Abstract
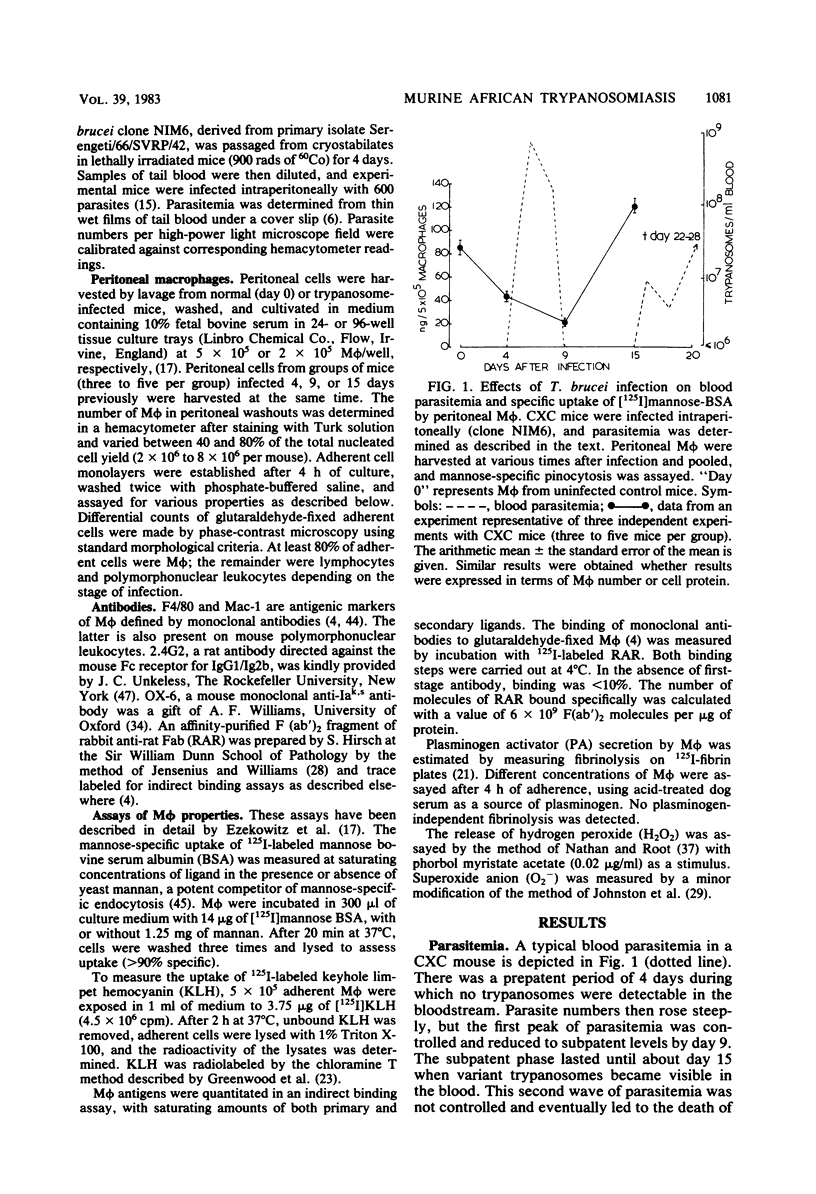
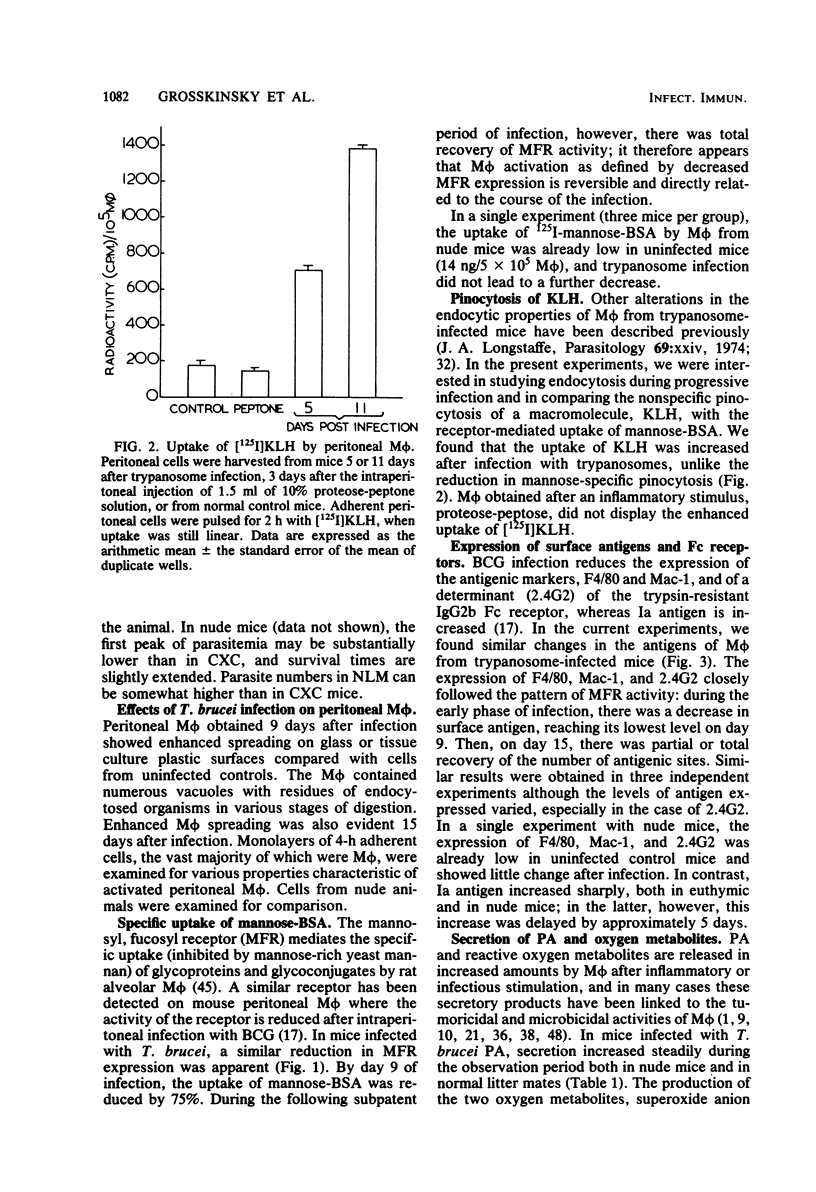
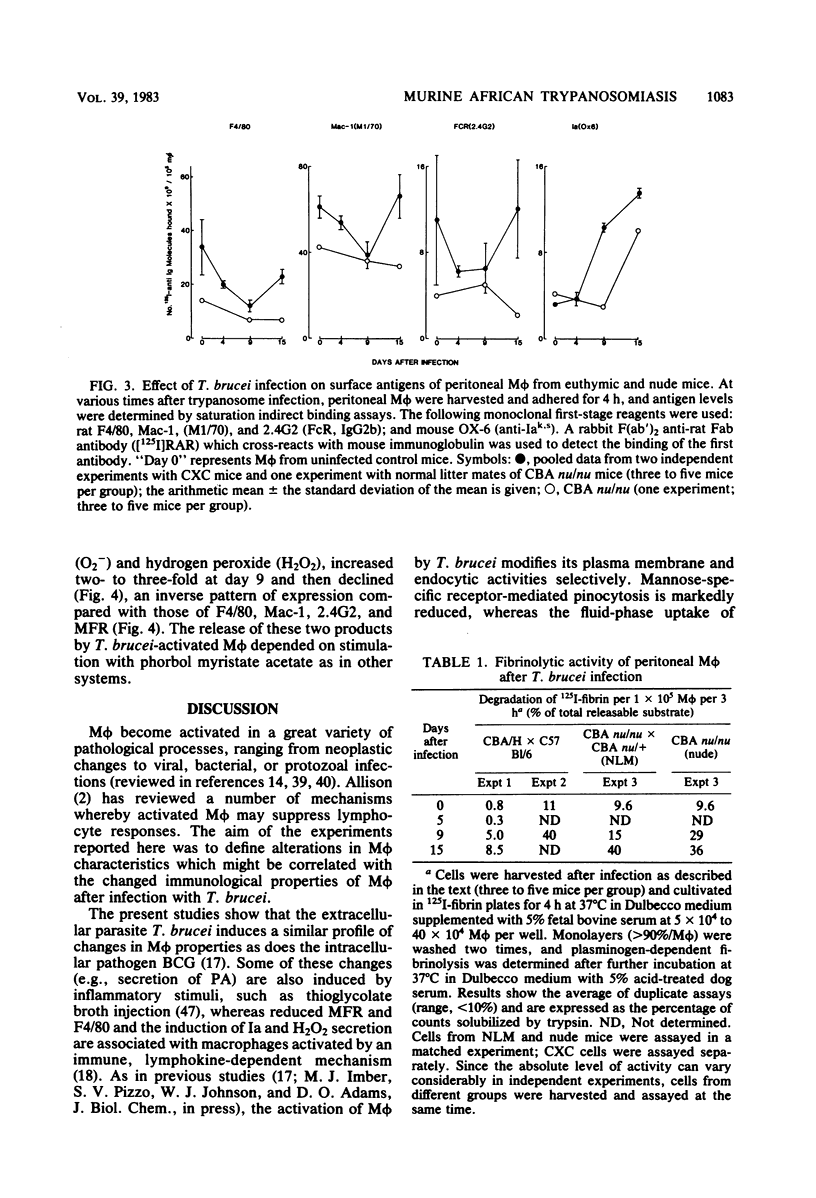
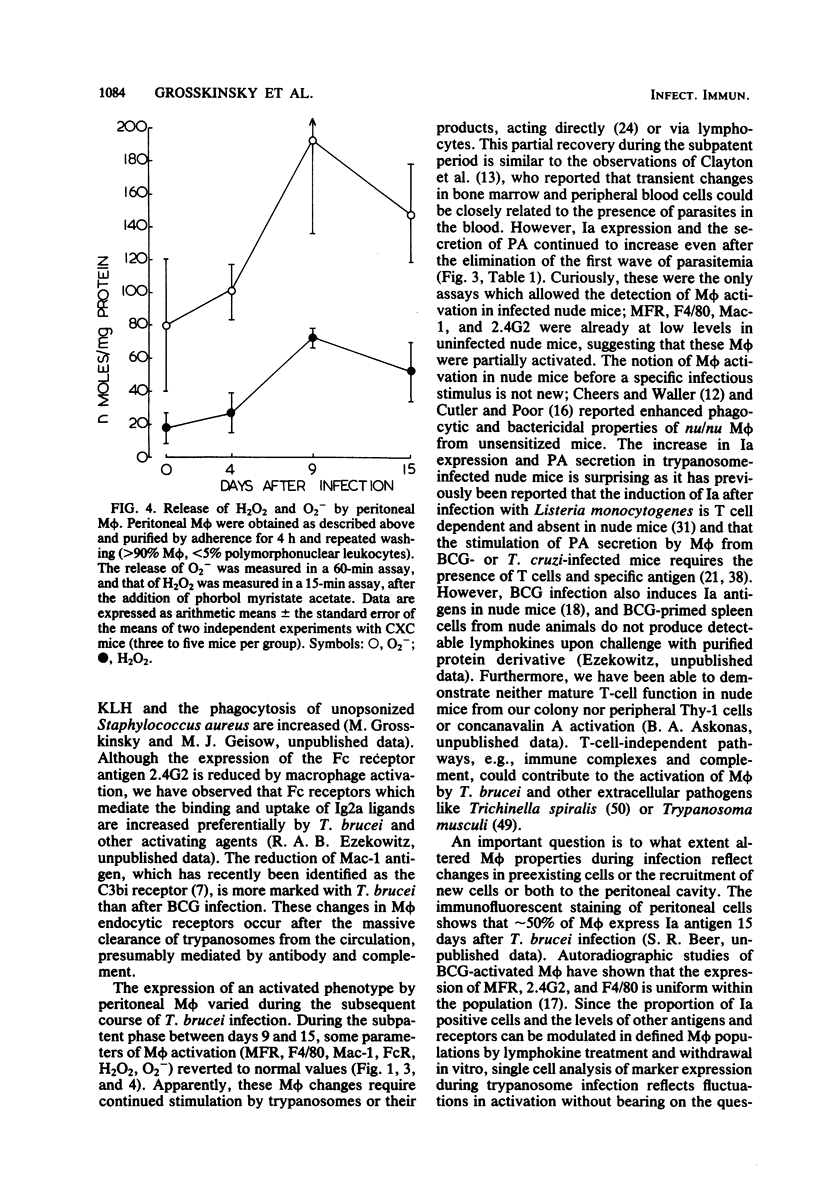
African trypanosomiasis is accompanied by a profound general immunosuppression in which both suppressive T cells and macrophages (M phi) have been implicated. The present studies define changes in the M phi surface, endocytic and secretory properties, during the infection of mice by Trypanosoma brucei. Peritoneal M phi obtained after the control of the first wave of parasitemia displayed characteristics similar to those activated by intracellular pathogens, such as Mycobacterium bovis bacillus Calmette-Guérin, e.g., the enhanced expression of Ia antigen, decreased M phi-specific antigens, receptors mediating the pinocytosis of mannose-terminal glycoproteins, and an increased ability to secrete plasminogen activator, superoxide anion, and H2O2. Some markers of macrophage activation persisted during the subpatent period before the recurrence of parasitemia, whereas others reverted to normal. Mature T cell function appears not to be essential for M phi activation by T. brucei since the infection of athymic nude mice also induced Ia antigens and plasminogen activator. These studies show that M phi activated by different pathways express common features which may contribute to immune dysfunction observed in trypanosomiasis, as well as in other infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. Effector mechanisms of cytolytically activated macrophages. I. Secretion of neutral proteases and effect of protease inhibitors. J Immunol. 1980 Jan;124(1):286–292. [PubMed] [Google Scholar]
