Abstract
Diabetes is the fifth leading cause of death worldwide and contributes to leading causes of death, cancer and cardiovascular disease including coronary heart disease, stroke, peripheral vascular disease and other vascular disease. While glycemic management remains a cornerstone of diabetes care, the co-management of hypertension, atherosclerosis, cardiovascular risk reduction and prevention of long-term consequences associated with diabetes are now well recognized as essential to improve long-term survival. Clinical trial evidence substantiates the importance of glycemic control, LDL-cholesterol lowering therapy, blood-pressure lowering, control of albuminuria, and comprehensive approaches targeting multiple risk factors to reduce cardiovascular risk. This article presents a review of the role of diabetes in pathogenesis of atherosclerosis and cardiac dysfunction, recent evidence regarding degree of glycemic control and mortality, and available evidence for a multi-faceted approach to improve long-term outcomes for patients.
Introduction
Diabetes is a devastating disease that affects more than 25.8 million people in the United States and more than 330 million people worldwide (http://www.diabetes.niddk.nih.gov/dm and http://www.who.int). The Centers for Disease Control and Prevention estimates that nearly 26% of US adults are obese and nearly 8% of the population has diabetes (http://www.cdc.gov/diabetes). Diabetes is the fifth leading cause of death worldwide and contributes to leading causes of death, cancer and cardiovascular disease. More than 90% of individuals with diabetes have type 2 diabetes mellitus, which could be prevented by behavioral modification including improved nutrition and increased physical activity1–4. People with diabetes have a nearly 40 percent incidence of cardiovascular disease including coronary heart disease, stroke, peripheral vascular disease, and other vascular disease (http://www.cdc.gov/diabetes). In addition, there is an excess risk of cancer. In 2001, the estimated decrease in life expectancy for people with diabetes in the UK was 10 years for people with type 2 diabetes and 20 years for people with type 1diabetes (www.dh.gov.uk/en/Publicationsandstatistics). More recent statistics for the United States suggest a decrease in life expectancy of 8.5 years5. The excess mortality attributable to diabetes in 2000 was 2.9 million (5.2%) globally, and up to 8% in the US6. Age of onset, duration of diabetes, and functional status are predictors of excess mortality7, 8. This background defends the need to prevent diabetes and treat preventable causes of premature death in people with diabetes.
The majority of clinical trials assessing cardiovascular and all-cause mortality in people with diabetes have focused on individual interventions including treatment of glucose, hypertension, cholesterol and anti-platelet therapy. In each instance, intervention has proven beneficial. Multi-pronged interventional studies provide evidence for coordinated treatment of cardiovascular risk factors resulting in cardiovascular risk reduction9–11. We will provide an overview of the pathophysiology of cardiovascular disease in diabetes and outline the evidence for treatment of multiple cardiovascular risk factors in the prevention of morbidity and mortality related to diabetes.
Epidemiological evidence supports the thesis that diabetes adds to the impact of individual risk factors such as hypertension and hyperlipidemia for the prediction of excess cardiovascular disease. Prospective longitudinal studies such as Framingham, MRFIT, the Physicians Health Study, and the Nurses' Health Study each demonstrated that in the context of diabetes, there was a greater risk of cardiovascular disease for a given blood pressure or cholesterol level12–14. These observations led to prospective investigation of cohorts with and without diabetes for cardiovascular disease. In these studies, such as the East-West study, the incidence of cardiovascular disease in people with greater than 5 years of diabetes and in the 55 to 65-year-old age range, was equivalent to cardiovascular risk in persons with previous myocardial infarction15. These data sets were replicated in some, albeit not all, other studies and populations, leading to the clinical impression that diabetes should be considered a cardiovascular risk equivalent. While this may not be universally true in younger populations of people with diabetes, for the general clinician, patients with diabetes should be treated expectantly and cardiovascular risk should be addressed aggressively. Recently, a post hoc analysis of the ADVANCE trial indicated that routine clinical parameters including age at diagnosis, duration of diabetes, sex, pulse pressure, treated hypertension, atrial fibrillation, retinopathy, HbA1c, urinary albumin/creatinine ratio and non-HDL cholesterol predicted CVD in diabetes16.
