Summary
Thymic Stromal Lymphopoietin (TSLP), a cytokine implicated in induction of T helper 2 (Th2)-mediated allergic inflammation, has recently been shown to stimulate solid tumor growth and metastasis. Conversely, studying mice with clonal loss of Notch signaling in their skin revealed that high levels of TSLP released by barrier-defective skin caused a severe inflammation, resulting in gradual elimination of Notch-deficient epidermal clones and resistance to skin tumorigenesis. We found CD4+ T cells to be both required and sufficient to mediate these effects of TSLP. Importantly, TSLP overexpression in wild-type skin also caused resistance to tumorigenesis, confirming that TSLP functions as a tumor suppressor in the skin.
Keywords: TSLP, Notch, inflammation, tumor suppressor, CD4+ T cell, Calcipotriol
Introduction
The mammalian skin is a model organ used for decades in chemical carcinogenesis studies and contributed to the recognition that carcinogenesis is a step-wise process (Hanahan and Weinberg, 2000). As in other models of cancer, skin tumors arise as a consequence of intrinsic changes in the initiated cells that are amplified through interactions with the tumor microenvironment (Campisi, 2005; Kessenbrock et al., 2010; Sneddon and Werb, 2007). Although immune cells can suppress tumor growth in the tumor microenvironment, their carcinogenesis-promoting role is becoming increasingly appreciated (Coussens and Werb, 2002; Hanahan and Weinberg, 2000; Hanahan and Weinberg, 2011). Because skin is the largest barrier organ in the body, it is under tight surveillance by the immune system, and even subtle changes in its cellular differentiation program can alter the overall susceptibility to cancer by eliciting a persistent inflammatory response (Quigley et al., 2009). This is partly due to a direct epidermal contribution to inflammation via secretion of multiple cytokines, such as interleukin (IL) -1, IL-6 and TGF-β (Morasso and Tomic-Canic, 2005).
Studies in mice with clonal deletion of Notch pathway components uncovered a role for Notch in tumor promotion via induction of a non-cell autonomous feed-forward loop between the epidermis, dermal fibroblasts and the immune system (Demehri et al., 2008; Demehri et al., 2009b). Notch is a transmembrane receptor that mediates short-range communication between adjacent cells (Kopan and Ilagan, 2009). Upon binding to the ligand presented by a neighboring cell, Notch undergoes proteolysis by γ-secretase enzyme to release its intracellular domain. Subsequently, Notch intracellular domain (NICD) translocates into the nucleus and binds to its DNA-binding partner, RBPj, and regulates its downstream targets in a context-dependent manner (Kopan and Ilagan, 2009). Notch signaling plays multiple roles in skin development (Mascia et al., 2011), but in the context of carcinogenesis, the most relevant role is in promoting supra-basal differentiation (Blanpain et al., 2006; Demehri et al., 2009b; Nguyen et al., 2006; Nicolas et al., 2003; Pan et al., 2004; Rangarajan et al., 2001). Reduction in Notch signaling leads to aberrant epidermal differentiation and defective barrier formation, which creates a chronic wound-like environment prone to spontaneous skin tumors. Consistent with these findings, mice and humans lacking components of the γ-secretase complex (presenilin 1 and 2 (PS1/2), presenilin enhancer 2 (PEN2), APH1 or Nicastrin) have elevated rates of spontaneous tumor development (Lapins et al., 2001; Li et al., 2007; Wang et al., 2010; Xia et al., 2001). Additionally, the γ-secretase inhibitor Semagacestat (LY450139) led to elevated incidence of skin cancer among the participants in a phase III clinical trial (Extance, 2010). Importantly, the latency for spontaneous tumor formation in the epidermis is determined by the degree of disruption in its differentiation program caused by Notch signaling loss (Demehri et al., 2009b).
An epidermal-derived cytokine whose level rises as more Notch signaling is lost in the skin is TSLP, which could contribute to the susceptibility of Notch-deficient skin to tumorigenesis (De Monte et al., 2011; Demehri et al., 2008; Demehri et al., 2009b; Olkhanud et al., 2011; Pedroza-Gonzalez et al., 2011). TSLP is an interleukin (IL)-7-like cytokine studied mainly in the context of T helper 2 (Th2)-mediated allergic inflammation in the skin and lung (Leonard, 2002; Rochman et al., 2009; Ziegler, 2010); overexpression of TSLP is sufficient to promote the development of atopic dermatitis and asthma, respectively (Ziegler, 2010). Importantly, transient exposure to an allergen in the presence of TSLP is sufficient to prime the skin and lung immune cells, creating long-lasting T cells that can trigger allergic inflammation at a later time (Han et al., 2012; Zhang et al., 2009). Although TSLP is not expressed in the skin under physiological conditions, keratinocytes are powerful secretors of TSLP in both humans (Lee et al., 2010) and mice; chronic and severe barrier disruption can result in TSLP release into the serum up to 5000 folds over its baseline levels (Demehri et al., 2008; Dumortier et al., 2010; Zhang et al., 2009). Interestingly, systemic TSLP drives a leukemia-like B cell lymphoproliferative disease in newborn mice (Demehri et al., 2008), and constitutively active TSLPR causes acute B-lymphoblastic leukemia in children (Cario et al., 2010; Hertzberg et al., 2010; Shochat et al., 2011). TSLP has also emerged recently as a pro-growth cytokine in breast and pancreatic cancers (De Monte et al., 2011; Olkhanud et al., 2011; Pedroza-Gonzalez et al., 2011). These findings suggest a therapeutic opportunity for TSLP-blocking agents that are in development for the treatment of allergic diseases (Schmitt, 2010) as cancer immunotherapeutic agents.
Considering the emerging role of TSLP as a potential therapeutic target in cancer therapy, we set out to investigate the role of epidermal-derived TSLP in skin carcinogenesis.
Results
Mice lacking all canonical Notch signaling in the epidermis are resistant to skin carcinogenesis
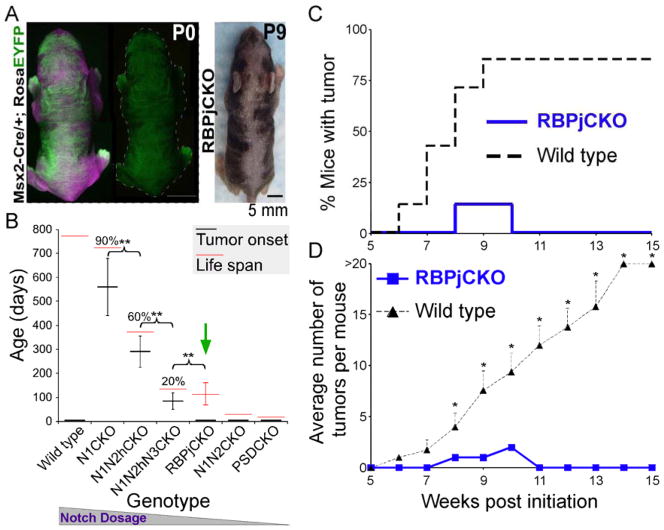
The Msx2-Cretg/+ line expresses Cre recombinase early, transiently and only in the dorsal and ventral midline regions of the skin, generating a calico pattern of gene deletion (Figure 1A). Step-wise removal of Notch alleles in epidermal keratinocytes by Msx2-Cretg/+ is associated with a corresponding decline in differentiation, creation of a wound-like environment and increased susceptibility to carcinogenesis ((Demehri et al., 2009b) and Figure 1A–B). Animals lacking all Notch signaling in their skin (e.g., Msx2-Cretg/+; Ps1flox/flox; Ps2−/− (PSDCKO; loss of γ-secretase enzyme function)) die shortly after birth, not allowing enough time for spontaneous tumor development (Figure 1B). RBPj-deficient animals (Msx2-Cretg/+; Rbpjflox/flox or RBPjCKO), however, live for ~100 days on average, which is comparable to animals lacking all but one Notch2 allele in their skin (Msx2-Cretg/+; Notch1flox/flox; Notch2flox/+; Notch3−/− or N1N2hN3CKO). Surprisingly, while 20% of N1N2hN3CKO mice developed spontaneous skin tumors, none (0/40) of the RBPjCKO mice that have been examined developed spontaneous tumors (Figure 1B).
