Abstract
Surgical placement of a left ventricular epicardial pacing lead is a valuable alternative to the standard approach of endovascular placement of a pacing lead in the coronary sinus for cardiac resynchronization therapy. Despite higher perioperative morbidity, surgically placed leads perform well with lower revision and dislocation rates. Moreover, surgery is the only option when an endovascular approach proves to be unsuccessful. We report a successful implantation of an epicardial left ventricular lead through an ultrasound-guided lateral left mini-thoracotomy in a patient with a severely disturbed thoracic anatomy due to left pneumonectomy.
Keywords: Cardiac resynchronization therapy; Epicardial left ventricular lead; Pneumonectomy; Implantable cardioverter, defibrillator
INTRODUCTION
Surgical implantation of a left ventricular (LV) pacing lead is usually straightforward. In most patients, an anterolateral mini-thoracotomy provides ample access to the patient's pericardium and LV wall. In the present case, however, left pneumonectomy dramatically altered the intrathoracic position of the heart, making the usual anatomical landmarks useless. We report on the implantation of an LV lead by ultrasound-guided lateral mini-thoracotomy.
CASE REPORT
A 76-year old patient with chronic renal impairment and severe chronic obstructive pulmonary disease was referred to our centre for invasive treatment of worsening heart failure. In 1981, the patient underwent successful left pneumonectomy for lung carcinoma and has been free of malignancy ever since. In 1997, a coronary artery bypass graft was performed for coronary three-vessel disease. Over the past few years, the patient developed signs of progressive ischaemic heart failure and remained in functional NYHA class IV, despite optimal medical therapy.
A recent coronary angiogram shows severe ventricular dysfunction with an ejection fraction (EF) of <15% despite three patent bypasses. An electrocardiogram depicts a regular sinus rhythm of 77 bpm with a prolonged atrioventricular interval (PR: 320 ms), and complete left bundle branch block with a widened QRS-complex of 142 ms.
Echocardiography revealed a dilated left ventricle with an LV end-diastolic dimension of 62 mm, EF of 10%, functional mitral regurgitation (grade III) and echographic signs of intra- and inter-ventricular dyssynchrony using teal-time 3D echocardiography (Fig. 1b) [1].
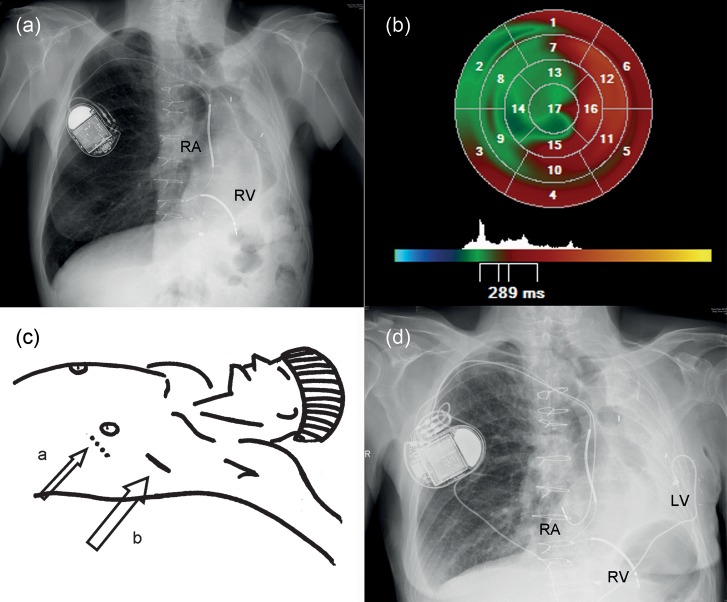
Figure 1:
(a) Chest X-ray showing an extreme leftward displacement of the heart, right atrial (RA) and right ventricular (RV)—shock lead connected to the internal cardiac defibrillator-CRT in the right infraclavicular region. (b) LV dyssynchrony analysis from an RT3DE data set using parametric images. The global time to minimum systolic volume is used as timing reference; early segments are coded in blue, whereas late segments are coded in red. (c) a: conventional site for incision and; b: actual skin incision based on ultrasound guidance. (d) Postoperative chest X-ray with the LV-lead tunnelled around the xyphoid processus.
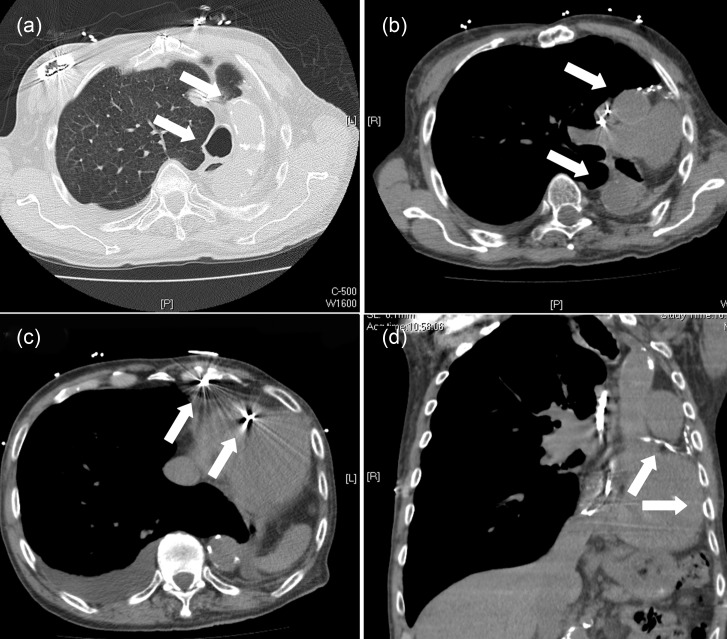
Chest X-ray (Fig. 1a) and computed tomography (CT) scan of the thorax (Fig. 2a–d) illustrate the extreme leftward displacement of the heart and the great vessels as a result of the left pneumonectomy.
