Abstract
Background
Renal function is a strong predictor of adverse events in heart failure. Current renal function measures are imperfect and Cystatin C (CysC) is promoted as a better marker of glomerular filtration rate (GFR). This study compares the prognostic utility of CysC and derived GFR estimates with other measures of renal function in patients with chronic heart failure.
Methods and Results
We measured serum CysC levels in 823 heart failure patients undergoing coronary angiography with follow-up of major adverse cardiovascular events (MACE = death, myocardial infarction, stroke). Cystatin C levels strongly correlated with creatinine (r = 0.73), blood urea nitrogen (r = 0.70), and eGFRMDRD (r = −0.62) (all p < 0.001). However, the correlation was lower in eGFR ≥ 60ml/min/1.73m2. CysC-based measures significantly improved areas under the ROC curve for the prediction of MACE, especially in eGFR ≥ 60ml/min/1.73m2 (p < 0.01). Net reclassification improvement was 22.2% (p < 0.001) in this group. CysC remained an independent predictor of MACE (p < 0.001) after adjustment for traditional risk factors and BNP.
Conclusions
Cystatin C is an independent predictor of adverse events in chronic heart failure. It adds prognostic value to creatinine, particularly in patients with “preserved” renal function.
Keywords: heart failure, kidney, prognosis
Although risk prediction in individual patients with heart failure remains challenging, many factors have shown to be predictive of adverse long-term outcomes. Renal dysfunction and elevated natriuretic peptides are among the strongest predictors 1–3. Renal insufficiency has often been considered as a reduction in glomerular filtration rate (GFR), and is traditionally quantified based on serum creatinine (sCr) levels or equations that are derived from sCr measurements. However, the prognostic utility of these sCr-based estimates in the chronic heart failure population remains inferior to GFR, directly quantified by 125I-iothalamate clearance, suggesting that confounders exist 4. Nevertheless, cardiovascular risk is believed to be significantly elevated below an estimated GFR of 60 ml/min/1.73m2 based on these equations 5.
In recent years, cystatin C (CysC) has emerged as a sensitive measure of renal function. Cystatin C is a cysteine protease inhibitor produced by nearly all nucleated human cells at a fairly constant rate (housekeeping gene) and is subsequently circulated in the bloodstream. Compared to creatinine, levels of CysC are less influenced by age, gender, or race. Because of its low molecular weight (13 kDa), it is freely filtered and neither secreted from nor reabsorbed back into the bloodstream (although it is metabolized by the proximal tubular cells)6. All these properties render CysC a potentially better estimate of GFR than sCr-based estimates. In the chronic kidney disease population, estimates of GFR have incorporated CysC as part of the new equations 7. CysC has also proven to be a better predictor of mortality and cardiovascular events than sCr-based estimates in different populations studied (elderly, coronary heart disease, acute and chronic heart failure) 8–11.
Natriuretic peptides have found their way to everyday cardiology practice for diagnostic as well as prognostic purposes in patients with heart failure. Natriuretic peptides reflect myocardial stress, and have proven to predict outcome after coronary syndromes, after hospitalization for acute heart failure and in chronic heart failure 2. Despite BNP levels being somewhat dependent on renal function, BNP and CysC potentially constitute a unique biomarker combination for risk prediction, particularly in the setting of relatively preserved renal function.
The aims of this study were: 1) to examine the prognostic ability of CysC and derived equations, independent of BNP; 2) to examine its incremental value over traditional markers of renal function; and 3) to investigate risk stratification by the combination of BNP and CysC in a chronic stable heart failure population undergoing cardiac evaluation.
Methods
Patient Population and Study Design
We evaluated 823 stable patients with a clinical history of heart failure undergoing elective coronary angiography as part of the GeneBank study. GeneBank was a large prospective study, conducted at the Cleveland Clinic between 2001 and 2006 (NCT 00590200) that established a well-characterized clinical repository with clinical and longitudinal outcomes. Inclusion criteria were: Age ≥ 18 years and planned coronary angiography, There were no specific exclusion criteria. All participants gave written informed consent approved by the Cleveland Clinic Institutional Review Board. At the time of cardiac catheterization, blood samples were collected, processed, and stored at −80°F until analyses.