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Askonas B. A., Corsini A. C., Clayton C. E., Ogilvie B. M. Functional depletion of T- and B-memory cells and other lymphoid cell subpopulations-during trypanosomiasis. Immunology. 1979 Feb;36(2):313–321. [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Schell R. F., Le Frock J. L. Evidence for depletion of Ia+ macrophages and associated immunosuppression in African trypanosomiasis. Infect Immun. 1981 Apr;32(1):188–193. doi: 10.1128/iai.32.1.188-193.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell H. S., Sharrow S. O., Singer A. Role of accessory cells in B cell activation. I. Macrophage presentation of TNP-Ficoll: evidence for macrophage-B cell interaction. J Immunol. 1980 Feb;124(2):989–996. [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation: possible involvement of oxygen metabolites in killing of Leishmania enrietti by activated mouse macrophages. J Reticuloendothel Soc. 1981 Mar;29(3):181–192. [PubMed] [Google Scholar]
- Charoenvit Y., Campbell G. H., Tokuda S. Suppression of parasite antigen-specific lymphoid blastogenesis in African trypanosomiasis. J Immunol. 1981 Dec;127(6):2350–2354. [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Clayton C. E., Selkirk M. E., Corsini C. A., Ogilvie B. M., Askonas B. A. Murine trypanosomiasis: cellular proliferation and functional depletion in the blood, peritoneum, and spleen related to changes in bone marrow stem cells. Infect Immun. 