Abstract
OBJECTIVES
The increased use of computed tomography has led to an increasing proportion of lung cancers that are identified when still less than 1 cm in diameter. However, there is no defined treatment strategy for such cases. The aim of this study was to investigate the surgical outcomes of small lung cancers.
METHODS
A total of 143 patients were retrospectively evaluated, who had undergone a complete surgical resection for lung cancer less than 1 cm in diameter between January 1995 and December 2011.
RESULTS
The 143 study subjects included 62 male and 81 female patients. The mean age was 64.0 years (43–82 years). The mean tumour size was 0.8 cm (0.3–1.0 cm). Seventy-seven patients (53.8%) underwent lobectomy. Thirty-two patients (22.4%) underwent segmentectomy and 34 patients (23.8%) underwent wedge resection. The 3-, 5- and 10-year survival rates were 95.7, 92.2 and 85.7%, respectively, after resection for sub-centimetre lung cancer. There were no significant differences between sub-lobar resection and lobectomy. However, two patients (1.4%) had recurrent cancer and seven (4.9%) had lymph node metastasis.
CONCLUSIONS
The selection of the surgical procedure is important and a long-term follow-up is mandatory, because lung cancer of only 1 cm or less can be associated with lymph node metastasis and distant metastatic recurrence.
Keywords: Lung cancer, Less than 1 cm, Surgical outcome
INTRODUCTION
The seventh edition of the TNM Classification of Malignant Tumours, with the new lung cancer staging system, was published in 2010 [1]. One of the major changes in the new staging was a subdivision of T-status in the TNM classification. The previous cut-off value regarding tumour size, to distinguish T1 from T2, was 3 cm. However, the new TNM classification includes some tumours measuring less than 2 cm in diameter, such as T1a. The increased utilization of computed tomography (CT) has led to an increasing proportion of lung cancers being identified when measuring less than 1 cm in diameter. However, such small lung cancers may not always have a good prognosis, as there are some reports of malignant lymphadenopathy and recurrence with distant metastasis after surgery [2, 3]. There is no definitive treatment strategy or surgical procedure for lung cancers smaller than 1 cm. The selection of minimally invasive surgery, such as a sub-lobar resection or video-assisted thoracoscopic surgery (VATS), for such cancers has been discussed. The aim of this study was to investigate the surgical outcomes of lung cancers under 1 cm in diameter.
PATIENTS AND METHODS
A total of 1881 patients underwent complete surgical resection for primary lung cancer at our institution from January 1995 to December 2011. There were also 86 cases of resection for benign nodules smaller than 1 cm during this period. One hundred and forty-three of these 1881 patients had sub-centimetre primary lung cancer. All 143 patients underwent careful preoperative evaluation with CT scan. We think there were no significant differences between clinical size and pathological size for small-sized lung cancer. Generally, if the nodules smaller than 1 cm were unchanged in size or density when observed by CT scan at 3-month intervals, they should be candidates for operation. All patients usually undergo careful preoperative staging with CT scan alone, but positron emission tomography (PET) scan has been available since January 2007. Endobronchial ultrasonography (EBUS), endscopic ultrasonography (EUS), or mediastinoscopy were not always performed for patients with sub-centimetre nodules, except in patients with suspicion of lymphadenopathy on CT or PET scan.
For solid lung cancer nodules under 1 cm in size, without ground-glass opacity (GGO), or part-solid type with GGO on CT findings, we performed standard lobectomy with lymph node systematic dissection. For pure GGO, suggesting Noguchi's type A or B, and localization of tumour close to the visceral pleura, we performed wedge resection without lymph node dissection. For non-peripherally located, small, non-invasive adenocarcinoma that had been diagnosed as N0 by intraoperative frozen-section examination, segmentectomy was undertaken with free surgical margin.
Basically, we selected the video-assisted thoracoscopic surgery (VATS) approach because the lung cancer was smaller than 1 cm. There were conversion cases from VATS to an open thoracotomy because of severe intrathoracic adhesion and insufficient interlobular fissure. One hundred and forty-three of those cases were pathologically confirmed to have a tumour diameter of 1 cm or less. Follow-up for all patients was arranged to take place at our institution. Postoperatively, patients were given a 3-monthly blood test and chest X-ray, in addition to a CT chest scan every 6 months. We diagnosed recurrence based on compatible physical examination and diagnostic imaging, and confirmed the diagnosis histologically when clinically feasible. If any findings of recurrence were observed, magnetic resonance imaging (MRI) for metastatic brain tumour or whole body PET scan was performed. The date of recurrence was defined as the date of histological proof or, in cases in which diagnosis was based on clinico-radiologic findings, the date of identification by a physician. Survivals were calculated using the Kaplan-Meier method and differences in survival were determined by a log-rank analysis. Statistically significant differences were accepted at P <0.05.