Renal function was expressed as sCr, urea levels (BUN), CysC, estimated GFR by either the four-variable MDRD equation (eGFRMDRD = 186 × sCr−1.154 × age−0.203 × [0.742 if female] × [1.21 if black]), the CKD-EPI equation for Cystatin C (eGFRCysC = 127.7 × CysC−1.17 × age−0.13 × [0.91 if female] × [1.06 if black]), or the eGFRsCr+CysC equation (eGFRsCr+CysC = 177.6 × sCr−0.65 × CysC−0.57 × age−0.20 × [0.82 if female] × [1.11 if black]). “Preserved” and “impaired” renal function were defined as eGFRMDRD ≥ 60 ml/min/1.73m2 and eGFRMDRD < 60 ml/min/1.73m2 respectively.
The endpoint for this study was a composite of major adverse cardiovascular events (MACE), including all-cause mortality, non-fatal myocardial infarction and non-fatal cerebrovascular accident (CVA). Non-fatal events were defined as MI or CVA in patients who survived at least 48 hours following the onset of symptoms. These clinical outcomes were prospectively ascertained over the ensuing 3 years for all subjects after enrollment, adjudicated and verified by source documentation.
Cystatin C Assay
Plasma CysC level was determined with a particle enhanced immunoturbidimetric immunoassay on the Architect ci8200 platform (Abbott Laboratories, Abbott Park, IL, USA). Briefly, latex particles are coated with anti-human CysC antibody and agglutinate with CysC present in the patient’s sample. The result is a change in absorbance which is proportional to the amount of CysC present in the sample. The analytical range spans 0.05mg/L to the highest calibration point. Intra- and inter-assay coefficients are 3.1 and 6% respectively. B-type natriuretic peptide, high-sensitivity C-reactive protein (hsCRP), sCr, BUN, fasting blood glucose and lipid profiles were all measured by clinically-approved assays on the same platform. The intra- and inter-assay coefficients were respectively 0.9% and 0.8% for BUN, 1.7% and 1% for sCr, 2.6% and 3.5% for BNP and 4% and 2.4% for hsCRP,
Statistical Analyses
Continuous variables were expressed as mean ± standard deviation if normally distributed or as median and inter-quartile range for non-normally distributed data. Categorical data were summarized as proportions and frequencies. Baseline characteristics between patients with preserved and decreased renal function were compared, using the student t-test, the Wilcoxon rank sum test or the chi-square test as appropriate. Spearman’s rank correlation method was used as a nonparametric measure of association for correlations between CysC and the different measures of renal function (sCr, BUN, eGFR equations). Areas under the ROC curves (c-statistics) for the prediction of MACE were compared between different measures of renal function and net reclassification improvement was calculated. Univariate Cox proportional-hazards models were used to evaluate the association of the different measures of renal function along other variables, with the composite outcome. Measures of renal function were entered either as continuous variables (logarithmic transformed when non-normally distributed), either categorized in quartiles. Since sCr levels differ substantially between genders, sex-specific quartiles for sCr were used to equalize the distribution of men and women. Afterwards two types of multivariate Cox proportional-hazards models were constructed; a shorter model, using one measure of renal function in combination with BNP, and a longer model with a measure of renal function adjusted for all variables with a p-value < 0.05 in univariate analysis. The 3-year outcome was evaluated by Kaplan-Meier survival analyses, using the aforementioned quartiles. Differences between groups were evaluated with the log-rank test. Statistical significance was set at a 2-tailed probability level <0.05. All statistical analyses were performed using JMP Pro 9.0 (SAS Institute, Cary, NC). All the authors had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agree to the manuscript as written.
Results
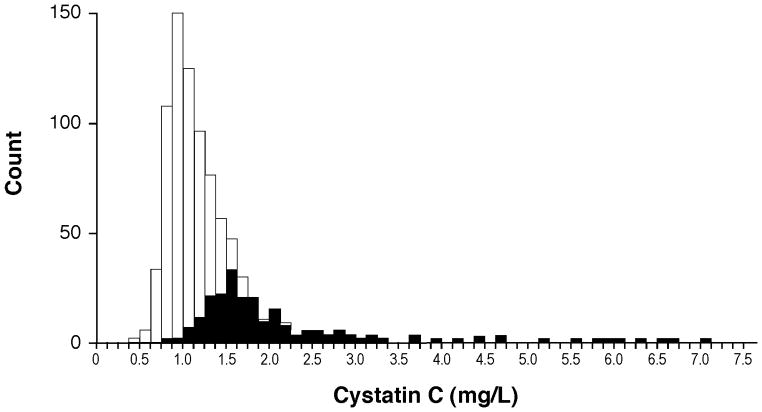
Table 1 illustrates the baseline characteristics of our study population, which included 35% of subjects with LVEF > 50%. In our study cohort, 208 subjects (25%) had impaired renal function as defined by eGFRMDRD < 60ml/min/m2. Subjects with impaired renal function were likely older, female, with more co-morbid conditions, and higher median BNP levels (Table 1). Cystatin C levels demonstrated a non-normal distribution (Figure 1), with a median level of 1.11 mg/L (interquartile range of 0.92 – 1.41 mg/L). As expected, CysC had a strong correlation with other markers of renal function. However in the subgroup with preserved renal function, such correlations were less robust with non-CysC-based measures of renal function (Online-only Data Supplement Table). There were no significant differences in baseline CysC levels between those with impaired versus preserved LVEF.