1980 Jun;28(3):824–831. doi: 10.1128/iai.28.3.824-831.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E., Poor A. H. Effect of mouse phagocytes on Candida albicans in in vivo chambers. Infect Immun. 1981 Mar;31(3):1110–1116. doi: 10.1128/iai.31.3.1110-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Austyn J., Stahl P. D., Gordon S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981 Jul 1;154(1):60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Gordon S. Down-regulation of mannosyl receptor-mediated endocytosis and antigen F4/80 in bacillus Calmette-Guérin-activated mouse macrophages. Role of T lymphocytes and lymphokines. J Exp Med. 1982 Jun 1;155(6):1623–1637. doi: 10.1084/jem.155.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin L. G., Green D. G., Guy M. W., Voller A. Immunosuppression during trypanosomiasis. Br J Exp Pathol. 1972 Feb;53(1):40–43. [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Cohn Z. A. Bacille Calmette-Guérin infection in the mouse. Regulation of macrophage plasminogen activator by T lymphocytes and specific antigen. J Exp Med. 1978 Apr 1;147(4):1175–1188. doi: 10.1084/jem.147.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Whittle H. C., Molyneux D. H. Immunosuppression in Gambian trypanosomiasis. Trans R Soc Trop Med Hyg. 1973;67(6):846–850. doi: 10.1016/0035-9203(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Grosskinsky C. M., Askonas B. A. Macrophages as primary target cells and mediators of immune dysfunction in African trypanosomiasis. Infect Immun. 