One of the most consistent and unacceptable observations across interventions in people with and without diabetes is that while both groups benefit from blood pressure lowering or cholesterol-lowering, excess mortality remains in the diabetic cohort. This observation indicates the need for research into additional targets for intervention in diabetes and the importance of comprehensive in risk factor modification for people with diabetes. Multiple risk factor intervention studies such as Steno-2 and Look AHEAD suggest an impressive impact of simultaneous multiple risk factor intervention11, 17. These studies do not have a non-diabetic comparator group, so it is not possible to tell whether they decreased cardiovascular risk to that of the general population. In this review, we will provide background data on the efficacy of risk factor intervention singly and in combination for people with diabetes to support our recommendation for coordinated risk factor modification in all people with diabetes.
Role of diabetes in progression of cardiac and vascular disease
Atherosclerosis is more common in people with diabetes, the atherosclerotic burden is higher and the mortality is greater after a cardiovascular event, acute myocardial infarction, stroke or peripheral vascular disease18, 19. In addition, people with diabetes have a higher incidence of congestive heart failure and greater loss in cardiac contractile function for the same size myocardial infarction leading to excess congestive heart failure20–22. Each of these conditions will be briefly reviewed in relationship with diabetes.
Atherosclerosis in diabetes
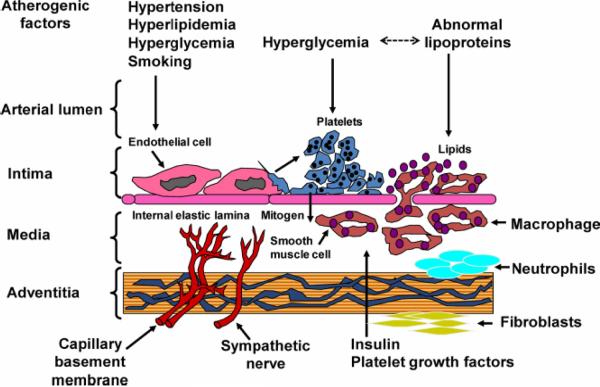
Atherosclerosis is the development of complex lipid-laden lesions in the vasculature with the potential to contribute to cardiovascular events including myocardial infarction, stroke and peripheral vascular disease (reviewed in 23–25). The blood vessel wall is lined by endothelium which overlies vascular smooth muscle cells while the vessel itself is surrounded by adventitia which includes fibroblasts, adipocytes, and other cells. The classical model of atherosclerosis progression includes injury to the endothelium by circulating factors such as hyperglycemia, hyperlipidemia, inflammatory cytokines, reactive oxygen species, excess flow-mediated stress (hypertension), cigarette smoke or environmental toxins. Injured endothelium changes vascular cell phenotype. The normal endothelial cell phenotype is anti-atherogenic: healthy endothelial cells have tight junctions preventing entry of lipoproteins into the subendothelial space; in response to sheer stress, endothelial cells secrete nitric oxide leading to flow-mediated vasodilation. Furthermore, platelets and monocytes will not adhere to healthy endothelial cells; tissue plasminogen activator, a thrombolytic protein, is secreted by healthy endothelial cells. With injury, endothelial cell tight junctions are weakened, allowing egress of lipoproteins into the subendothelial space; there is decreased function of endothelial nitric oxide synthase leading to decreased vasodilatory nitric oxide secretion and increased expression of endothelin-1leading to increased vasoconstriction; endothelial cells express adhesion molecules which attract and activate platelets and monocyte/ macrophages; in addition, production of plasminogen activator inhibitor type I, a pro-thrombotic protein, is increased in injured endothelium26–33. Taken together, this series of changes in endothelial function leads to a feed-forward pro-inflammatory phenotype (Fig 1 – Figure 1 from Reusch/Wang 2011). In addition to the specific changes outlined above, there is increased reactive oxygen species, cytokines and growth factors leading to smooth muscle cell proliferation and foam cell formation34. More recently, the role of the adventitia, recruitment of inflammatory cells, and stimulation of adventitial neovascularization have added to the complexity of progression of the atherosclerotic plaque35–37. Diabetes accelerates plaque progression in hypertensive and dyslipidemic animal models. In human subjects, endothelial dysfunction predicts cardiovascular disease (reviewed in 38).