RESULTS
Patient characteristics
The patients and tumour characteristics of 143 patients for lung cancer smaller than 1 cm are summarized in Table 1. There were 62 male (43.4%) and 81 female (56.6%) patients in this study. The mean age was 64.0 years (43–82 years). The mean tumour size was 0.8 cm (0.3–1.0 cm). Seventy-eight cases (54.5%) were right lung cancers, and 65 cases (45.5%) were left lung cancers. The location of the cancer was the upper lobe in 76 cases (53.1%), the middle lobe in 14 cases (9.8%), and the lower lobe in 53 cases (37.1%). The histopathological diagnosis of lung cancer was adenocarcinoma (Ad) in 127 patients (88.8%), squamous cell carcinoma (Sq) in 7 (4.9%), and others in 9 (6.3%) [small cell carcinoma (Sm) in 6 (4.2%), pleomorphic carcinoma (Pl) in 2 (1.4%), adenosquamous carcinoma (AdSq) in 1 (0.7%)]. Seven patients (4.9%) had lymph node metastases. Four patients (2.8%) and 3 patients (2.1%), respectively, had N1 and N2 malignant lymphadenopathy (Table 1). These node positive cases were not evaluated by mediastinoscopy or EBUS preoperatively, because of clinical N0.
Table 1:
Patient and tumour characteristics (n = 143)
| Age (years) | mean 64.0 (43–82) |
| Gender | |
| Male | 62 (43.4%) |
| Female | 81 (56.6%) |
| Tumour size (cm) | mean 0.8 (0.3–1.0) |
| Location | |
| Right | 78 (54.5%) |
| Upper lobe | 49 (34.2%) |
| Middle lobe | 14 (9.8%) |
| Lower lobe | 15 (10.5%) |
| Left | 65 (45.5%) |
| Upper lobe | 27 (18.9%) |
| Lower lobe | 38 (26.6%) |
| Histology | |
| Ad | 127 (88.8%) |
| Sq | 7 (4.9%) |
| Others | 9 (6.3%) |
| Sm | 6 (4.2%) |
| Pl | 2 (1.4%) |
| AdSq | 1 (0.7%) |
| pStage | |
| pIA (pT1aN0) | 136 (95.1%) |
| pIIA (pT1aN1) | 4 (2.8%) |
| pIIIA (pT1aN2) | 3 (2.1%) |
Ad: adenocarcinoma; Sq: squamous cell carcinoma; Sm: small-cell carcinoma; Pl: pleomorphic carcinoma; AdSq: adenosquamous carcinoma.
The disease stage was pIA (pT1aN0) in 136 (95.1%), pIIA (pT1aN1) in 4 (2.8%), pIIIA (pT1aN2) in 3 (2.1%) (Table 1).
Methods of diagnosis
Only about 8.4% (12) of the cases were preoperatively diagnosed by transbronchial lung biopsy (TBLB). Most of the diagnoses depended on the intraoperative pathological diagnosis. There were 120 cases with an intraoperative pathological diagnosis. One hundred and thirteen of the 120 were associated with an intraoperative pathological diagnosis of a specimen following a partial resection of the lung. The seven other cases (4.9%) were diagnosed by intraoperative needle aspiration cytology (NAC). There were 11 foci (7.7%) that were never confirmed due to the very small tumour size or its location apart from the visceral pleura, or the wide extent of GGO. Such cases were treated by segmentectomy or lobectomy without a pathological diagnosis.
An accurate resection of small and non-superficial pulmonary nodules was usually performed by VATS, using the hook-wire marking system (21G × 150 mm) (Guiding-Marker System, Hakko Co. Ltd, Tokyo, Japan) with CT just before the operation. A total of 68 patients (47.5%) were treated using the hook-wire technique in this study. The location of pulmonary nodules was detected with palpation in 33 patients (23.1%) and with only while inspecting for signs of pleural change or a lung prominence by the pulmonary nodules during VATS in 23 patients (16.1%) (Table 2).