1981 Jul;33(1):149–155. doi: 10.1128/iai.33.1.149-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J., McConnell I., Raniwalla J. Antigen-induced non-specific suppressor factor in sheep efferent lymph is prostaglandin E2. Immunology. 1981 May;43(1):205–212. [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Jensenius J. C., Williams A. F. The binding of anti-immunoglobulin antibodies to rat thymocytes and thoracic duct lymphocytes. Eur J Immunol. 1974 Feb;4(2):91–97. doi: 10.1002/eji.1830040207. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Lu C. Y., Calamai E. G., Unanue E. R. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature. 1979 Nov 15;282(5736):327–329. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M. Immunobiology of African trypanosomiasis. Cell Immunol. 1978 Aug;39(1):204–210. doi: 10.1016/0008-8749(78)90094-1. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M., Levine R. F., Dempsey W. L., Wellhausen S. R., Hansen C. T. Lymphocyte function in experimental African trypanosomiasis. IV. Immunosuppression and suppressor cells in the athymic nu/nu mouse. Cell Immunol. 1981 Sep 1;63(1):210–215. doi: 10.1016/0008-8749(81)90043-5. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Monoclonal antibodies to Ia antigens from rat thymus: cross reactions with mouse and human and use in purification of rat Ia glycoproteins. Immunol Rev. 1979;47:117–137. doi: 10.1111/j.1600-065x.1979.