 7.3 (Grandin and Charbonneau, 1989). Furthermore, a strong pH dependence of protein synthesis has been shown in polyribosome preparations from maize root tips by Webster et al. (1991), who suggested that the pH dependence of root-tip protein synthesis could provide a mechanism that enables the cell's translational machinery to sense changes in pHc, leading directly to selective gene expression. In mammalian cells, a role for cytosolic acidification in signaling programmed cell death is unlikely (Schrode et al., 1997), but changes in cytosolic aK have been implicated (Hughes et al., 1997). Under these K+-starved conditions, the seminal root cells may be showing programmed cell death so that specific sink root meristems are removed and K+ is made available to supply the emerging adventitious roots. Proof of apoptosis would require the identification of the morphological and biochemical markers (for review, see Harvel and Durzan, 1996) that are associated with this process in the root cells.
An additional role for K+ is in balancing the net anionic charge of proteins within the cytosol (Maathuis and Sanders, 1996). The cytosolic acidification that is associated with depletion of cytosolic K+ could result from H+ substituting in this function. However, the depletion of cytosolic K+ from 80 to 45 mm (Fig. 2A) was associated with a relatively small increase in H+ concentration from 40 to 100 nm (pH 7.4–7.0, Fig. 2B). Changes in pHc will occur only after the H+-buffering capacity of the cytosol has been exceeded, and estimates of this parameter range from 18 to 40 mm H+ per pH unit (Roos and Boron, 1981; Reid et al., 1989); thus, H+ can only partially substitute for K+ in balancing the charge within the cytosol.
For K+-starved barley seedlings, simultaneous measurements of growth, cytosolic aK, and pHc in cells of the root-growth zone have allowed the relationship among these parameters to be determined. There was a direct dependence of growth on both pHc and cytosolic aK in expanding cells (Fig. 3). Protein synthesis may be altered by changes in cytosolic ion concentrations, and when this process was assayed in vivo, like growth, it was found to be dependent on both pHc and cytosolic ak (Fig. 4). However, artificial manipulation of pHc by treatment with butyrate or procaine showed that it is this parameter, rather than cytosolic aK, that is critical for protein synthesis and therefore growth.
ACKNOWLEDGMENT
We wish to thank Dr. Emyr Davies (IACR-Rothamsted) for advice regarding assaying rates of protein synthesis.
Abbreviations:
- aK
K+ ion activity
- Em
trans-plasma membrane electrical potential
- FNS
full nutrient solution
- [K+]o
external K+ concentration
- pHc
cytosolic pH
Footnotes
IACR-Rothamsted receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
LITERATURE CITED
- Asher CJ, Ozanne PG. Growth and potassium content of plants in solution cultures maintained at constant potassium concentrations. Soil Sci. 1967;103:155–161. [Google Scholar]
- Blatt MR, Slayman CL. Role of “active” potassium transport in the regulation of cytoplasmic pH by nonanimal cells. Proc Natl Acad Sci USA. 1987;84:2737–2741. doi: 10.1073/pnas.84.9.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Lepper R., Jr Effect of boron on cell elongation and division in squash roots. Plant Physiol. 1977;59:884–887. doi: 10.1104/pp.59.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram WJ. Negative feedback regulation of transport in cells. The maintenance of turgor, volume and nutrient supply. In: Lüttge U, Pitman MG, editors. Encyclopaedia of Plant Physiology, New Series, Vol 2: Transport in Plants II, Part A. Berlin: Springer-Verlag; 1976. pp. 284–316. [Google Scholar]
- Curl EA, Truelove B. The Rhizosphere. Berlin: Springer-Verlag; 1986. [Google Scholar]
- Davies TGE, Steele SH, Walker DJ, Leigh RA. An analysis of vacuole development in oat aleurone protoplasts. Planta. 1996;198:356–364. [Google Scholar]
- Felle H, Bertl A. Light-induced cytoplasmic pH changes and their interrelation to the activity of the electrogenic proton pump in Riccia fluitans. Biochim Biophys Acta. 1986;848:176–182. [Google Scholar]
- Fry CH, Hall SK, Blatter LA, McGuigan JAS. Analysis and presentation of intracellular measurements obtained with ion-selective microelectrodes. Exp Physiol. 1990;75:187–198. doi: 10.1113/expphysiol.1990.sp003393. [DOI] [PubMed] [Google Scholar]
- Grandin N, Charbonneau M. Intracellular pH and the increase in protein synthesis accompanying activation of Xenopus eggs. Biol Cell. 1989;67:321–330. [PubMed] [Google Scholar]
- Hackett C. A study of the root system of barley. I. Effects of nutrition on two varieties. New Phytol. 1968;67:287–299. [Google Scholar]
- Harvel L, Durzan DJ. Apoptosis in plants. Bot Acta. 1996;109:268–277. [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Calif Agric Exp Stn Bull. 1950;347:1–32. [Google Scholar]
- Huang CX, van Steveninck RFM. Longitudinal and transverse profiles of K+ and Cl− concentration in “low-” and “high-salt” barley roots. New Phytol. 1989;112:475–480. doi: 10.1111/j.1469-8137.1989.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Hughes FM, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- Jeschke WD, Stelter W. Measurement of longitudinal ion profiles in single roots of Hordeum and Atriplex by use of flameless atomic absorption spectroscopy. Planta. 1976;128:107–112. doi: 10.1007/BF00390311. [DOI] [PubMed] [Google Scholar]