Table 2:
Diagnosis of the tumour (n = 143)
| Preoperative diagnosis | |
| Yes | |
| Transbronchial lung biopsy | 12 (8.4%) |
| No | |
| Intraoperative diagnosis | |
| Frozen section | 113 (79.0%) |
| Needle aspiration cytology | 7 (4.9%) |
| Postoperative diagnosis | 11 (7.7%) |
| Fix on tumour location in operation | |
| Yes | |
| Hook-wire marking (with computed tomography) | 68 (47.5%) |
| Palpation | 33 (23.1%) |
| Inspection | 23 (16.1%) |
| No | 19 (13.3%) |
Surgery, morbidity and mortality
Seventy-seven (53.8%) patients were treated by lobectomy, 32 (22.4%) underwent segmentectomy and 34 (23.8%) underwent wedge resection. Lung resection with VATS was performed in 85 patients (59.4%), open thoracotomy in 44 patients (30.8%). There were 14 conversion cases (9.8%) from VATS to an open thoracotomy because of severe intrathoracic adhesion and insufficient interlobular fissure (Table 3). The mean operative time and estimated blood loss were 185.7 min (30–385 min) and 113.9 ml (5–1440 ml), respectively. Postoperative morbidity included pneumonia in four cases, atelectasis in four cases, asthma attack in four cases, prolonged air leakage (longer than seven days) in one case, wound infection in two cases. No patients required surgery for postoperative bleeding or respiratory failure requiring ventilation. There was no surgical mortality within 30 days after surgery (Table 3).
Table 3:
Perioperative outcomes (n = 143)
| Operative time (min) | mean 185.7 (30–385) |
| Estimated blood loss (ml) | mean 113.9 (5–1440) |
| Surgical procedure | |
| Lobectomy | 77 (53.8%) |
| Segmentectomy | 32 (22.4%) |
| Wedge resection | 34 (23.8%) |
| Approach | |
| Video-assisted thoracoscopic surgery | 85 (59.4%) |
| Thoracotomy | 44 (30.8%) |
| Conversion case | 14 (9.8%) |
| Morbidity | |
| Pneumonia | 4 (2.8%) |
| Atelectasis | 4 (2.8%) |
| Asthma attack | 4 (2.8%) |
| Prolonged air leakage | 1 (0.7%) |
| Wound infection | 2 (1.4%) |
| Mortality, 30-day | 0 |
Recurrence and survival pattern
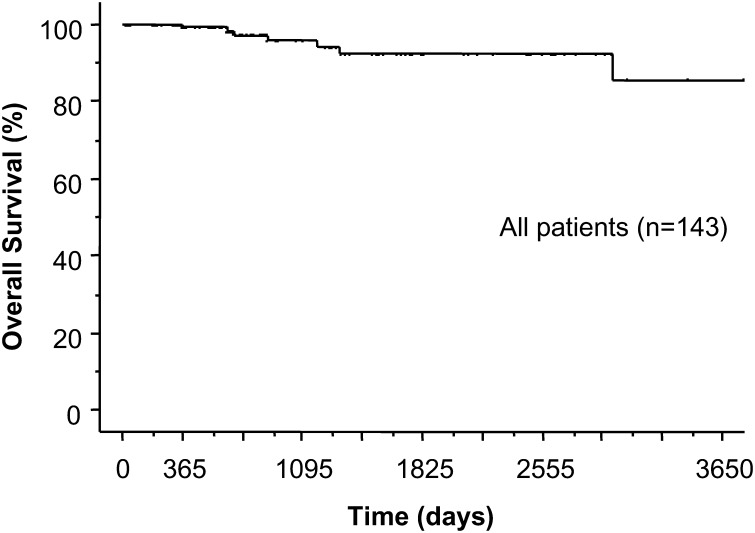
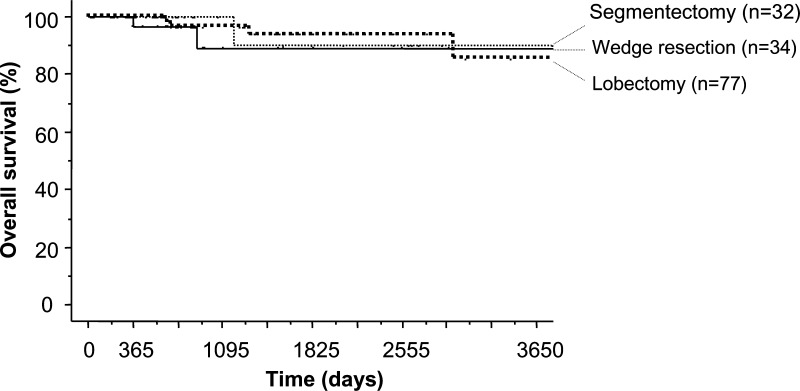
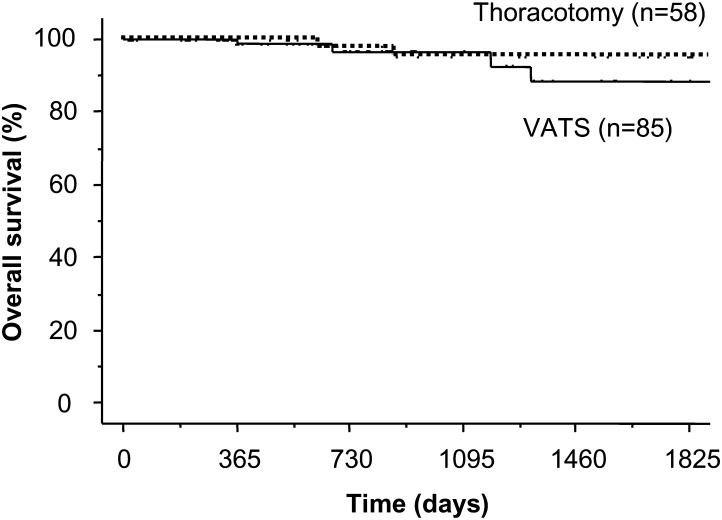