Table 1.
Baseline Characteristics.
| Total Cohort (n=823) | eGFRMDRD ≥60ml/min/1.73m2 (n=615) | eGFRMDRD <60ml/min/1.73m2 (n=208) | P-value§ | |
|---|---|---|---|---|
| Demographics: | ||||
| Age (Years) | 66 ± 11 | 65 ± 11 | 71 ± 9 | < 0.0001 |
| Male Gender (%) | 60 | 64 | 48 | < 0.0001 |
| BMI (kg/m2) | 29.5 ± 6.7 | 29.7 ± 6.7 | 28.9 ± 6.6 | 0.12 |
| Co-morbidities: | ||||
| Diabetes mellitus (%) | 29 | 23 | 45 | < 0.0001 |
| Hypertension (%) | 76 | 72 | 86 | < 0.0001 |
| Hyperlipidemia (%) | 80 | 80 | 80 | 0.68 |
| Current smoker (%) | 12 | 13 | 8 | 0.05 |
| CAD (%) | 76 | 74 | 84 | 0.002 |
| Echocardiographic Data: | ||||
| LVEF (%) | 35 (25–55) | 35 (25–50) | 40 (25–55) | 0.10 |
| Laboratory Data: | ||||
| Urea (mg/dl) | 21 (16–27) | 19 (15–23) | 35 (27–44) | < 0.0001 |
| Creatinine (mg/dl) | 0.94 (0.8–1.17) | 0.87 (0.76–1.15) | 1.41 (1.22–1.81) | < 0.0001 |
| Cystatin C (mg/dl) | 1.11 (0.92–1.41) | 1.01 (0.88–1.18) | 1.69 (1.42–2.13) | < 0.0001 |
| eGFRMDRD (ml/min/1.73m2) | 77 (60–93) | 85 (72–97) | 44 (36–53) | < 0.0001 |
| eGFRCysC (ml/min/1. 73m2) | 63 (47–80) | 71 (58–84) | 37 (30–46) | < 0.0001 |
| eGFRsCr+CysC (ml/min/1. 73m2) | 70 (53–86) | 77 (66–90) | 40 (32–48) | < 0.0001 |
| BNP (pg/ml) | 300 (118–675) | 259 (106–540) | 557 (220–1108) | < 0.0001 |
| Hemoglobin (gr/dl) | 13.2 ± 1.8 | 13.5 ± 1.7 | 12.4 ± 1.8 | < 0.0001 |
| CRP (mg/dl) | 3.8 (1.6–9) | 3.2 (1.4–7.8) | 5.5 (2.6–14.1) | < 0.0001 |
| Medications: | ||||
| ACE-I or ARB (%) | 68 | 68 | 66 | 0.49 |
| Beta-blockers (%) | 67 | 67 | 66 | 0.67 |
| Diuretic (%) | 58 | 53 | 72 | < 0.0001 |
| Aspirin (%) | 62 | 62 | 63 | 0.80 |
| Statin (%) | 59 | 60 | 57 | 0.36 |
Abbreviations: BMI, Body Mass Index; CAD, Coronary Artery Disease; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide; CRP, C-Reactive Protein; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
P-value for difference between eGFRMDRD ≥ 60ml/min/1.73m2 and eGFRMDRD < 60ml/min/1.73m2.
Figure 1. Histogram of serum Cystatin C Concentrations.
Patients with eGFRMDRD < 60ml/min/1.73m2 are projected in darker shade.
