Abstract
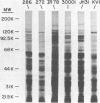
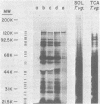
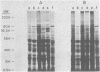
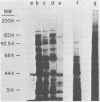
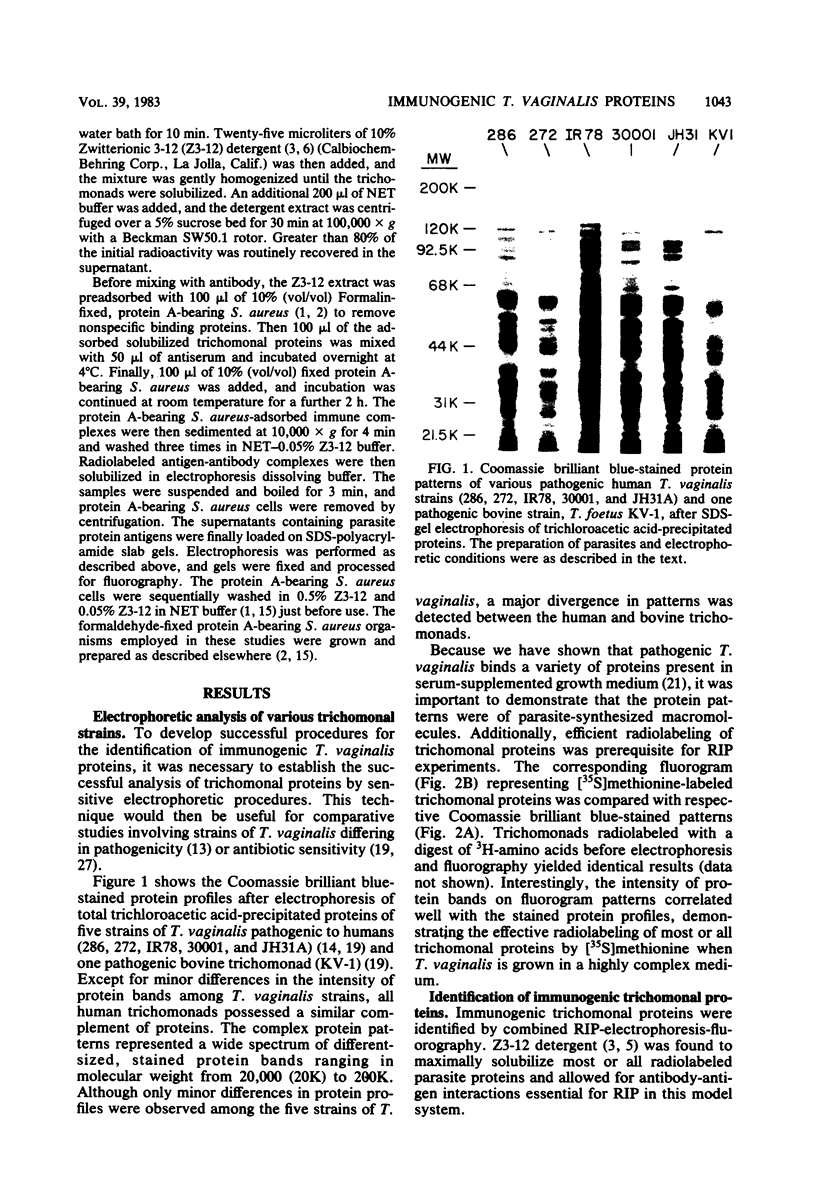
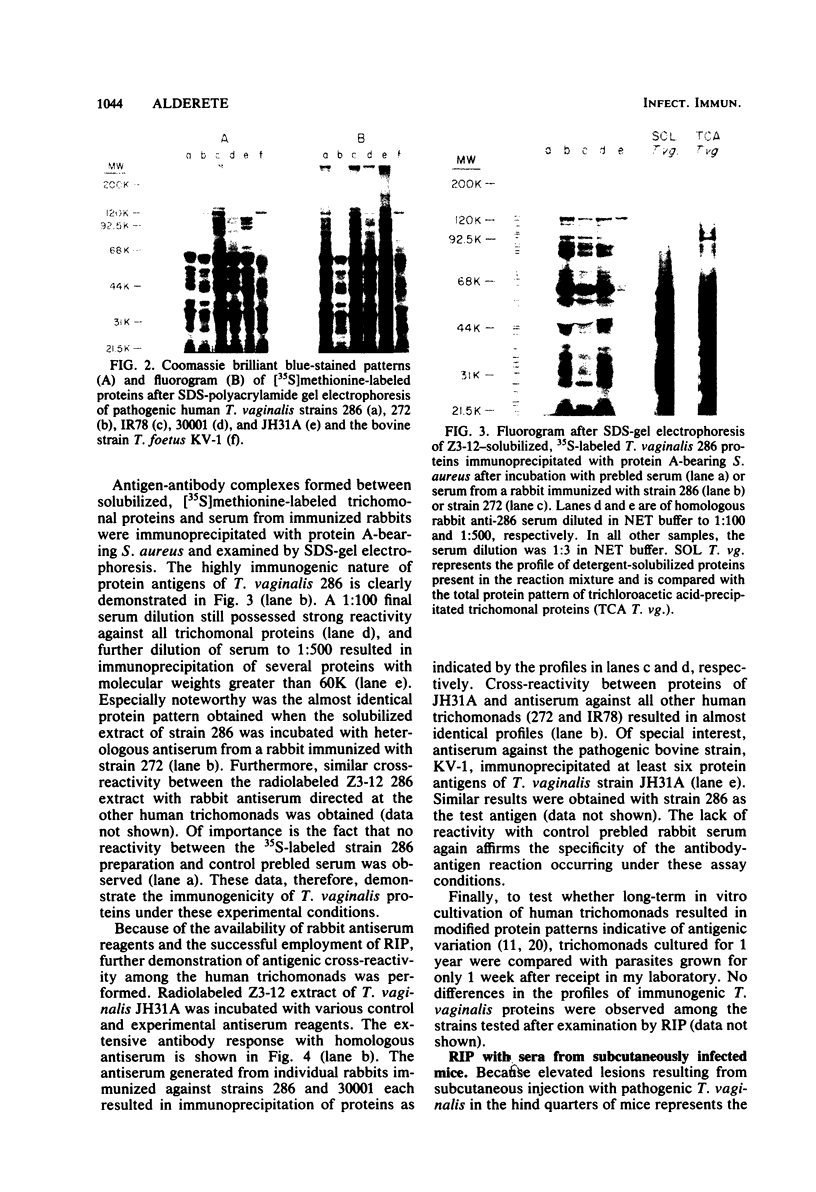
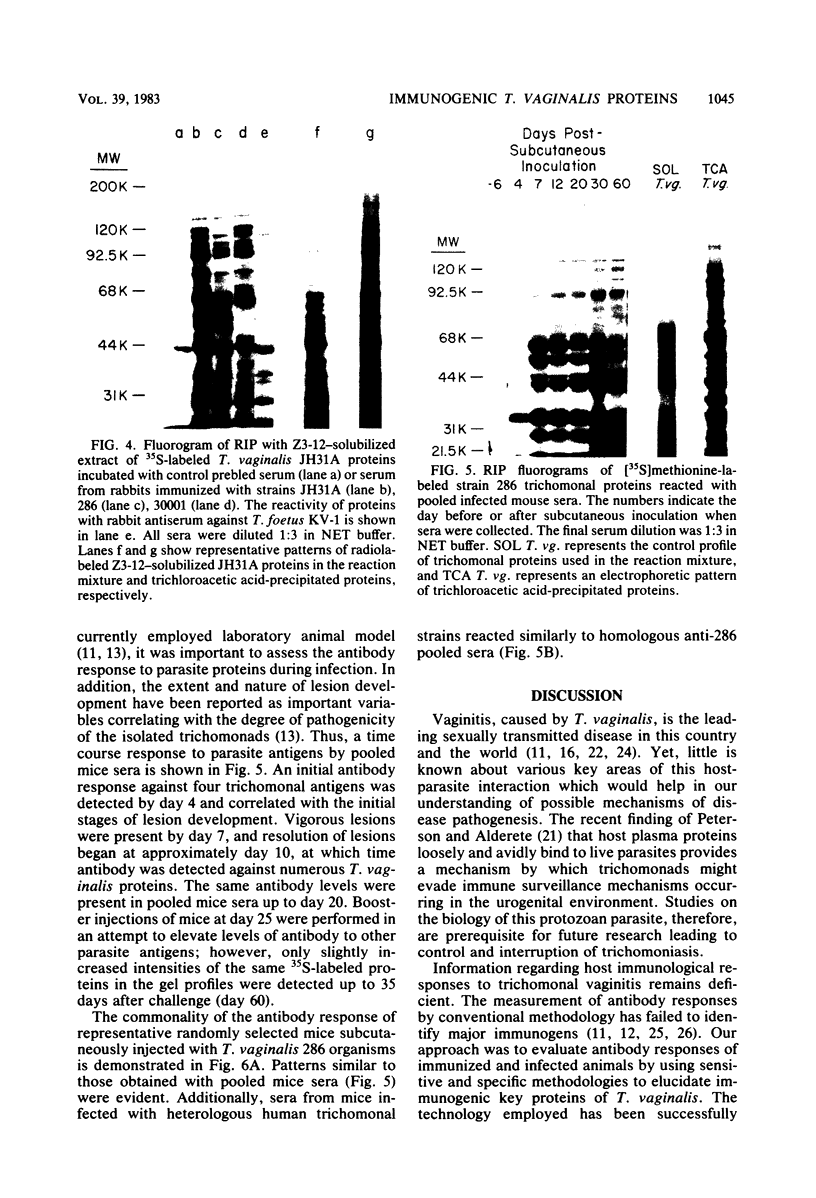
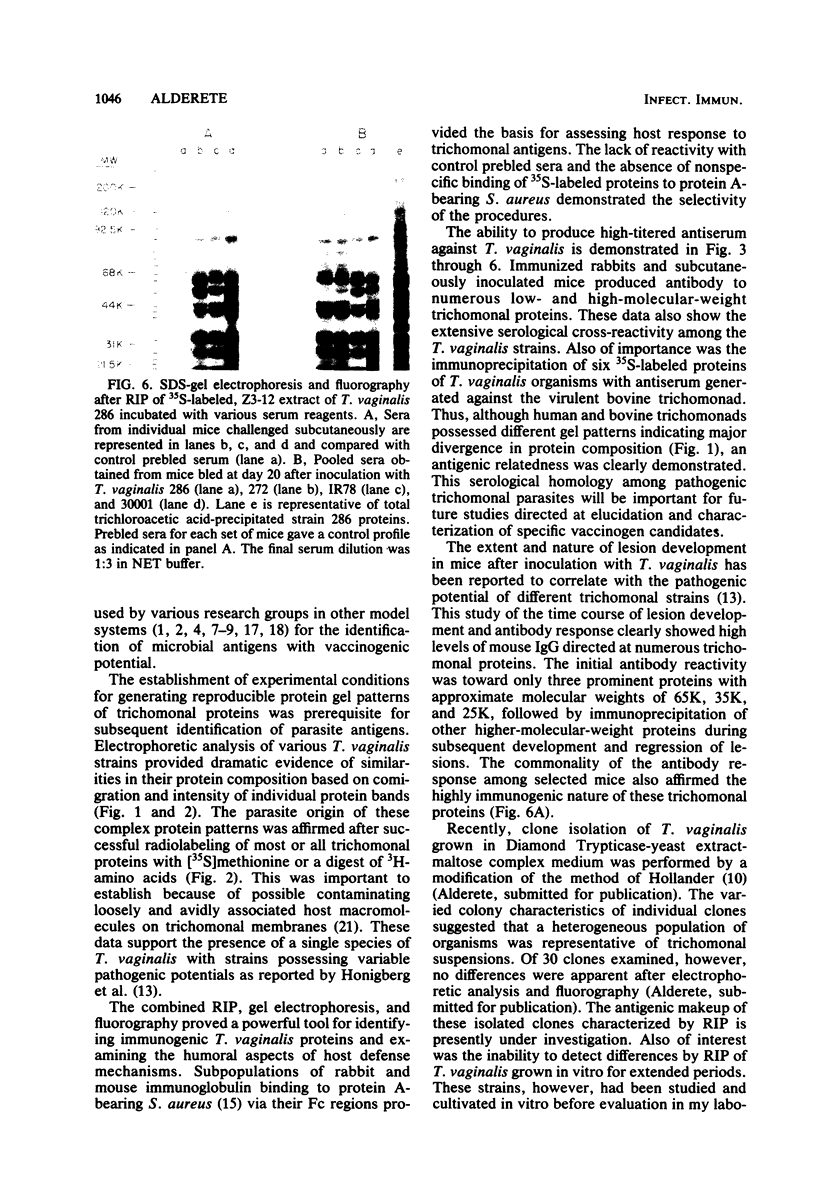
Analysis of several human strains of Trichomonas vaginalis and one bovine strain of Tritrichomonas foetus was accomplished with standard sodium dodecyl sulfate-gel electrophoresis and fluorography technology. Highly motile, live trichomonads were radiolabeled, and total trichloroacetic acid-precipitated proteins were electrophoresed. Complex protein profiles of the various human strains of T. vaginalis were obtained with proteins ranging in molecular weight from 20,000 to greater than 200,000. The parasite biosynthesis of the Coomassie brilliant blue-stained protein bands was demonstrated by efficient radiolabeling of trichomonads with [35S]methionine or a 3H-amino acid digest before electrophoresis and fluorography. Immunogenic trichomonal proteins were then identified by a radioimmunoprecipitation method. A detergent extract of [35S] methionine-labeled T. vaginalis proteins was mixed with serum from an immunized rabbit or pooled sera from subcutaneously infected