Stage IA patients did not receive any adjuvant therapy. Although four patients with N1 disease received chemotherapy, three patients with N2 disease, who underwent surgery before 1997, did not receive any adjuvant therapy. Following their surgical removal, there was no locoregional recurrence of these sub-centimetre cancers. However, there was distant metastatic recurrence in two patients (1.4%). One patient had multiple metastatic brain tumours and the other had multiple metastatic bone and lung tumours 17 months after surgery (Table 4). The 3-, 5- and 10-year overall survival rates after the resection for these small lung cancers were 95.7, 92.2 and 85.7%, respectively (Fig. 1). The 10-year overall survival rates for lobectomy, segmentectomy and wedge resection was 85.3, 90.0 and 89.2%, respectively (Fig. 2). There was no significant difference in the overall survival between lobectomy with sublobar resection (P = 0.635), segmentectomy or wedge resection. The 5-year overall survival rates for lung resection with VATS and with open thoracotomy (i.e. without VATS) were 88.5 and 95.3%, respectively (Fig. 3). There was no significant difference in overall survival between VATS and open thoracotomy (P = 0.322). The 10-year overall survival rates, following resection for lung cancer smaller than 1 cm, with N0, N1 and N2 diseases were 85.5% (n = 136), 75.0% (n = 4) and 100% (n = 3), respectively.
Table 4:
Recurrence and survival patterns (n = 143)
| Overall recurrence | |
| Locoregional | 0 |
| Distant | 2 (1.4%) |
| Brain | 1 (0.7%) |
| Bone + lung | 1 (0.7%) |
| Overall survival (%) | |
| 3 years | 95.7% |
| 5 years | 92.2% |
| 10 years | 85.7% |
Figure 1:
Kaplan-Meier survival curve shows overall survival rates after resection for lung cancer measuring less than 1 cm in size. The 3-, 5-, 10-year survival rates were 95.7, 92.2, 85.7%, respectively.
Figure 2:
Kaplan-Meier survival curve shows 10-year overall survival rates for patients undergoing lobectomy, segmentectomy, and wedge resection for lung cancer measuring less than 1 cm (95.7, 92.2, 85.7%, respectively). There were no significant differences between the three groups.
Figure 3:
Kaplan-Meier survival curve shows 5-year overall survival rates for patients with VATS and without VATS (thoracotomy) for lung cancer measuring less than 1 cm (95.3, 88.5%, respectively). There were no significant differences between the two groups.