Figure 1.
Mice lacking RBPj in portions of their epidermis are resistant to skin tumorigenesis. (A) The calico pattern of EYFP expression (green) induced by Msx2-Cre-mediated gene deletion in a Msx2-Cretg/+; Rosa (LoxP_Stop_LoxP)-Eyfp (Msx2-Cre/+; RosaEYFP) newborn is shown. Image taken under tungsten illumination is shown in magenta. After birth, mutant clones become evident due to hair phenotypes. (B) Reduction in Notch signaling dosage in the skin correlates with shortening of life span and time to spontaneous tumor formation. This trend, however, does not extend to mice lacking RBPj (green arrow), which live ~100 days yet do not develop any skin tumors. n > 20 in each group; % indicates the percentage of mice that developed skin tumors; **: p <0.01, student’s t-test; error bars represent +/− SD. This figure is modified from (Demehri et al., 2009b). (C,D) Time to tumor onset (C; p <0.0001, log-rank test) and average tumor number (D) of RBPjCKO and wild-type littermates treated with the standard DMBA-TPA protocol from 3 to 18 weeks of age are shown. n = 7 for each group; *: p <0.05, student’s t-test; error bars represent +/− SEM. Genotypes: Msx2-Cretg/+; Notch1flox/flox (N1CKO), Msx2-Cretg/+; Notch1flox/flox; Notch2flox/+ (N1N2hCKO), Msx2-Cretg/+; Notch1flox/flox; Notch2flox/+; Notch3−/− (N1N2hN3CKO), Msx2-Cretg/+; Notch1flox/flox; Notch2flox/flox (N1N2CKO), Msx2-Cretg/+; Ps1flox/flox; Ps2−/− (PSDCKO), Msx2-Cretg/+; Rbpjflox/flox (RBPjCKO).
To further rule out the possibility that the short life span of RBPjCKO mice masked incipient tumors, we subjected RBPjCKO mice, maintained through >6 generations of intercrossing in a mixed genetic background (C57BL/6 with FVB, CD1 contribution), to a multistage chemical skin carcinogenesis model (Yuspa et al., 1994). Three-week-old RBPjCKO and their wild-type littermates (defined as all mice not inheriting the Cre transgene) were treated with 25 μg 7,12-dimethylbenz[α]anthracene (DMBA), followed by a twice-weekly dose of 12-O-tetradecanoylphorbol-13-acetate (TPA) for 14 weeks. Surprisingly, no tumors were detected in the DMBA/TPA-treated RBPjCKO animals after 15 weeks of follow-up, whereas the majority of wild-type littermates developed more than 20 papillomas/mouse (n = 7 in each group, p <0.0001, Figure 1C–D). These results contrast starkly to the enhanced tumor susceptibility seen in other Notch-deficient animals (Figure 1B; (Demehri et al., 2009b)). Resistance to DMBA/TPA might reflect a reduced growth potential of RBPj-deficient keratinocytes (Blanpain et al., 2006). However, even if this were the case, we would expect initiated wild-type cells in this environment to form tumors (Demehri et al., 2009b). Alternatively, resistance to tumorigenesis in RBPjCKO skin may be due to a switch from a tumor-promoting environment in N1CKO (Demehri et al., 2009b) to a tumor-suppressing environment in RBPjCKO skin.
Wild-type keratinocytes replace their Notch signaling-deficient neighbors over time
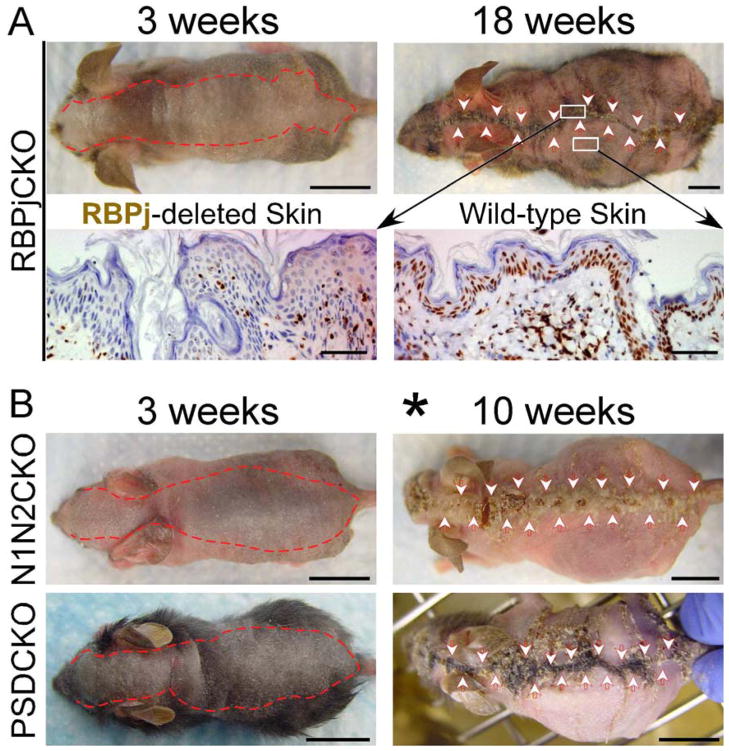
The calico pattern of gene deletion in RBPjCKO animals allowed us to notice a second, potentially related phenotype. As these mice aged, the mutant epidermal clones on their dorsal and ventral surfaces shrank, and RBPj-deficient epidermis eventually disappeared in the oldest individuals (Figure 2A and data not shown). Although Msx2-Cretg/+; Notch1flox/flox; Notch2flox/flox (N1N2CKO) and PSDCKO mice die post weaning due to a lethal B-lymphoproliferative disorder (Demehri et al., 2008), they did survive longer if we controlled their B-LPD with a sublethal dose of irradiation (Demehri et al., 2008). When lethality was rescued in this manner, we observed a similar regression of Notch1/2- or Ps1/2-deleted epidermis as N1N2CKO and PSDCKO animals aged (Figure 2B). This too could reflect a proliferative disadvantage of Notch signaling-deficient keratinocytes or the active process of rejection.
Figure 2.
Notch signaling-deficient epidermal clones regress with age. (A) The red dotted line and arrowheads delineate the boundaries of the RBPj-deficient dorsal epidermis, as determined by hair/epidermal phenotype. Bottom panel shows α-RBPj antibody staining of RBPj-depleted midline skin (left) and wild-type skin in the periphery (right) of a 18-week-old RBPjCKO animal. (B) The red dotted line and arrowheads delineate the boundaries of the mutant dorsal epidermis in N1N2CKO and PSDCKO animals. Asterisk marks the recipients of a sublethal dose of irradiation in the second week of life; representative pictures are shown in all panels; scale bars: 1 cm (mice pictures); 50 μm (histology).