mice and soluble antibody-antigen complexes isolated by adsorption to protein A-bearing Staphylococcus aureus. The radiolabeled protein antigens were then identified by gel electrophoresis and fluorography. Immunized rabbit serum and pooled sera from challenged mice contained high-titered antibody which reacted with numerous high- and low-molecular-weight proteins. Individual subcutaneously infected mice were found to possess identical antibody responses to these immunogenic trichomonal proteins. A high degree of serological cross-reactivity among the various trichomonads was demonstrated. No differences in the composition of immunogenic proteins were observed among cultures grown in vitro for various lengths of time under the experimental conditions employed. Finally, electrophoretic analysis of cloned colonies of T. vaginalis organisms revealed no differences in their protein composition. The biological relevance of these observations is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Analysis of serum IgG against Treponema pallidum protein antigens in experimentally infected rabbits. Br J Vener Dis. 1981 Oct;57(5):302–308. doi: 10.1136/sti.57.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Thompson T. E. Solubilization of bacterial membrane proteins using alkyl glucosides and dioctanoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Mar 25;382(3):276–285. doi: 10.1016/0005-2736(75)90270-9. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Handman E., Remington J. S. Antibody responses to toxoplasma antigens in mice infected with strains of different virulence. Infect Immun. 1980 Jul;29(1):215–220. doi: 10.1128/iai.29.1.215-220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D. H. Colonial morphology of Trichomonas vaginalis in Agar. J Parasitol. 1976 Oct;62(5):826–828. [PubMed] [Google Scholar]
- Honigberg B. M., Goldman M. Immunologic analysis by quantitative fluorescent antibody methods of the effects of prolonged cultivation on Trichomonas gallinae. J Protozool. 1968 Feb;15(1):176–184. doi: 10.1111/j.1550-7408.1968.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Krieger J. N. Urologic aspects of trichomoniasis. Invest Urol. 1981 May;18(8):411–417. [PubMed] [Google Scholar]
- Machamer C. E., Hayes E. C., Gollobin S. D., Westfall L. K., Zweerink H. J. Antibodies against the measles matrix polypeptide after clinical infection and vaccination. Infect Immun. 1980 Mar;27(3):817–825. doi: 10.1128/iai.27.3.817-825.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Meingassner J. G., Miller W. A., Ledger W. J. Three metronidazole-resistant strains of Trichomonas vaginalis from the United States. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 1):808–812. doi: 10.1016/s0002-9378(16)32741-7. [DOI] [PubMed] [Google Scholar]