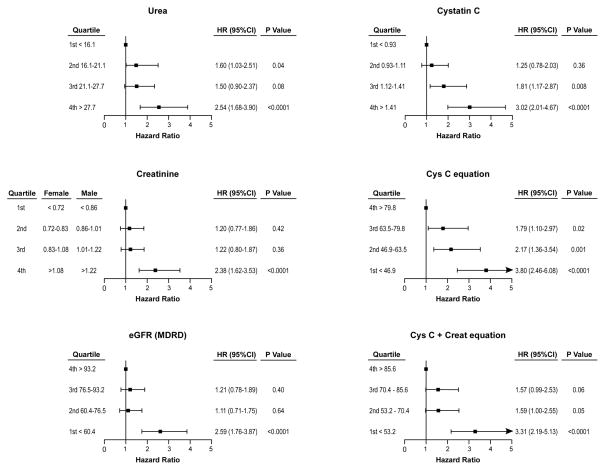
During the 3-year follow-up period, the combined endpoint of MACE occurred in 201 (24%) of the patients, including 20% in the preserved renal function group (121 patients, 132 events: 80 deaths, 41 MI and 11 CVA) and 39% in the impaired renal function group (80 patients, 93 events: 62 deaths, 25 MI and 6 CVA). Areas under the ROC curves for Cys C and Cys C-based GFR estimations are significantly better than eGFRMDRD both in the total cohort and in the subgroup of patients with eGFRMDRD ≥ 60 ml/min/1.73m2 but not in patients with eGFRMDRD < 60 ml/min/1.73m2 (Table 2). Nevertheless in the eGFRMDRD ≥ 60 ml/min/1.73m2 group, net reclassification improvement (compared to eGFRMDRD) was 22.2% with Cys C, 22.8% with eGFRCysC and 22.2% with eGFRsCr+CysC (all P < 0.001). In univariate analysis, elevated levels of all renal measures were significantly associated with higher risks for the combined outcome in the overall cohort, both when entered as a continuous variable and when divided in quartiles (Table 3 and Figure 2). Age, body mass index, diabetes mellitus, coronary artery disease, hemoglobin, hsCRP, and BNP were also significantly associated with risk of future MACE in univariate analysis (data not shown). Increasing quartiles of CysC and decreasing quartiles for eGFRCysC demonstrated progressively higher risk of future MACE (Figure 3). In particular, by adjusting for age, gender, and race in the eGFRCysC equation, there was a better separation of patients with higher eGFRCysC values. However in an analysis with covariate information (BNP, age, body mass index, history of coronary artery disease, history of diabetes mellitus, hemoglobin, and hsCRP), the difference in C-statistics was not significant anymore.
Table 2.
AUC-ROC for different measures of renal function as predictors of MACE.
| AUC-ROC
|
|||
|---|---|---|---|
| Total Cohort n=823 | eGFR ≥60ml/min/1.73m2 n=615 | eGFR<60ml/min/1.73m2 n=208 | |
| eGFRMDRD | 0.615 | 0.529 | 0.550 |
| Cys C | 0.648 (p=0.07) | 0.600 (p=0.006) | 0.586 (p=0.18) |
| eGFRCysC | 0.652 (p=0.01) | 0.603 (p=0.001) | 0.589 (p=0.18) |
| eGFRsCr+CysC | 0.641 (p=0.02) | 0.581 (p=0.02) | 0.573 (p=0.25) |
P-values are for comparison with eGFRMDRD.
MACE = Death, myocardial infarction or stroke
Table 3.
Uni- and multivariate Cox proportional hazard models for different renal measures as predictors of MACE.
| Log-transformed | COX PROPORTIONAL HAZARD
|
|||||
|---|---|---|---|---|---|---|
| UNADJUSTED | MODEL 1§ | MODEL 2¶ | ||||
| Hazard Ratio# | P-value | Hazard Ratio# | P-value | Hazard Ratio# | P-value | |
| Urea | 1.40 (1.22–1.59) | <0.0001 | 1.25 (1.09–1.42) | 0.0016 | 1.14 (0.96–1.28) | 0.17 |
| Creatinine | 1.34 (1.21–1.48) | <0.0001 | 1.22 (1.09–1.35) | 0.0008 | 1.17 (1.03–1.32) | 0.015 |
| eGFRMDRD | 0.72 (0.65–0.80) | <0.0001 | 0.80 (0.72–0.90) | 0.0002 | 0.85 (0.75–0.96) | 0.012 |
| Cystatin C | 1.45 (1.30–1.60) | <0.0001 | 1.29 (1.15–1.44) | <0.0001 | 1.20 (1.05–1.36) | 0.0085 |
| eGFRCysC | 0.69 (0.62–0.77) | <0.0001 | 0.77 (0.68–0.87) | <0.0001 | 0.82 (0.73–0.94) | 0.006 |
| eGFRsCr+CysC | 0.70 (0.63–0.78) | <0.0001 | 0.78 (0.70–0.88) | <0.0001 | 0.83 (0.73–0.94) | 0.006 |
Abbreviations as in Table 1. P-value for log(BNP) <0.001 in every model.