H-Ras-infected Notch-deficient keratinocytes are highly tumorigenic in immune-compromised mice
To ask if the resistance of RBPjCKO mice to carcinogenesis and the loss of mutant keratinocytes are due to a low proliferative capacity, we evaluated their tumor-forming potential in response to the activated H-Ras oncogene in the nude mouse environment (Nicolas et al., 2003). First, we isolated keratinocytes from newborn Rbpjflox/flox and Rbpj+/flox littermates. Cells were then infected with activated H-Ras-expressing retrovirus, allowed to recover for 24 hours and then infected with Cre-expressing adenovirus. H-Ras-infected, Cre expressing, Rbpj−/− (RBPjKO) or Rbpj+/− (wild type) cells (1.5 × 106) were injected subcutaneously into nude mice and tumor development was monitored over time. Importantly, RBPjKO keratinocytes formed large tumors with histological features of moderately differentiated squamous cell carcinomas in 30 days, while wild-type keratinocytes did not form a significant tumor mass (Figure 3). Similar results were obtained using γ-secretase-deficient (PSDKO) keratinocytes (Figure 3). These results demonstrate that Notch signaling-deficient cells are highly proliferative and competent to form tumors in a T cell-deficient environment. Therefore, we hypothesized that the apparent resistance of Notch signaling-deficient animals to skin tumorigenesis is most likely due to the activation of a tumor-suppressing environment in their skin.
Figure 3.
H-Ras-infected RBPjKO or PSDKO keratinocytes are highly tumorigenic in nude mice. (A) Schema showing the experimental procedure. Cells are infected with retrovirus containing oncogenic H-Ras and then with Adeno-Cre to delete the floxed alleles. H-Ras-infected RBPjKO or PSDKO and wild-type cells injected subcutaneously into the right and left flanks of the nude mice, respectively. (B) Nude mice with palpable tumors (red circles) are harvested 30 days after the injection. Hematoxylin and eosin (H&E) and α-RBPj antibody stainings on a tumor formed by RBPjKO keratinocytes are shown. Representative pictures are shown; scale bars: 1 cm (mice pictures), 50 μm (histology).
Notch-deficient animals develop severe allergic skin inflammation caused specifically by epidermal TSLP overexpression
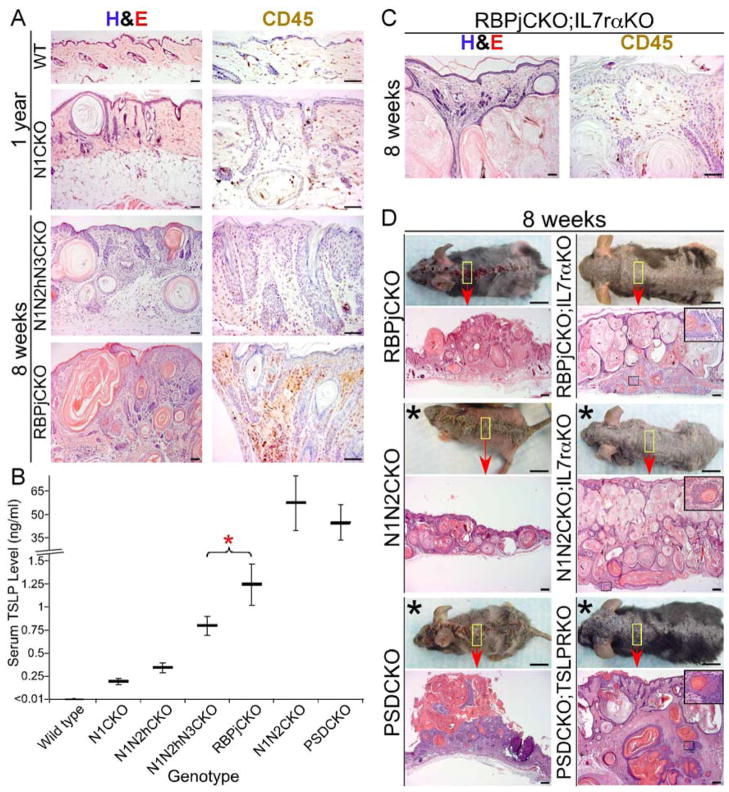
Inflammation can either promote or suppress tumorigenesis depending on its magnitude and the immune cells involved (Coussens and Werb, 2002; Schreiber et al., 2011). In sharp contrast to the mild inflammation, dermal fibroplasia and angiogenesis, which generated a tumor-promoting environment in N1CKO skin (Demehri et al., 2009b), RBPjCKO skin exhibited a significant accumulation of leukocytes (CD45+ cells) beneath the RBPj-deficient epidermal clones (Figure 4A). To test if this high level of dermal inflammation could have a suppressing effect on tumor growth (Coussens and Werb, 2002), we first examined the effect of immunosuppressant drugs on DMBA-treated RBPjCKO mice. Treatment with the maximum tolerable doses of Dexamethasone or Methotrexate did not significantly reduce inflammation in RBPjCKO mice, nor did it affect the rejection of mutant skin cells or allow tumor formation (Figure S1A–B). Next, we examined if TNFα, a prominent proinflammatory cytokine also overexpressed in Notch signaling-deficient skin (Dumortier et al., 2010), contributes to skin inflammation in RBPjCKO animals. The levels of skin inflammation, tumor resistance and mutant skin patch rejection in Msx2-Cretg/+; Rbpjflox/flox; TnfrI−/−; TnfrII−/− mice were indistinguishable from RBPjCKO littermates (Figure S1C), indicating that TNFα was not necessary for theses phenotypes.
Figure 4.
Blunting the skin inflammation by blocking TSLP signaling in Notch signaling-deficient animals results in mutant skin expansion and tumorigenesis. (A) H&E and CD45 antibody stainings show the level of skin inflammation in RBPjCKO animals compared to the tumor-prone N1CKO and N1N2hN3CKO mice. Scale bars: 50 μm. (B) The circulating TSLP levels during the second week of life are compared among the allelic series of Notch-deficient animals. *: p <0.05, student’s t-test; error bars represent +/− SD. This figure is modified from (Demehri et al., 2008). (C) H&E and CD45 antibody stainings highlight the level of dermal inflammation in RBPjCKO;IL7rαKO animals that lack TSLP signaling. Scale bars: 50 μm. (D) Gross and microscopic features of RBPjCKO;IL7rαKO, N1N2CKO;IL7rαKO and PSDCKO;TSLPRKO skin are compared to those of RBPjCKO, N1N2CKO and PSDCKO littermates, respectively. Asterisks mark the recipients of a sublethal dose of irradiation in the second week of life; insets: tumors penetrating the muscle layer; scale bars: 1 cm (mice pictures), 200 μm (histology); representative pictures are shown in all panels. See also Figure S1.
RBPjCKO keratinocytes produced significantly higher levels of TSLP than those seen in the tumor-prone N1CKO and N1N2hN3CKO animals (Figure 4B), driving the severe Th2 inflammation seen in RBPjCKO skin (Demehri et al., 2009a; Dumortier et al., 2010; He et al., 2008). Therefore, we next examined the effect of loss of TSLP signaling on inflammation by generating RBPjCKO mice that lack the TSLP receptor by deleting genes encoding its subunits (Il7rα or Crlf2 (Tslpr)). Il7rα −/− (IL7rαKO) mice were used in place of Tslpr−/− (TSLPRKO) mice because Rbpj and Tslpr are linked on the same arm of chromosome 5, and thus we were unable to generate RBPjCKO;TSLPRKO animals. Deleting Il7rα in RBPjCKO or N1N2CKO ((Msx2-Cretg/+; Rbpjflox/flox; Il7rα−/− or RBPjCKO;IL7rαKO, Msx2-Cretg/+; Notch1flox/flox; Notch2flox/flox; Il7rα−/− or N1N2CKO;IL7rαKO) mice led to a marked reduction in skin inflammation (Figure 4C and Figure S1D). To confirm that this effect was specific to TSLP and not an indirect consequence of reduced lymphocyte numbers in IL7rαKO mice (Peschon et al., 1994), we deleted Tslpr in PSDCKO animals (Msx2-Cretg/+; Ps1flox/flox; Ps2−/−; Tslpr−/− or PSDCKO;TSLPRKO), which significantly prolonged their lifespan (Dumortier et al., 2010). As with N1N2CKO;IL7rαKO and RBPjCKO;IL7rαKO, inflammation was greatly reduced in PSDCKO;TSLPRKO animals (Figure S1D). Taken together, these results demonstrate a central role for TSLP in regulating the level of inflammation in Notch-deficient skin.