DISCUSSION
The increased use of CT also increased detection and treatment of small-sized lung cancers [4–6]. A tumour under 2 cm in size is recognized as small-sized lung cancer by a subdivision of the ‘T’ status in the seventh edition of the TNM classification of lung cancer. Surgical management, including the method for the clinical diagnosis of small-sized lung cancer, is very important. It is very difficult to determine whether a microscopic tumour with GGO and size less than 1 cm is a benign or malignant tumour. Since we do not have a good system for preoperatively distinguishing benign from malignant nodules, 86 patients with benign sub-centimetre nodules underwent lung resection at our institution during this time period. As it was difficult to diagnose a tumour smaller than 1 cm, we performed preoperative TBLB in only 20 patients (14%). In fact, a preoperative diagnosis was achieved in only 12 patients (8.4%) in this study. Almost all of these diagnoses were determined by intraoperative evaluations, combined with VATS. A superficial pulmonary tumour with pleural change and lung prominence is easy to detect using palpation or inspection. However, it is difficult to detect the accurate location of a tumour characterized by GGO. We used a hook-wire needle marker with CT preoperatively in 68 patients. Furthermore, as we were unable to reach a pathological diagnosis without resection, we performed major lung resection, including segmentectomy, in a further 11 cases.
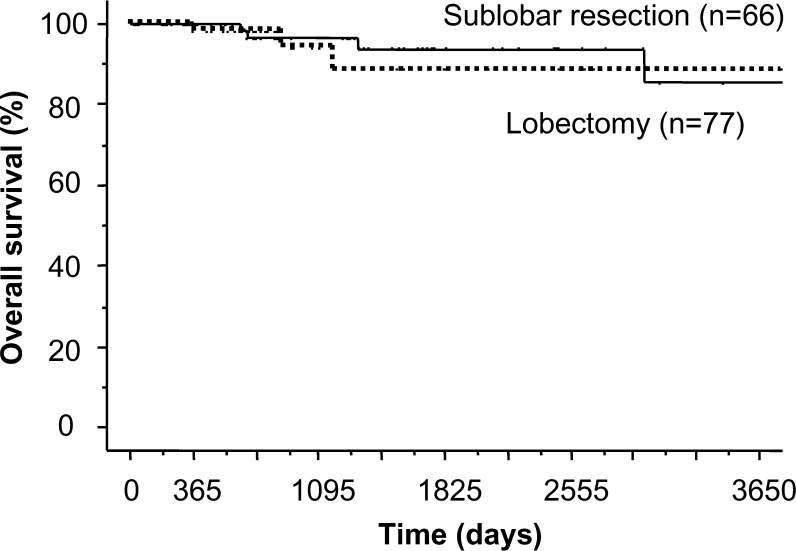
Thomas et al. reported that lung operations with VATS are safe surgical procedures [7]. Mathew et al. reported that there is no significant difference in the prognosis between lobectomy and sublobar resection (segmentectomy or wedge resection) for lung cancer smaller than 1 cm [3]. The present study also found no significant difference between VATS and open approaches, and no significant difference was observed between the lobectomy and sublobar resection (Fig. 4). Miller et al. reported that seven of 100 patients with sub-centimetre lung cancer had lymph node metastasis and suggested that lobectomy with lymph node dissection should be performed even if non-small-cell lung cancer is less than 1 cm diameter [2].
Figure 4:
Kaplan-Meier survival curve shows 10-year overall survival rates for patients undergoing lobectomy and sublobar resection for lung cancer measuring less than 1 cm (88.5, 85.3%, respectively). There were no significant differences between the two groups.
The rate of lymph node metastasis was 4.9% in the current series and was similar to the previous report. It may be necessary to perform intraoperative diagnosis of lymph node metastasis for sublobar resections. Lymph node metastases occurred in seven patients (4.9%), six who underwent the standard lobectomy with lymph node dissection and one who received basal segmentectomy with an intraoperative diagnosis of the lymph nodes. Therefore, five of those patients were underwent the operation before December 2004 survived and two had died, one due to cerebral infarction after 165 months and one from oesophageal cancer 39 months after lung resection. The absence of locoregional recurrence in sublobar resections may be due to ample free surgical margin because the lung cancers were smaller than 1 cm. Six out of seven cases with nodal involvement underwent lobectomy with systematic node dissection. Even if only one case underwent basal segmentectomy, hilar and mediastinal lymph node dissection were completed. Both ample free surgical margin and lymph node dissection are the reasons why we did not find locoregional recurrence in sublobar resection.