Model 1 is adjusted for log-transformed BNP
Model 2 is further adjusted for age, BMI, history of coronary artery disease, history of diabetes mellitus, hemoglobin, and hsCRP.
Hazard ratios are per 1-standard deviation (Urea 12.7, Creatinine 0.89, eGFRMDRD 26.2, Cystatin C 0.73, eGFRCysC 23.7, eGFRsCr+CysC 24.8)
Figure 2. Forrest Plots (unadjusted) For Quartiles of Different Measures of Renal Function.
Hazards ratio compared to reference for quartiles of urea, cystatin, creatinine (all in mg/dL) as well as estimated glomerular filtration rate equations (all in ml/min/1.73m2).
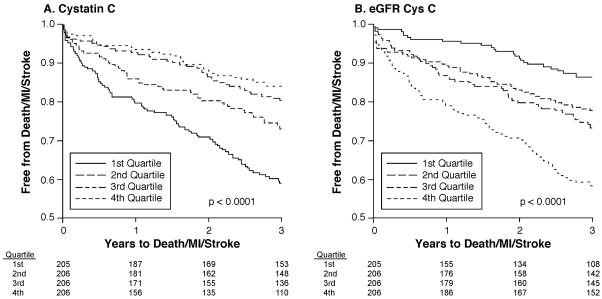
Figure 3.
Kaplan-Meier curves for Death/MI/Stroke according to Quartiles of Cystatin C (A) and eGFRCysC (B).
Figure 2 also demonstrates that, when looking at eGFRMDRD, the risk of MACE seems to increase only when eGFRMDRD < 60ml/min/1.73m2, in agreement with classic teaching. But when patients are stratified by CysC or derived equation, the risk of MACE seems to increase earlier and looks more like a continuum. Fifty-two percent (212/409 subjects) in the two higher CysC quartiles and 67% (415/617 subjects) in the 3 lower eGFRCysC quartiles had eGFRMDRD ≥ 60 ml/min/1.73m2 and were not deemed to be at higher risk of MACE based on their eGFRMDRD. However CysC based measurements showed they were at risk. This is particularly apparent when comparing different measures of renal function in those with eGFRMDRD ≥60ml/min/1.73m2, indicating a progressive increased risk for future MACE with increasing quartiles of CysC and decreasing quartiles of eGFRCysC (p= 0.002 and 0.001 respectively) but not other measures (Online-only Data Supplement Figure).
In different multivariate Cox proportional hazard models (each containing one measure of renal function), all renal function measures (except urea) were associated with higher risk of future MACE when adjusted for BNP (model 1) and other risk factors (model 2) (Table 3).
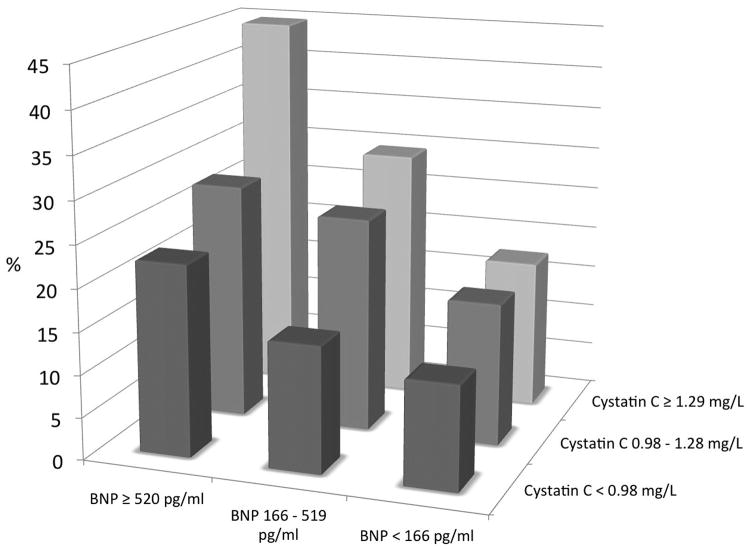
Figure 4 depicts the additive value of CysC and BNP in overall risk prediction. Patients in the highest tertile of both variables had a 45% risk to experience death, myocardial infarction, or stroke after 3 years as compared to only 12% when both variables were in the lowest tertile (p<0.0001).
Figure 4. Risk Stratification in Patients with Heart Failure According to Tertiles of Cystatin C and BNP.