Blocking TSLP signaling in Notch-deficient animals results in the expansion of the mutant skin and tumorigenesis
RBPjCKO;IL7rαKO animals showed a clear reversal of the two phenotypes unique to RBPjCKO mice. First, RBPj-deficient epidermal clones expanded dramatically in RBPjCKO;IL7rαKO mice, forming numerous hypertrophic cysts (Figure 4D). Together with the data in Figure 3, this result excludes a proliferative defect in RBPj-deficient keratinocytes. Second, RBPjCKO;IL7rαKO mice developed spontaneous, invasive dermal and exophytic tumors over time (Figure 4D and S1E). N1N2CKO;IL7rαKO and PSDCKO;TSLPRKO animals also showed expansion of their mutant skin territories (Figure 4D, (Dumortier et al., 2010) and the accompanying paper by Di Piazza et al.). All RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO animals eventually developed cancerous lesions that invaded through the subcutaneous muscle layer at 10 to 15 weeks of age (Figure 4D and S1E). Treating the skin of these animals with a single dose of DMBA further revealed their susceptibility to tumorigenesis (Figure S1E). Therefore, eliminating TSLP reception reduced inflammation, restored a tumor-promoting environment reminiscent of the N1CKO mice, and uncovered the underlying cancer-prone phenotype in mice lacking canonical Notch signaling in their skin. Whether viruses contributed to tumor formation in these animals remains to be determined.
The adaptive immune system mediates the effects of TSLP on skin rejection and tumor resistance
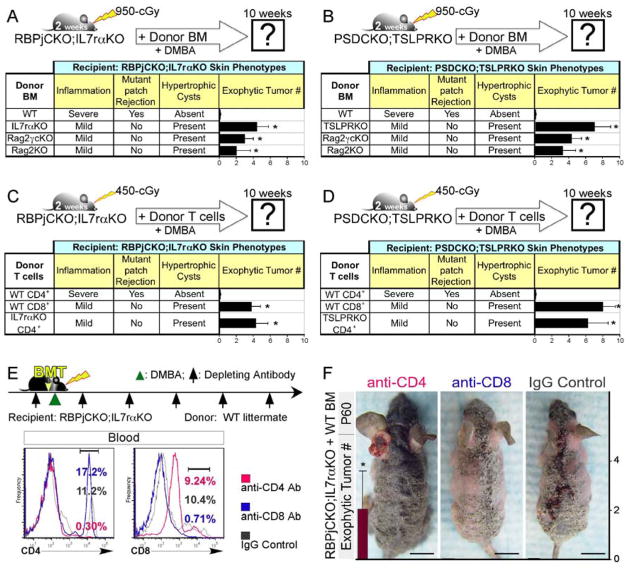
In order to determine if bone marrow (BM)-derived immune cells mediated the effects of TSLP on skin rejection and resistance to tumorigenesis, we used littermates with identical major MHC class I haplotypes (Figure S2A–C) and reconstituted the immune system of lethally irradiated RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO mice with BM from their wild-type littermates. We monitored their response to DMBA treatment following BM transplantation (BMT). Interestingly, wild-type BM restored resistance to DMBA-induced tumors in RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO mice; concomitantly, the mice gained the ability to eliminate their mutant skin cells (Figure 5A–B and S2D). In a complementary set of experiments, we reconstituted the immune system of lethally irradiated RBPjCKO and PSDCKO mice with BM from their IL7rαKO and TSLPRKO littermates, respectively, to determine if a pro-tumor environment would be re-established. This immune reconstitution, however, failed to establish tolerance, and no DMBA-induced tumors formed (Figure S2E). This could be explained by the persistence of irradiation-resistant activated and memory T cells in the mutant animals at the time of transplantation (Figure S2F). Together, these findings demonstrate that BM-derived immune cells act downstream of TSLP, and suggest that the effector cell type(s) formed in RBPjCKO and PSDCKO are resistant to lethal doses of irradiation.
Figure 5.
CD4+ T cells mediate the effects of TSLP on Notch-deficient skin rejection and tumor resistance. (A,B) The skin phenotypes of RBPjCKO;IL7rαKO (A) and PSDCKO;TSLPRKO (B) animals are analyzed 8 weeks after BMT from wild-type littermates, Rag2−/−,γc−/− (Rag2γcKO) or Rag2−/− (Rag2KO) animals. Note that Rag2γcKO and Rag2KO donors lack T and B cells. The level of dermal inflammation, the rejection of the mutant keratinocytes, the formation of hypertrophic cysts and the development of DMBA-dependent exophytic tumors are scored in the recipient animals at 10 weeks of age. BMT from TSLP receptor-deficient littermates are used as controls. *: p <0.05 compared to wild-type BM donor group. (C,D) 2-week-old RBPjCKO;IL7rαKO (C) and PSDCKO;TSLPRKO (D) pups were irradiated with 450-cGy and underwent adoptive T cell transfer using wild-type CD4+, wild-type CD8+ or TSLPR-deficient CD4+ T cells isolated from the spleens of their littermates as shown in the schematic diagrams. The level of dermal inflammation, the rejection of the mutant keratinocytes, the formation of hypertrophic cysts and the development of DMBA-dependent exophytic tumors are documented in the recipient animals at 10 weeks of age. *: p <0.05 compared to wild-type CD4+ T cell donor group. (E) 2-week-old RBPjCKO;IL7rαKO mice were treated with depleting antibodies (anti-CD4 or anti-CD8α; black arrows), lethally irradiated 2 days later and transplanted with T cell-depleted c-Kit+ BM progenitor cells from their wild-type littermates as shown in the schematic diagram. 2 days later the animals were treated with one dose DMBA while continuing to receive a weekly intraperitoneal injection of the indicated depleting antibody for 10 weeks. Flow cytometry shows the status of CD4+ and CD8+ T cells in the peripheral blood of RBPjCKO;IL7rαKO mice transplanted with c-Kit+ wild-type BM and treated with anti-CD4 or anti-CD8α antibody one week after the last antibody injection. Blood CD45+ leukocytes are traced in the figure. Note that PE-conjugated anti-CD8β antibody is used to detect CD8+ cells. (F) RBPjCKO;IL7rαKO mice transplanted with c-Kit+ BM cells and treated with anti-CD4, anti-CD8α or IgG control antibodies are compared. Bar graphs show the average number of DMBA-dependent exophytic tumors in each treatment group. *: p <0.05 compared to IgG control-treated group. For all experiments, mice were followed up to 90 days of age for exophytic tumor count; at least five mice were analyzed in each group; error bars on all bar graphs represent +/− SD; representative pictures are shown; scale bars: 1 cm. See also Figure S2.