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Nathan C. F., Arrick B. A., Murray H. W., DeSantis N. M., Cohn Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Gordon S., Cohn Z. Trypanosoma cruzi: the immunological induction of macrophage plasminogen activator requires thymus-derived lymphocytes. J Exp Med. 1977 Jul 1;146(1):172–183. doi: 10.1084/jem.146.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. Mechanisms of macrophage activation. Clin Exp Immunol. 1980 May;40(2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. W., Roelants G. E., Lundin L. B., Mayor-Withey K. S. Immune depression in trypanosome-infected mice. I. Depressed T lymphocyte responses. Eur J Immunol. 1978 Oct;8(10):723–727. doi: 10.1002/eji.1830081010. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Askonas B. A. Trypanosome-induced suppression of anti-parasite responses during experimental African trypanosomiasis. Eur J Immunol. 1980 Dec;10(12):971–974. doi: 10.1002/eji.1830101216. [DOI] [PubMed] [Google Scholar]
- Scott J. M., Pegram R. G., Holmes P. H., Pay T. W., Knight P. A., Jennings F. W., Urquhart G. M. Immunosuppression in bovine trypanosomiasis: field studies using foot-and-mouth disease vaccine and clostridial vaccine. Trop Anim Health Prod. 1977 Aug;9(3):159–165. doi: 10.1007/BF02236590. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P., Caristan A., Pautrizel R. Macrophage function during Trypanosoma musculi infection in mice. Infect Immun. 1981 Nov;34(2):378–381. doi: 10.1128/iai.34.2.378-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]