The risk of the combined outcome (death, myocardial infarction, stroke) increases from 12.3% in patients with both biomarkers in the lowest tertile to 44.8% in patients with both biomarkers in the highest tertile (p<0.0001).
Discussion
There is a wealth of literature demonstrating the ability of CysC as a reliable and sensitive measure of renal dysfunction, and we and others have demonstrated the prognostic value of CysC in the setting of acute heart failure 10, 12–14 as well as in stable chronic heart failure 9, 15–17. However, the majority of the studies have been limited to relatively small sample sizes that precluded adequately powered subgroup analyses with covariate adjustments to shed the light on how to best utilize this measure of renal function in the clinical setting. As the largest study looking at the clinical utility of CysC in stable chronic heart failure using contemporary statistical methodologies, we observed that elevated CysC may identify a large number of patients at increased cardiovascular risk with what was previously labeled as ”preserved renal function” (eGFRMDRD > 60ml/min/1.73m2). Combining CysC with BNP provides excellent risk stratification, with an incidence of 12% of MACE at 3 years when both variables were in the lowest tertiles, versus an incidence of 45% of MACE at 3 years when both variables were in the highest tertiles. Taken together, our results imply that there is incremental prognostic value of CysC in patients with chronic stable heart failure, particularly in those with relatively preserved renal function based on creatinine-based estimates. This demonstrates that even in the setting of mild renal dysfunction there appears to be heightened risk for adverse cardiovascular outcomes.
Our findings corroborated prior studies 9, 15 that suggested CysC may improve overall risk prediction in heart failure. The limitations of sCr’s reciprocal relationship with GFR are well known, largely due to variable production (as a function of age, gender, muscle mass, and ethnicity), diet and tubular secretion 18. Creatinine-based equations try to correct for this by including determinants of muscle mass (age, weight, race, gender) in their formulas 19. The equations were developed in patients with chronic kidney disease where they are reasonably accurate 20. It remains intriguing to see, in this and other studies 21, that CysC is consistently better in risk prediction whereas it is either equivalent or only slightly improves eGFRMDRD estimation 7. We see two potential explanations for this observation. First, CysC and derived equations are significantly better than sCr derived equations in estimating GFR in the non-chronic kidney disease population. This is very well possible because in these non-chronic kidney disease populations (elderly, heart failure, coronary patients), mean GFR is higher, patients are older and have more comorbidities. Current creatinine-based GFR equations tend to be less accurate in the higher GFR ranges as nicely demonstrated in the diabetic population and in the heart failure population 4, 22, 23. In addition, in the aforementioned settings, the dependence of creatinine on other factors than GFR (muscle mass, diet and general health condition) becomes more and more important. These influences are not all captured by correcting for age, gender and race. A second possibility is that CysC represents other factors aside renal impairment. There is indeed some mechanistic evidence to suggest that CysC, being an inhibitor of cysteine proteases, may be intricately involved in the pathogenesis of atherosclerosis 24. In addition it has been shown that the levels of CysC can be influenced by inflammation, glucocorticoid use, and thyroid function 25. All this may explain why the incremental prognostic value of CysC is mainly confined to those with relatively “preserved” renal function, whereas the role of measuring CysC in those with already impaired renal function as determined by sCr-based estimates is limited.
We have also applied for the first time the CysC-derived equation for estimating GFR in the heart failure population, and found eGFRCysC to be reliably predictive of long-term outcomes (and potentially superior to eGFRsCr+CysC). The observation that the eGFRCysC equation further improved risk prediction as compared to CysC was unexpected. In fact, the correlation between CysC and eGFRCysC equation is very high (r = 0.98), and the equation only reclassified 14% of patients in a different quartile. However, in addition to a play of chance, it remains possible that adjusting for age, gender and race is important in this population. Interestingly, the addition of creatinine to the CysC-based equation (eGFRsCr+CysC) weakened the prognostic ability again. This suggests that, once GFR is estimated on the base of CysC, any potential improvement of GFR estimation by adding sCr is outweighed by its association with muscle mass or general health condition.
One important observation from our study is the fact that one third of subjects in the subset with eGFRMDRD ≥ 60 ml/min/1.73m2, (or up to one quarter of subjects in our entire study cohort) may demonstrate an elevated CysC level that is associated with heightened risk for future adverse cardiac events. This represents a relatively large population at risk. In this subgroup, CysC and CysC-derived equation were the only measures of renal function that stratified risk of adverse events. Taken together, the present data suggest that a decrease in GFR increases cardiovascular risk throughout its spectrum without a clear “normal” cut-off by sCr or sCr-based equations.