Based on the findings above, we focused on the immune cell repertoire in RBPjCKO (Figure S2G; (Demehri et al., 2009b)) and targeted cell types that are known to be resistant to irradiation (Murphy et al., 1987), express TSLP receptors, and mediate tumor resistance and skin rejection (Vesely et al., 2011). Based on these criteria, we chose to delete CD8+ Cytotoxic T lymphocytes (CTLs) and mast cells in RBPjCKO animals. Genetic depletion of these cells in RBPjCKO;CD8α−/− and RBPjCKO;Kitwsh/wsh did not cause any alteration in the RBPjCKO skin phenotypes, suggesting that neither cell type is necessary to mediates the effects of TSLP (Figure S2H). In a separate set of experiments, we used previously described depleting antibodies (Rogers and Unanue, 1993; Shankaran et al., 2001) to deplete CD4+ T cells, CD8+ T cells, natural killer (NK) cells, or granulocytes in RBPjCKO animals. Although we achieved the complete depletion of these cell types in the blood, we failed to alter rejection or tumor resistance phenotypes in RBPjCKO mice (Figure S2I), most likely due to the resistance of activated T cells to depletion (Figure S2J). From these sets of experiments, we concluded either that (a) the cell type(s) mediating the effects of TSLP were resistant to antibody depletion and irradiation, such as activated/memory T cells (Jamali et al., 1992; Murphy et al., 1987), or (b) not present among the cell types depleted with the antibodies we used.
To distinguish these possibilities systematically, we focused on RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO mice, which never establish TSLP-dependent tumor resistance and thus lack activated effector cells. Having established that Wild-type BM can reconstitute the skin rejection and tumor resistance in RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO animals (Figure 5A–B), we reconstituted the immune cells of lethally irradiated RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO mice with BM from Rag2−/− donors that lack adaptive immunity. DMBA-treated RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO animals transplanted with Rag2−/− BM failed to reject mutant skin, which formed hypertrophic cysts and developed tumors (Figure 5A–B and S2K). This clearly demonstrates that adaptive immune cells responding to TSLP are required for the tumor resistance and mutant skin rejection in Notch signaling-deficient animals.
CD4+ T cells are both required and sufficient to mediate the effects of TSLP on skin rejection and tumor resistance
Among adaptive immune cell types, activated T cells are known to be resistant to irradiation and persist after antibody depletion (Jamali et al., 1992; Murphy et al., 1987). Considering that mice lacking CD8+ CTLs retained their tumor resistance and skin rejection phenotypes (RBPjCKO;CD8α −/−; Figure S2H), CD4+ T cells emerged as the prime candidate mediating the effects of TSLP in our model of Notch signaling-deficient skin. To test this hypothesis, 2 × 106 CD4+ or CD8+ T cells from wild-type littermates were transferred to sublethally irradiated RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO newborns. Adoptive transfer of wild-type CD4+ T cells to RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO mice re-established the tumor resistance and mutant skin rejection phenotypes in these animals, but adoptive transfer of wild-type CD8+ CTLs did not (Figure 5C–D and S2L). As expected, wild-type CD4+ T cells transferred into RBPjCKO;IL7rαKO animals underwent Th2 differentiation (Figure S2M). This finding demonstrates that CD4+ Th2 cells are sufficient to receive TSLP signal and reconstitute the effects of TSLP in Notch signaling-deficient skin.
To ask if CD4+ T cells were required to initiate the effects of TSLP, lethally irradiated RBPjCKO;IL7rαKO mice were transplanted with c-Kit+ BM cells from their wild-type littermates and treated continually with anti-CD4, anti-CD8 or IgG control antibodies (Figure 5E). Injecting the RBPjCKO;IL7rαKO animals with depleting antibodies weekly resulted in effective and sustained depletion of targeted cell types (Figure 5E). Importantly, only the RBPjCKO;IL7rαKO mice that received wild-type BM progenitors plus anti-CD4 antibody retained their mutant cells, formed hypertrophic cysts, and developed skin tumors in response to DMBA (Figure 5F), despite the possible presence of a few mature CD4+ cells which differentiated in the donor environment. In all BMT and adoptive T cell transfer experiments, the presence of donor-derived hematopoietic cells were confirmed at the conclusion of the study (Figure S2N). Together, these data demonstrate that naïve CD4+ T cells constitute the cell type receiving the TSLP signal and coordinating the immune response necessary to reject Notch signaling-deficient skin and prevent tumorigenesis in this context.
Epidermal TSLP overexpression in wild-type skin prevents skin tumorigenesis
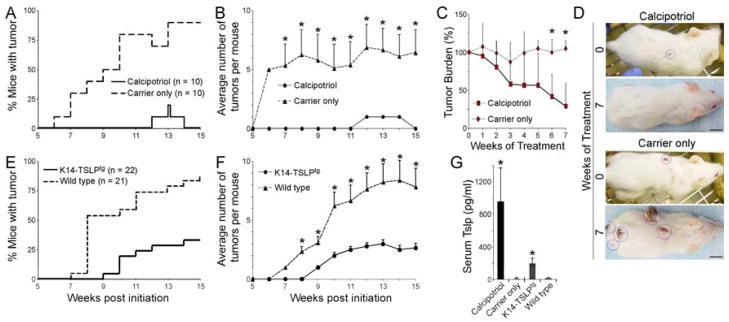
To test if TSLP overexpression can mobilize the anti-tumor immune response in a background free of Notch mutations, we used chemical and genetic approaches to upregulate epidermal TSLP expression in wild-type animals subjected to the multistage chemical skin carcinogenesis model. First, we used the topical application a low-calcemic Vitamin D3 analog (calcipotriol; known also as MC903 or Dovonex) to induce epidermal TSLP expression in CD1 female mice (Li et al., 2006). CD1 genetic background was chosen for these studies because the skin inflammation caused by TSLP overexpression downstream of calcipotriol treatment did not result in a full-blown AD-like disease (Demehri et al., 2009a). Mice were treated with a single initiating dose of DMBA (125 μg), followed by a twice-weekly dose of TPA (4 μg) for 14 weeks. The test group was also treated with topical calcipotriol (32 nmol) and controls with carrier only (EtOH) five times a week during the 14 weeks of TPA application. Strikingly, the majority of carrier-only treated animals developed papillomas, whereas only two of the calcipotriol-treated animals transiently developed papillomas during 15 weeks of follow-up (n = 10 for each group, p <0.0001, Figure 6A–B).
Figure 6.
TSLP creates a tumor-suppressing environment in the wild-type skin. (A,B) DMBA-TPA treated CD1 wild-type animals were topically treated with calcipotriol or carrier (EtOH), (A) time to tumor onset (p <0.0001, log-rank test) and (B) average number of tumors among tumor-bearing animals are shown. n = 10 for each group; error bars represent +/− SEM; *: p <0.01, student’s t-test. (C,D) The 8 tumor-bearing carrier-treated mice shown in “A&B” were randomly divided into two groups at the end of the 15-week DMBA-TPA treatment course. The “test” group was treated with 32 nmol calcipotriol while the “control” group continued to receive carrier alone 5 times per week. (C) After an additional 7-week treatment period, the tumor burdens of the calcipotriol-treated and carrier-treated mice are compared. n = 4 in each group; *: p <0.05, student’s t-test; error bars represent +/− SD. (D) The representative pictures show the size of the remaining tumors in calcipotriol-treated versus carrier-treated mice at the end of the 7-week follow-up period. Tumors are highlighted with blue circles; scale bars: 1 cm. (E,F) K14-TSLPtg female mice and their wild-type female littermates treated with DMBA-TPA are compared for (E) time to tumor onset (p <0.0001, log-rank test) and (F) average tumor number among tumor-bearing animals. n = 22 for K14-TSLPtg group and n = 21 for wild-type group; error bars represent +/− SEM; *: p <0.01, student’s t-test. (G) Circulating TSLP levels in calcipotriol-treated and K14-TSLPtg animals are compared to their controls. Error bars represent +/− SD; *: p <0.0001 compared to wild-type group, student’s t-test.