Figure 2:
(a) CT scan: enlargement of the right lung, lateral displacement of trachea and calcified aorta. (b) CT scan: lateral displaced of the heart and great vessels. (c) CT scan: pacemaker leads in the right atrium and shock-lead in the right ventricle. (d) CT scan: calcified venous bypass, left ventricle adjacent to the thoracic wall.
An attempt to perform endovascular deployment of an LV lead in the coronary sinus (CS) was unsuccessful due to the tortuosity of the cardiac veins. The patient was therefore evaluated by a multidisciplinary heart team and scheduled for conventional surgical lead implantation.
We assumed that a standard anterolateral mini-thoracotomy would not provide us with the correct approach to the left ventricle due to the extreme leftward displacement of the heart. Therefore, we used ultrasound to identify the position of the lateral LV-wall in relation to the thorax. In this case, the entry site for optimal mini-thoracotomy ended up being one intercostal spacer higher and 11 cm more towards the posterior (Fig. 1c; large arrow (b)) than a conventional entry point would have been (Fig. 1c; small arrow (a)). We obtained easy access to the lateral LV wall through a 6 cm skin incision, an Enpath® screw-in lead was implanted, showing a low threshold voltage, and a subcutaneous tunnel was created around the xyphoid processus towards the right infraclavicular pocket (Fig. 1d). No lead extensions were necessary.
Transient worsening of renal failure and bronchopneumonia complicated the immediate postoperative course and kept the patient in the hospital for 2 weeks.
One year after the intervention, the patient is in NYHA class III, with an EF of 25% and mitral insufficiency grade 2.
DISCUSSION
Biventricular pacing is nowadays a widespread technique for the treatment of advanced heart failure despite a 30% non-responder-rate [2]. Although the transvenous approach of deploying a cardiac resynchronization therapy (CRT) lead in the CS is technically demanding, frequently requiring extensive fluoroscopy times, we as well as many others prefer this approach because of its less-invasive nature and lower postprocedural complications compared with surgery [3].
Success, however, depends largely on the coronary venous anatomy, the ability to obtain adequate capture, possible lead dislodgement and the risk of perforation and phrenic nerve stimulation. Most authors report an early and late failure rate of ∼10–12% [4].
Surgically deployed LV leads have lower dislocation and revision-rates and provide similar functional benefits and long-term outcomes despite a higher periprocedural risk, including transient renal failure and infection [3, 5].
In our case, the surgical approach was mandatory after a failed attempt to insert a CRT-lead in the CS, bearing in mind that the dramatically distorted thoracic anatomy would make a standard anterolateral mini-thoracotomy inadequate to expose the lateral side of the left ventricle. However, ultrasound proved to be a fast and adequate tool in determining the correct entry point for thoracotomy in such a way that the incision could be kept minimal.
Echocardiography in the operating room, after the patient has been properly installed, can aid the surgeon in optimizing the optimal location for thoracotomy, hence keeping the incision as small as possible.
Conflict of interest: none declared.
REFERENCES
- 1.Marsan NA, Bleeker GB, Ypenburg C, Ghio S, van de Veire NR, Holman ER, et al. Real-time three-dimensional echocardiography permits quantification of left ventricular mechanical dyssynchrony and predicts acute response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2008;19:392–9. doi: 10.1111/j.1540-8167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 2.Coceani M. Guideline challenge: has CRT earned a Class I recommendation? Circ Heart Fail. 2010;3:460–1. doi: 10.1161/CIRCHEARTFAILURE.110.956334. [DOI] [PubMed] [Google Scholar]
- 3.Miller AL, Kramer DB, Lewis EF, Koplan B, Epstein LM, Tedrow U. Event-free survival following CRT with surgically implanted LV leads versus standard transvenous approach. Pacing Clin Electrophysiol. 2011;34:490–500. doi: 10.1111/j.1540-8159.2010.03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navia JL, Atik FA, Grimm RA, Garcia M, Vega PR, Myhre U, et al. Minimally invasive left ventricular epicardial lead placement: surgical techniques for heart failure resynchronization therapy. Ann Thorac Surg. 2005;79:1536–44. doi: 10.1016/j.athoracsur.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Ailawadi G, Lapar DJ, Swenson BR, Maxwell CD, Girotti ME, Bergin JD, et al. Surgically placed left ventricular leads provide similar outcomes to percutaneous leads in patients with failed coronary sinus lead placement. Heart Rhythm. 2010;7:619–25. doi: 10.1016/j.hrthm.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]