The current usage of CysC in clinical practice is limited despite its approval, clinical availability, and extensive nephrology and epidemiology literature to support its value. This is in part because no prior studies have relied on CysC as an inclusion criterion for treatment decisions, and limited therapeutic studies have provided analyses to test the differential impact of interventions in high versus low CysC subgroups. Nevertheless, the ability to identify a vulnerable population in a cohort of chronic stable heart failure is an opportunity to test therapeutic strategies that may potentially provide incremental benefit to existing therapies. Current clinical trials have utilized sCr-based cut-off values as exclusion criterion either due to therapeutic contraindications or to remove confounding due to concomitant end-organ dysfunction that may indirectly affect the demonstration of therapeutic efficacy. In contrast, natriuretic peptide-based cut-off values, identifying a more at-risk sub-population, have been increasingly utilized in clinical studies to facilitate the successful demonstration of the benefits of drug therapy (such as eplerenone in mild heart failure 26). At the other end of the spectrum, post-hoc subgroup analysis has implied that lower rather than higher natriuretic peptide levels may demonstrate therapeutic benefits of statin therapy in ischemic cardiomyopathy 27. Based on the inter-relationship between CysC and BNP, it is conceivable to identify those with elevated natriuretic peptides and/or elevated CysC levels to test therapeutic strategies towards combined or separate targets in the setting of mild heart failure.
Study Limitations
The biggest limitation of this study is that there was no direct “gold standard” measurement of GFR (e.g., inulin or 125I-iothalamate clearance), yet recent reports of relationships between iothalamate-derived GFR measures and CysC or eGFRMDRD have been reported 17. Consequently, it remains speculative to state that the CysC-derived equation may better reflect GFR in the heart failure population. Second, there was some degree of selection bias as the population studied included patients undergoing elective cardiac catheterization for symptom evaluation, and there were no prospective outcomes regarding heart failure hospitalizations. Furthermore, the particle-enhanced turbidimetric immunoassay used to determine CysC levels may have resulted in values that are 20% to 30% higher than in the common particle-enhanced nephelometric immunoassay method 28. Similarly, the eGFRCysC equation was derived with CysC values determined by the nephelometric and not turbidimetric method, thus resulting in lower absolute eGFRCysC values. However, this should not alter the correlation of CysC or eGFRCysC with other measures of renal function, nor its risk predictive ability. Finally, we were not able to examine how CysC adds to other renal biomarkers (e.g., neutrophil gelatinase-associated lipocalin (NGAL)) that reflect complementary renal processes (e.g. tubular injury).
Conclusion
Cystatin C is a strong predictor of adverse events in stable chronic heart failure, independent of traditional risk factors and BNP. Cystatin C and its derived equation to estimate glomerular filtration rate add significant prognostic value to creatinine, predominantly in patients with relatively preserved renal function.
Supplementary Material
Clinical Summary.
Renal dysfunction is a well-known predictor of adverse events in heart failure patients. Renal function is most often expressed in terms of serum creatinine values. However, due to its dependence on age, diet and muscle mass, serum creatinine is probably not the ideal filtration marker. In this analysis of 823 patients with clinically stable heart failure, we demonstrate that cystatin C, a cysteine protease inhibitor, and derived equations have incremental value in predicting adverse events (all-cause death and non-fatal myocardial infarction or stroke) as compared to creatinine-based GFR estimation (eGFRMDRD). The benefit is however mostly limited to patients with relative preserved renal function (eGFRMDRD > 60ml/min/1.73m2). The consequence is that even mild renal dysfunction seems to be a risk factor for adverse cardiovascular outcomes as opposed to the classic notion that it only becomes a significant risk factor if eGFRMDRD < 60 ml/min/1.73m2.
Acknowledgments
Sources of Funding
This research was supported by National Institutes of Health grants P01HL076491-055328 (S.L.H.), 5P01HL103453 (S.L.H.), P01HL098055-01 (S.L.H.), R01HL103866 (S.L.H.), 1P20HL113452-01 (S.L.H./W.H.T.), 1R01HL103931-02 (W.H.T.), and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439-06), as well as the Fondation Leducq. (S.L.H.). Dr. Dupont is supported by a research grant from the Belgian American Educational Foundation (BAEF). Supplies and funding for performance of fasting lipid profiles, blood glucose, creatinine, BNP, CysC, and hsCRP were provided by Abbott Laboratories Inc.