To investigate if calcipotriol application can affect existing skin tumors, we randomly divided the tumor-bearing CD1 animals from the carrier-only treatment group (Figure 6A–B) into two groups at the end of the TPA treatment period. One subgroup received calcipotriol five times weekly, whereas the other received carrier only. Both were monitored for an additional 7 weeks. While tumors on the carrier-treated animals continued to grow in size, tumors on the calcipotriol-treated animals shrank over time (p <0.05 at weeks 6 and 7, Figure 6C–D).
It is possible that the effects observed above were related to vitamin D signaling, independent of TSLP expression in the skin. To confirm that the anti-tumor effects are mediated through TSLP, we examined the tumor susceptibility of transgenic animals that overexpress TSLP in basal keratinocytes. Consistent with the proposed role for TSLP, K14-Tslptg/+ (K14-TSLPtg) female animals treated once with 100 μg DMBA followed by 4 μg TPA twice weekly for 14 weeks showed significant resistance to tumorigenesis compared to their wild-type littermates (n = 22 for K14-TSLPtg group and 21 for wild-type group, p <0.0001 log-rank test, Figure 6E–F). The serum TSLP measurements confirmed the overexpression of TSLP in calcipotriol-treated wild-type and K14-TSLPtg animals (Figure 6G). These results clearly demonstrate that the tumor-suppressor effect of TSLP in the skin can be extended to wild-type animals and that TSLP can prevent tumorigenesis as well as inhibit growth of existing tumors.
Discussion
The main finding of this report is that upregulation of epidermal TSLP can generate a dominant and lasting anti-tumor CD4+ T cell response in a Th2 inflammatory microenvironment (Demehri et al., 2009a), protecting animals from spontaneous and chemically-induced skin tumors. These cells orchestrate the recognition and elimination of proliferating pre-cancerous cells. This is in stark contrast to the previously described pro-tumor function of Th2-polarized inflammation (Johansson et al., 2008) and of TSLP (De Monte et al., 2011; Olkhanud et al., 2011; Pedroza-Gonzalez et al., 2011). Importantly, we show that chemical induction of TSLP expression in wild-type animals with DMBA-induced papillomas results in tumor regression. This latter finding suggests that TSLP upregulation may provide therapeutic benefits in treating skin tumors and perhaps for other solid tumors.
TSLP is a pleiotropic cytokine involved in several immune processes (Ziegler and Artis, 2010). In the skin and the lung, TSLP is expressed in response to barrier disruption (Demehri et al., 2008), where it can skew CD4+ T cell differentiation towards a Th2 subtype and mediate allergic inflammation (Al-Shami et al., 2004; Tanaka et al., 2009; Ziegler and Artis, 2010) (Leonard, 2002). TSLP serum levels can be a sensitive readout for the degree of disruption in epidermal differentiation/barrier integrity (Demehri et al., 2008). Epidermal Notch1 deletion results in mild disruption in epidermal differentiation, which forms a tumor-promoting environment dominated by Gr-1+ CD11b+ myeloid suppressor cells and soluble factors (Demehri et al., 2009b). The contribution of TSLP to this tumor-promoting skin environment remains to be determined. Others have shown that TSLP is responsible for promoting tumor growth and metastasis in pancreatic and breast cancer (De Monte et al., 2011; Olkhanud et al., 2011; Pedroza-Gonzalez et al., 2011). However, once all Notch-signaling is inactivated, high TSLP levels help establish a potent tumor-suppressing response. The observation that calcipotriol treatment is capable of shrinking pre-existing tumors argues against a model in which TSLP may have a protective effect on the early stages of tumorigenesis but a promoting effect on later stages of tumor growth and metastasis, but this is still feasible. There are other possible explanations for these conflicting findings: TSLP tumor-suppressor effect may be skin-specific; alternatively, based on observations in an allelic series of Notch-deficient mice, TSLP levels may determine whether it promotes or inhibits tumor development and growth. The tipping point may vary according to the strength of the pro-tumor environment in a specific context. Support for the latter comes from the observations that higher TSLP levels are needed to establish a tumor-resistant phenotype in the tumor-prone Notch mutant animals than in the otherwise wild-type K14-TSLPtg animals. Below this hypothetical threshold, TSLP will have a neutral or tumor-promoting effect; above it, TSLP acts as a potent tumor-suppressor. These concepts need to be formally tested in future studies. Nonetheless, here we report that, in both a neutral and a pro-tumor microenvironment, TSLP concentrations capable of creating a CD4+ T cell-mediated anti-tumor response can be reached. Once achieved, activated CD4+ T cells not only identify and prevent growth of transformed cells but also mediate the rejection of Notch signaling-deficient cells.
In the Msx2-Cre animals, large territories of mutant tissue are embedded in wild-type skin. Therefore, the global resistance of calico RBPjCKO skin to chemical carcinogens suggests that the wild-type keratinocytes harboring an activated H-Ras allele are being suppressed alongside mutant cells with three different Notch-deficient genotypes (i.e. RBPjCKO, N1N2CKO or PSDCKO). Importantly, TSLP-activated CD4+ T cells do not target the normal skin cells in the same animal as they proliferate to replace the rejected mutant ones. Based on these observations, we conclude that CD4+ T cells conditioned by high local TSLP concentrations must recognize immunogenic epitope(s) arising independent of Notch signaling in cells with abnormal differentiation. This protective activity does not arise in animals that have CD4+ T cells that lack the TSLP receptor, that have low local TSLP concentrations, or that do not have CD4+ T cells. It is important to note that CD4+ T cell activation by TSLP can occur even if all other tissues and hematopoietic lineages, including dendritic and Langerhans cells, are rendered blind to TSLP by germline deletion of either TSLP receptor arms (Il7rα or Crlf2/Tslpr), as shown in the context of allergic disease where TSLP acts directly on CD4+ T cells (Al-Shami et al., 2005; Rochman et al., 2007; Rochman and Leonard, 2008). These data establish that TSLP-responsive CD4+ T cells are both sufficient and required to create the tumor suppressing microenvironment, likely by recruiting several cytotoxic immune effector cells, including CTLs, NK cells and macrophages to the skin. Considering that TSLP promotes Th2 differentiation in Notch signaling-deficient mice (data not shown (Al-Shami et al., 2004; Demehri et al., 2009a; Tanaka et al., 2009; Ziegler and Artis, 2010)), we propose that the CD4+ Th2 cells are initiating the effects of TSLP. The exact nature of the antigens they are reacting to, and whether they are keratinocyte-specific, remain to be addressed in future studies. Once activated, the CD4+ Th2 cells home to the skin and form a lasting pool of “memory” cells that could not be eradicated with anti-CD4 antibody or irradiation (Figures S4D). This observation is very exciting because it demonstrates that TSLP induces a lasting anti-tumor immunity that is achieved by targeting antigens specific to at least some tumor cells. This protection can be achieved by application of a TSLP-inducer (calcipotriol) to fully developed skin tumors in wild-type animals. It remains to be determined if TSLP can also stimulate the regression of other solid tumors besides the ones formed in the skin.