- Nielsen M. H. The ultrastructure of Trichomonas vaginalis donné before and after transfer from vaginal secretion to Diamonds medium. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):581–589. doi: 10.1111/j.1699-0463.1975.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. F., Chapel T. A. Trichomoniasis, candidiasis, and the minor venereal diseases. Clin Obstet Gynecol. 1975 Mar;18(1):73–88. doi: 10.1097/00003081-197503000-00008. [DOI] [PubMed] [Google Scholar]
- Soendjojo A., Pindha S. Trichomonas vaginalis infection of the median raphe of the penis. Sex Transm Dis. 1981 Oct-Dec;8(4):255–257. doi: 10.1097/00007435-198110000-00003. [DOI] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Stepkowski S., Honigberg B. M. Antigenic analysis of virulent and avirulent strains of Trichomonas gallinae by gel diffusion methods. J Protozool. 1972 May;19(2):306–315. doi: 10.1111/j.1550-7408.1972.tb03465.x. [DOI] [PubMed] [Google Scholar]
- Thurner J., Meingassner J. G. Isolation of Trichomonas vaginalis resistant to metronidazole. Lancet. 1978 Sep 30;2(8092 Pt 1):738–738. doi: 10.1016/s0140-6736(78)92739-3. [DOI] [PubMed] [Google Scholar]