Footnotes
Disclosures
Drs. Dupont and Wu had no relationships to disclose. Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: Abbott Diagnostics, BG Medicine Inc., Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens. Dr. Tang reports having received research grant support from Abbott Laboratories, Inc.
References
- 1.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 2.Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in val-heft. Eur Heart J. 2004;25:292–299. doi: 10.1016/j.ehj.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 4.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 6.Coll E, Botey A, Alvarez L, Poch E, Quinto L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin c as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating gfr using serum cystatin c alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with ckd. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin c and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 9.Shlipak MG, Katz R, Fried LF, Jenny NS, Stehman-Breen CO, Newman AB, Siscovick D, Psaty BM, Sarnak MJ. Cystatin-c and mortality in elderly persons with heart failure. J Am Coll Cardiol. 2005;45:268–271. doi: 10.1016/j.jacc.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 10.Lassus J, Harjola VP, Sund R, Siirila-Waris K, Melin J, Peuhkurinen K, Pulkki K, Nieminen MS. Prognostic value of cystatin c in acute heart failure in relation to other markers of renal function and nt-probnp. Eur Heart J. 2007;28:1841–1847. doi: 10.1093/eurheartj/ehl507. [DOI] [PubMed] [Google Scholar]
- 11.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin c with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: Data from the heart and soul study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell CY, Clarke W, Park H, Haq N, Barone BB, Brotman DJ. Usefulness of cystatin c and prognosis following admission for acute heart failure. Am J Cardiol. 2009;104:389–392. doi: 10.1016/j.amjcard.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Manzano-Fernandez S, Boronat-Garcia M, Albaladejo-Oton MD, Pastor P, Garrido IP, Pastor-Perez FJ, Martinez-Hernandez P, Valdes M, Pascual-Figal DA. Complementary prognostic value of cystatin c, n-terminal pro-b-type natriuretic peptide and cardiac troponin t in patients with acute heart failure. Am J Cardiol. 2009;103:1753–1759. doi: 10.1016/j.amjcard.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Naruse H, Ishii J, Kawai T, Hattori K, Ishikawa M, Okumura M, Kan S, Nakano T, Matsui S, Nomura M, Hishida H, Ozaki Y. Cystatin c in acute heart failure without advanced renal impairment. Am J Med. 2009;122:566–573. doi: 10.1016/j.amjmed.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Arimoto T, Takeishi Y, Niizeki T, Takabatake N, Okuyama H, Fukui A, Tachibana H, Nozaki N, Hirono O, Tsunoda Y, Miyashita T, Shishido T, Takahashi H, Koyama Y, Kubota I. Cystatin c, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail. 2005;11:595–601. doi: 10.1016/j.cardfail.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Tang WH, Van Lente F, Shrestha K, Troughton RW, Francis GS, Tong W, Martin MG, Borowski AG, Jasper S, Starling RC, Klein AL. Impact of myocardial function on cystatin c measurements in chronic systolic heart failure. J Card Fail. 2008;14:394–399. doi: 10.1016/j.cardfail.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Damman K, van der Harst P, Smilde TD, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. Use of cystatin c levels in estimating renal function and prognosis in patients with chronic systolic heart failure. Heart. 2012;98:319–324. doi: 10.1136/heartjnl-2011-300692. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89:457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and cockcroft-gault equations in the estimation of gfr in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 21.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20:2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS. Comparative performance of the ckd epidemiology collaboration (ckd-epi) and the modification of diet in renal disease (mdrd) study equations for estimating gfr levels above 60 ml/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pucci L, Triscornia S, Lucchesi D, Fotino C, Pellegrini G, Pardini E, Miccoli R, Del Prato S, Penno G. Cystatin c and estimates of renal function: Searching for a better measure of kidney function in diabetic patients. Clin Chem. 2007;53:480–488. doi: 10.1373/clinchem.2006.076042. [DOI] [PubMed] [Google Scholar]
- 24.Maahs DM, Ogden LG, Kretowski A, Snell-Bergeon JK, Kinney GL, Berl T, Rewers M. Serum cystatin c predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes. 2007;56:2774–2779. doi: 10.2337/db07-0539. [DOI] [PubMed] [Google Scholar]
- 25.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin c levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 26.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 27.Cleland JG, McMurray JJ, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, Hjalmarson A, Korewicki J, Lindberg M, Ranjith N, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: Prediction of cardiovascular events and interaction with the effects of rosuvastatin: A report from corona (controlled rosuvastatin multinational trial in heart failure) J Am Coll Cardiol. 2009;54:1850–1859. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Laterza OF, Price CP, Scott MG. Cystatin c: An improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.