In an accompanying paper, a similar set of observations is reported. Both studies report an anti-tumor function for TSLP in Notch-deficient skin, both demonstrate that this function of TSLP can also be elicited in animals with intact Notch signaling, and both find an important role for CD4+ T cells. However, whereas our colleagues report that CD8+ CTLs mediate the tumor resistance phenotype, we do not find CD8+ T cells to be necessary in our model. In their study, Notch alleles are deleted globally in the skin after birth using an inducible basal keratinocyte Cre. Consequently, deletion occurs during the hair follicle growth phase, the hair follicle bulge remains intact and the immune system matures in the absence of TSLP or tumor antigens. This deletion paradigm precludes monitoring the rejection of mutant skin clones. In our mice, the immune system matures in the presence of TSLP, which begins to accumulate in utero at E16.5 (Demehri et al., 2008), and in the presence of the antigens presented by mutant cells and by tumor cells later on. Moreover, in our mice, the hair follicle is destroyed by P10 and the bulge never forms because the outer root sheath of the hair follicles adopts an epidermal fate right after birth (Demehri et al., 2008). It is conceivable that some of the difference between the two studies reflects methodological differences, but it is hard to explain why CD8+ CTLs do not contribute to our system, yet provide the bulk of protection for their Notch-deficient mice. The explanation may lie in the difference between the tumor cells of origin, the timing of the Notch signaling deletion, or the differences in background (ours is mixed, whereas theirs is pure C57/B6). The differential role of CTLs in these two experimental paradigms needs to be addressed in future studies. The accompanying study demonstrates that the cystic tumors arise via a Wnt/β-catenin-dependent mechanism from bulge-derived hair follicles, whereas our tumors originate from epidermal cells that did not accumulate nuclear β-catenin prior to tumor formation (Demehri et al., 2009b). Taken together, however, the differences between the studies support the conclusion that TSLP can provide immunological protection against skin cancer and support the speculation that it may do the same in other types of solid tumors.
In summary, this study and the accompanying paper by Di Piazza et al. identify a previously unrecognized role for TSLP in inducing a robust anti-tumor response in several experimental paradigms (Notch loss of function, Wnt gain of function (Di Piazza et al.) and DMBA-induced H-Ras dependent tumors). Moreover, these studies demonstrate that TSLP exerts its anti-tumor effects through a CD4+ Th2 inflammatory environment. Our findings may explain why some individuals who suffer from Th2-dominant allergic disorders display reduced risk of developing certain types of cancers, including non-melanoma skin cancers (Gandini et al., 2005; Hwang et al., 2012; Prizment et al., 2007; Vajdic et al., 2009; Wang and Diepgen, 2005). Importantly, therapeutic exploitation of this mechanism seems within reach given the efficacy of calcipotriol, an FDA-approved drug and a potent inducer of TSLP, in blocking DMBA-induced carcinogenesis.
Experimental Procedures
Mice
The mutant animals (listed in the supplemental methods section) were generated according to the methods outlined in our previous report (Pan et al., 2004). All the mice were housed in the Washington University animal facility and all experiments were performed in accordance with relevant institutional and national guidelines and regulations approved by the Animal Studies Committee at Washington University. The pedigreed RBPjCKO cohort was maintained in mixed FVB, C57BL/6 and CD1 genetic backgrounds, which were overall more susceptible to DMBA/TPA skin carcinogenesis. All other animals were kept in mixed C57BL/6 and CD1 genetic backgrounds and, therefore, were relatively more resistant to chemical skin carcinogens. In all cancer experiments, age-matched littermates were compared.
Msx2-Cretg/+; Rosa-LSL-Eyfp mouse was imaged at P0 using Leica stereoscopic fluorescence microscope with regular light (rendered magenta in Figure 1a) or YFP filter. In studies related to mutant skin rejection, spontaneous/DMBA-induced tumorigenesis and life span, mice were photographed with a digital camera weekly and monitored for onset, number and size of tumors and any sign of failure to thrive. Moribund mice were euthanized and skin, tumors, blood, spleen and lymph nodes were harvested.
Chemical Skin Carcinogenesis Studies
For RBPjCKO DMBA-TPA experiments, 3-week-old mutant mice and Cre-negative wild-type littermates were treated with the standard protocol for skin chemical carcinogenesis model, as previously described (Nicolas et al., 2003). RBPjCKO mice received one dose of 25 μg DMBA (Sigma, St. Louis, MO) followed in a week by biweekly treatment with 4 μg TPA (Sigma, St. Louis, MO) for 14 weeks. In K14-TSLPtg and CD1 carcinogenesis experiments, mice were treated with 125 μg DMBA (CD1, Calcipotriol treatment) or 100 μg DMBA (K14-TSLPtg). In all of the experiments, adult mice were shaved under anesthesia 2 days prior to treatment with DMBA to ensure the hair follicles were in the second telogen.
In studies where tumorigenesis was induced with one dose of DMBA, the mutant animals received a single dose of 125 μg DMBA in 100 μl of acetone during the second week of life, after the mice were subjected to any other experimental procedures including irradiation, BMT or adoptive T cell transfer.
Bone marrow transplantation (BMT)
The recipient mice were lethally irradiated with 950-cGy in second week of life and transplanted with unfractionated BM cells from their littermates, Rag2−/−;γc−/− or Rag2−/− animals as previously described (Zhang and Ren, 1998). All transplanted animals were maintained on antibiotics containing water to prevent infection.
Adoptive T cell transfer
For T cell isolation, splenocytes were positively selected for CD4 or CD8 surface expression using CD4 or CD8 MicroBeads, respectively, followed by negative selection to remove any CD11c+ dendritic cells using CD11c MicroBeads (Miltenyi Biotec Inc., Auburn, CA). RBPjCKO;IL7rαKO and PSDCKO;TSLPRKO mice were irradiated with 450-cGy during the second week of life and injected intravenously with ~2 × 106 CD4+ or CD8+ T cells isolated from the spleen of their littermates.
BMT and Antibody Depletion
RBPjCKO;IL7rαKO newborn mice received intraperitoneal injection of 750 μg anti-CD4 (GK1.5; Bio X Cell, West Lebanon, NH), anti-CD8 (YTS; Bio X Cell) or IgG Control antibody (Sigma, St. Louis, MO) in second week of life. 48 hr later, the mutant animals received BMT using c-Kit+ BM progenitors (lacking T cells) from their wild-type littermates isolated using CD117 MicroBeads and magnetic columns according to manufacturer’s protocol (Miltenyi Biotec Inc., Auburn, CA). Each recipient was injected intravenously with 2×106 c-Kit+ BM cells in 100μl PBS+2%FBS. The mutant animals were then treated topically with one dose of DMBA and continued to receive 250 μg of the depleting antibody weekly. Mice were monitored for skin rejection and tumor formation weekly and harvested at P90.
Statistical analysis
Except for Calcipotriol studies performed on wild-type CD1 animals, all the animals used in this report were kept on mixed genetic backgrounds in a pedigreed colony (i.e. all animals are logged into a database). To minimize the confounding effects of strain or family background on tumor outcomes, we only compared gender-matched littermates in each cancer study. Using the power analysis described previously (Demehri et al., 2009b), we determined the number of animals needed in each chemical skin carcinogenesis study. As the test of significance between the study groups, we used log-rank test for “Time to tumor onset” and Student’s t-test for tumor counts, serum TSLP levels and other quantitative measurements.
See supplemental material for further description of methods.
Supplementary Material
Significance.
We demonstrate unequivocally that TSLP triggers a dominant anti-tumor response in a Th2-polarized inflammatory microenvironment in the skin. Importantly, the anti-tumor microenvironment created by TSLP-inducers like low-calcemic vitamin D agonists (e.g. Calcipotriol) can prevent and eliminate skin tumors in wild-type mice. Although our findings may reflect a skin-specific effect, it is intriguing to postulate that TSLP plays a common tumor suppressor role during the early stages of solid tumor development. Considering the emergence of TSLP as a potential therapeutic target in treatment of solid cancers, this report points to an alternative utility for TSLP as an anti-tumor immune factor that can be utilized to optimally combat and ultimately prevent solid cancers.
Highlights.