Yamato [8] et al. and Kondo [9] et al. reported that only lung cancers less than 1 cm with GGO, classified pathologically as types A and B of Noguchi's classification, seemed to have a good prognosis following wedge resection without lymph node dissection. Most sub-centimetre lung cancers tend to be peripheral lung adenocarcinoma, as seen in this study. It is important to select the best surgical procedure—standard lobectomy or sublobar resection—for small-sized lung tumour based on the extent of GGO in the CT findings, or for a non-invasive type of lung cancer such as Noguchi's type A and B. A micropapillary pattern is associated with a poor prognosis, even with small-sized lung cancers measuring less than 2 cm [10, 11]. Therefore, it is important to assess not only the tumour size but also the biological characteristics of the tumour, when performing lung resection. We did not evaluate biological characteristics in this study. However, it may be important to investigate biological characteristics of small-sized lung cancer in the future.
CONCLUSIONS
Lung resection was performed for 143 primary lung cancers measuring 1 cm or less in size. There was no significant difference in the overall survival rate between sublobar resection and lobectomy. However, seven patients had hilar or mediastinal lymph node metastases and distant recurrent cancer occurred in two patients. Therefore, our findings lead us to recommend the careful selection of the surgical procedure, and a long-term follow-up is required, even if the lung cancer measures less than 1 cm in diameter.
Conflict of interest: none declared.
REFERENCES
- 1.Sobin LH, Gospodarowicz MK, Wittekind Ch. TNM Classification of Malignant Tumors. 7th edn. Unio Internationalis Contra Cancrum. Blackwell Publishing Ltd; 2010. [Google Scholar]
- 2.Miller DL, Rowland CM, Deschamps C, Allen MS, Trastek VF, Pairolero PC. Surgical treatment of non-small cell lung cancer 1 cm or less in diameter. Ann Thorac Surg. 2002;73:1545–50. doi: 10.1016/s0003-4975(02)03525-7. [DOI] [PubMed] [Google Scholar]
- 3.Matthew JS, Arman K, Arjun P, Katie SN, David OW, James DL, et al. Oncologic outcomes after surgical resection of subcentimeter non-small cell lung cancer. The Society of Thoracic Surgeons. 2011;91:1681–8. doi: 10.1016/j.athoracsur.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 4.MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax. 2004;59:237–41. doi: 10.1136/thx.2003.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin MS, Drucker EA, McLoud TC, Shepard JO. Small pulmonary nodules: detection at chest CT and outcome. Radiology. 2003;226:489–93. doi: 10.1148/radiol.2262010556. [DOI] [PubMed] [Google Scholar]
- 6.Altorki N, Kent M, Pasmantier M. Detection of early-stage lung cancer: computed tomographic scan or chest radiograph? J Thorac Cardiovasc Surg. 2001;121:1053–7. doi: 10.1067/mtc.2001.112827. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P, Doddoli C, Yena S, Thirion X, Sebag F, Fuentes P, et al. VATS is an adequate oncological operation for stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2002;21:1094–9. doi: 10.1016/s1010-7940(02)00179-3. [DOI] [PubMed] [Google Scholar]
- 8.Yamato Y, Tsuchida M, Watanabe T, Aoki T, Koizumi N, Umezu H, et al. Early result of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg. 2001;71:971–4. doi: 10.1016/s0003-4975(00)02507-8. [DOI] [PubMed] [Google Scholar]
- 9.Kondo D, Yamada K, Kitayama Y, Hoshi S. Peripheral lung adenocarcinomas. 10mm or less in diameter. Ann Thorac Surg. 2003;76:350–5. doi: 10.1016/s0003-4975(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami T, Nabeshima K, Makimoto Y, Hamasaki M, Iwasaki A, Shirakusa T, et al. Micropapillary pattern and grade of stromal invasion in pT1 adenocarcinoma of the lung: usefulness as prognostic factors. Mod Pathol. 2007;20:514–21. doi: 10.1038/modpathol.3800765. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami T, Nabeshima K, Hamasaki M, Iwasaki A, Shirakusa T, Iwasaki H. Small cluster invasion: a possible link between micropapillary pattern and lymph node metastasis in pT1 lung adenocarcinomas. Virchows Arch. 2009;454:61–70. doi: 10.1007/s00428-008-0695-5. [DOI] [PubMed] [Google Scholar]