- Lanzagorta JMA, de la Torre C, Aller P. The effect of butyrate on cell cycle progression in Allium cepa root meristems. Physiol Plant. 1988;72:775–781. [Google Scholar]
- Leigh RA, Wyn Jones RG. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984;97:1–13. [Google Scholar]
- Maathuis FJM, Sanders D. Mechanisms of potassium absorption by higher plant roots. Physiol Plant. 1996;96:158–168. [Google Scholar]
- Maathuis FJM, Verlin D, Smith FA, Sanders D, Fernández JA, Walker NA. The physiological relevance of Na+-coupled K+-transport. Plant Physiol. 1996;112:1609–1616. doi: 10.1104/pp.112.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon AR, Glass ADM. Genotypic differences in subcellular compartmentation of K+: implications for protein synthesis, growth and yield. In: Gabelman HW, Loughman BC, editors. Genetic Aspects of Plant Mineral Nutrition. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 323–329. [Google Scholar]
- Métraux J-P, Taiz L. Cell wall extension in Nitella as influenced by acids and ions. Proc Natl Acad Sci USA. 1977;74:1565–1569. doi: 10.1073/pnas.74.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritsugu M, Shibasaka M, Kawasaki T. Where is the most important and efficient site for absorption and translocation of cations in excised barley roots? Soil Sci Plant Nutr. 1993;39:299–307. [Google Scholar]
- Pritchard J. The control of cell expansion in roots. New Phytol. 1994;127:3–26. doi: 10.1111/j.1469-8137.1994.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Pritchard J, Tomos AD, Wyn Jones RG. Control of wheat root elongation growth. I. Effects of ions on growth rate, wall rheology and cell water relations. J Exp Bot. 1987;38:948–959. [Google Scholar]
- Reid RJ, Dejaegere R, Pitman MG. Regulation of electrogenic pumping in barley by pH and ATP. J Exp Bot. 1985a;36:535–549. [Google Scholar]
- Reid RJ, Field LD, Pitman MG. Effects of external pH, fusicoccin and butyrate on the cytoplasmic pH in barley root tips measured by 31P-nuclear magnetic resonance spectroscopy. Planta. 1985b;166:341–347. doi: 10.1007/BF00401171. [DOI] [PubMed] [Google Scholar]
- Reid RJ, Smith FA, Whittington J. Control of intracellular pH in Chara corallina during uptake of weak acid. J Exp Bot. 1989;40:883–91. [Google Scholar]
- Robinson RA, Stokes RH. Electrolyte Solutions, Ed 2. London: Butterworths; 1970. [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Russell RS. Root systems and plant nutrition. Endeavour. 1970;29:60–66. [Google Scholar]
- Sanders D, Hansen U-P, Slayman CL. Role of the plasma membrane proton pump in pH regulation in non-animal cells. Proc Natl Acad Sci USA. 1981;78:5903–5907. doi: 10.1073/pnas.78.9.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Shrode LD, Tapper H, Grinstein S. Role of intracellular pH in proliferation, transformation, and apoptosis. J Bioenerg Biomembr. 1997;29:393–399. doi: 10.1023/a:1022407116339. [DOI] [PubMed] [Google Scholar]
- Siddiqi MY, Glass ADM. Studies of the growth and mineral nutrition of barley varieties. I. Effect of potassium supply on the uptake of potassium and growth. Can J Bot. 1983;61:671–678. [Google Scholar]
- Spear SN, Asher CJ, Edwards DG. Response of cassava, sunflower, and maize to potassium concentration in solution. I. Growth and plant potassium concentration. Field Crops Res. 1978;1:347–361. [Google Scholar]
- Tomos AD, Leigh RA, Shaw CA, Wyn Jones RG. A comparison of methods for measuring turgor pressures and osmotic pressures of cells of red beet storage tissue. J Exp Bot. 1984;35:1675–1683. [Google Scholar]
- Tramontano WA, DeLillo AR, Yung SY, Natarajan C, Kearns CM. Short-chain fatty-acid-induced effects on the cell cycle in root meristems of Pisum sativum. Physiol Plant. 1991;82:79–84. [Google Scholar]
- Triboulot M-B, Pritchard J, Levy G. Effects of potassium deficiency on cell water relations and elongation of tap and lateral roots of maritime pine seedlings. New Phytol. 1997;135:183–190. [Google Scholar]
- Ulrich A, Hills FJ (1967) Principles and practices of plant analysis. In GW Hardy, ed, Soil Testing and Plant Analysis, Part II. Soil Science Society of America, Madison, WI, pp 11–24
- Walker DJ, Leigh RA, Miller AJ. Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA. 1996;93:10510–10514. doi: 10.1073/pnas.93.19.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Smith SJ, Miller AJ. Simultaneous measurement of intracellular pH and K+ or NO3− in barley root cells using triple-barreled, ion-selective microelectrodes. Plant Physiol. 1995;108:743–751. doi: 10.1104/pp.108.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing PF, Phillips DJ. Growth and Differentiation in Plants, Ed 3. Oxford, UK: Pergamon Press; 1981. [Google Scholar]
- Webster C, Kim C-Y, Roberts JKM. Elongation and termination reactions of protein synthesis on maize root tip polyribosomes studied in a homologous cell-free system. Plant Physiol. 1991;96:418–425. doi: 10.1104/pp.96.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. Relationship between the development and growth of rye (Secale cereale L.) and the potassium concentration in solution. Ann Bot. 1993;72:349–358. [Google Scholar]
- Wyn Jones RG, Brady CJ, Speirs J. Ionic and osmotic relations in plant cells. In: Laidman DL, Wyn Jones RG, editors. Recent Advances in the Biochemistry of Cereals. London: Academic Press; 1979. pp. 63–103. [Google Scholar]