RBPj-deficient skin with severe Th2 inflammation is resistant to tumorigenesis
TSLP signaling is responsible for tumor resistance in Notch-signaling deficient skin
TSLP acts through CD4+ T cells to establish tumor resistance
Induction of TSLP in wild-type skin can produce resistance to tumorigenesis
Acknowledgments
We would like to thank Drs. Robert Schreiber, Kenneth Murphy, Barry Sleckman, Emil Unanue, Wayne Yokoyama, Deepta Bhattacharya, Takeshi Egawa, Suellen Greco, Omar Jassim and David Denardo for providing us with many valuable experimental tools, ideas and comments on the manuscript, and the members of the Kopan laboratory for their suggestions during the course of this study. We wish to thank Dr. Freddy Radtke and his coworkers for sharing their unpublished results with us during the course of these experiments. We would like to thank Dr. Gail Martin for providing Msx2-Cretg/+ mice, Dr. Tom Gridley for Notch2flox/flox mice, Dr. Jie Shen for Ps1flox/flox mice, Dr. Tasuku Honjo for Rbpjflox/flox mice, Dr. Andrew Farr for the K14-TSLPtg mice, and Dr. Warren Leonard for Tslpr−/− mice. We would like to thank Rheumatic Disease Core Center’s Speed Congenics Lab at Washington University for their assistance with genotyping. SD, MT and RK are supported by grant GM55479-16 from NIH/NIGMS. SM and LY were supported with funds from the American Asthma Foundation (Grant 09-0234). AT was supported by funds from NIH 2 U19 AI070489-09. The flow cytometry core is supported by P30 CA091842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Cario G, Zimmermann M, Romey R, Gesk S, Vater I, Harbott J, Schrauder A, Moericke A, Izraeli S, Akasaka T, et al. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 2010;115:5393–5397. doi: 10.1182/blood-2009-11-256131. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. The Journal of experimental medicine. 2011 doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, Grigsby PW, Miner JH, Farr AG, Kopan R. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009a;7:e1000067. doi: 10.1371/journal.pbio.1000067. 1000010.1001371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer cell. 2009b;16:55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier A, Durham AD, Di Piazza M, Vauclair S, Koch U, Ferrand G, Ferrero I, Demehri S, Song LL, Farr AG, et al. Atopic Dermatitis-Like Disease and Associated Lethal Myeloproliferative Disorder Arise from Loss of Notch Signaling in the Murine Skin. PLoS ONE. 2010;5:e9258. doi: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extance A. Alzheimer’s failure raises questions about disease-modifying strategies. Nat Rev Drug Discov. 2010;9:749–751. doi: 10.1038/nrd3288. [DOI] [PubMed] [Google Scholar]
- Gandini S, Lowenfels AB, Jaffee EM, Armstrong TD, Maisonneuve P. Allergies and the risk of pancreatic cancer: a meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2005;14:1908–1916. doi: 10.1158/1055-9965.EPI-05-0119. [DOI] [PubMed] [Google Scholar]
- Han H, Xu W, Headley MB, Jessup HK, Lee KS, Omori M, Comeau MR, Marshak-Rothstein A, Ziegler SF. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer [Review] Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg L, Vendramini E, Ganmore I, Cazzaniga G, Schmitz M, Chalker J, Shiloh R, Iacobucci I, Shochat C, Zeligson S, et al. Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood. 2010;115:1006–1017. doi: 10.1182/blood-2009-08-235408. [DOI] [PubMed] [Google Scholar]
- Hwang CY, Chen YJ, Lin MW, Chen TJ, Chu SY, Chen CC, Lee DD, Chang YT, Wang WJ, Liu HN. Cancer risk in patients with allergic rhinitis, asthma and atopic dermatitis: A nationwide cohort study in Taiwan. International journal of cancer. 2012;130:1160–1167. doi: 10.1002/ijc.26105. [DOI] [PubMed] [Google Scholar]
- Jamali I, Field EH, Fleming A, Cowdery JS. Kinetics of anti-CD4-induced T helper cell depletion and inhibition of function. Activation of T cells by the CD3 pathway inhibits anti-CD4-mediated T cell elimination and down-regulation of cell surface CD4. J Immunol. 1992;148:1613–1619. [PubMed] [Google Scholar]
- Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunological reviews. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapins J, Ye W, Nyren O, Emtestam L. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730–734. [PubMed] [Google Scholar]
- Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21:e457–460. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- Leonard WJ. TSLP: finally in the limelight. Nature immunology. 2002;3:605–607. doi: 10.1038/ni0702-605. [DOI] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wen H, Brayton C, Laird FM, Ma G, Peng S, Placanica L, Wu TC, Crain BJ, Price DL, et al. Moderate reduction of gamma-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci. 2007;27:10849–10859. doi: 10.1523/JNEUROSCI.2152-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia F, Denning M, Kopan R, Yuspa SH. The Black Box Illuminated: Signals and Signaling. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97:173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. The Journal of experimental medicine. 1987;166:1499–1509. doi: 10.1084/jem.166.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, di Vignano AT, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, Van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ, Biragyn A. Thymic Stromal Lymphopoietin Is a Key Mediator of Breast Cancer Progression. J Immunol. 2011 doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Lin M, Tian X, Cheng H, Gridley T, Shen J, Kopan R. g-Secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7:731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. The Journal of experimental medicine. 2011 doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. The Journal of experimental medicine. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizment AE, Folsom AR, Cerhan JR, Flood A, Ross JA, Anderson KE. History of allergy and reduced incidence of colorectal cancer, Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2007;16:2357–2362. doi: 10.1158/1055-9965.EPI-07-0468. [DOI] [PubMed] [Google Scholar]
- Quigley DA, To MD, Perez-Losada J, Pelorosso FG, Mao JH, Nagase H, Ginzinger DG, Balmain A. Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature. 2009;458:505–508. doi: 10.1038/nature07683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Syal R, Selvarajah S, Chakrabarti O, Sarin A, Krishna S. Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology. 2001;286:23–30. doi: 10.1006/viro.2001.0867. [DOI] [PubMed] [Google Scholar]
- Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008;181:7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infection and immunity. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CA. Immunotherapy: TSLP fuels inflammation in breast and pancreatic tumors. Nat Rev Clin Oncol. 2010 doi: 10.1038/nrclinonc.2011.40. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, NY. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, Te Kronnie G, Cario G, Cazzaniga G, Kulozik AE, Stanulla M, et al. Gain-of-function mutations in interleukin-7 receptor-{alpha} (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011 doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Watanabe N, Kido M, Saga K, Akamatsu T, Nishio A, Chiba T. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy. 2009;39:89–100. doi: 10.1111/j.1365-2222.2008.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajdic CM, Falster MO, de Sanjose S, Martinez-Maza O, Becker N, Bracci PM, Melbye M, Smedby KE, Engels EA, Turner J, et al. Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Res. 2009;69:6482–6489. doi: 10.1158/0008-5472.CAN-08-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual review of immunology. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang W, Wen W, Sun J, Su B, Liu B, Ma D, Lv D, Wen Y, Qu T, et al. {gamma}-Secretase Gene Mutations in Familial Acne Inversa. Science. 2010;330:1055–1056. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2005;60:1098–1111. doi: 10.1111/j.1398-9995.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- Xia X, Qian S, Soriano S, Wu Y, Fletcher AM, Wang XJ, Koo EH, Wu X, Zheng H. Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10863–10868. doi: 10.1073/pnas.191284198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Dlugosz AA, Cheng CK, Denning MF, Tennenbaum T, Glick AB, Weinberg WC. Role of oncogenes and tumor suppressor genes in multistage carcinogenesis. The Journal of investigative dermatology. 1994;103:90S–95S. doi: 10.1111/1523-1747.ep12399255. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–3840. [PubMed] [Google Scholar]
- Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, Chambon P. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci U S A. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Current opinion in immunology. 2010;22:795–799. doi: 10.1016/j.coi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.