Figure 1.
Diagram of the vascular wall: atherogenesis in the setting of diabetes. Exposure to atherogenic factors such as hyperglycemia, hyperlipidemia, excess flow-mediated stress (hypertension), or cigarette smoke results in over-production of inflammatory cytokines and reactive oxygen species, injuring the endothelium, causing vascular smooth muscle cell phenotype switching , allowing egress of lipoproteins into the subendothelial space, increasing adhesion molecule expression which attracts and activates platelets and monocytes/ macrophages, and foam cell formation34. Diabetes accelerates plaque progression.
Reusch and Wang, JCEM 2011, Figure 1
Cardiac Dysfunction
Congestive heart failure is increased in diabetes as illustrated by the Framingham study and the Strong Heart Study39, 40. In addition, people with diabetes have an increased incidence of recurrent MI or hospitalization for heart failure after a first MI41. Individuals with diabetes without coronary artery disease experience an excess burden of cardiac dysfunction ranging from subclinical left ventricular hypertrophy to clinically apparent diabetic cardiomyopathy, which is more pronounced in women than in men42. Interestingly, in diabetes, especially in women, CHF hospitalization often occurs in people with increased LV mass and preserved systolic function43, 44. This excess burden of CHF contributes to disability and mortality in people with diabetes.
Much work has been done recently to clarify the etiological factors contributing to the progression of diabetic cardiomyopathy (reviewed in45, 46). In clinical terms, it appears that the inability of the myocardium to efficiently use fuel (lipid or carbohydrate) underlies diabetic cardiomyopathy. Free fatty acids are the preferred fuel of the myocardium at rest. With exertion, healthy myocardium switches to the use of carbohydrates which will generate more ATP for a given amount of oxygen. This is termed metabolic flexibility. In diabetes, there is decreased metabolic flexibility and increased incomplete oxidation of fatty acids leading to production of aldehydes and reactive oxygen species which are toxic to the myocardium46, 47. Much remains to be clarified regarding the precise etiology of this decreased metabolic flexibility, but it is suggested that insulin resistance in the myocardium (decreased reliance on insulin regulated glucose utilization) and limited perfusion of the myocardium contribute to the progression of diabetic cardiomyopathy. In its end stage, persistent excess of reactive oxygen species leads to inflammation and fibrosis much as it does in the adventitia of the vessel wall. In recent data from our laboratory, it is evident that subclinical cardiac dysfunction occurs early in diabetes and is present in adolescents with both type 1 and type 2 diabetes48, 49. Little is known about the impact of clinical interventions on cardiac dysfunction in diabetes. It is well established that hypertension and physical inactivity contribute to cardiac dysfunction. As such, it is expected that antihypertensive and physical activity interventions will improve cardiac function. A more recently appreciated contributor to cardiac dysfunction may be arterial stiffness. Arterial stiffness is often seen with hypertension, but can persist even when hypertension is treated. Overall, it predicts mortality50.
EVIDENCE FROM CLINICAL TRIALS
The East-West trial was a pivotal trial published 15 years ago that changed the way clinicians approached cardiovascular risk in diabetes15. In this trial, the 7-year incidence of myocardial infarction was examined in 2,432 subjects. Subjects with type 2 diabetes but no prior history of myocardial infarction had a risk of cardiovascular death that was similar to individuals with a prior history of myocardial infarction but no diabetes. Furthermore, subjects with both type 2 diabetes and a history of MI had a nearly 3-fold greater risk of cardiovascular death than individuals with a history of MI but no diabetes.
What is the evidence for the impact of glycemic control versus targeting other risk factors on long term survival in diabetes? The next section will outline major trial evidence for intervening in glycemia, cholesterol, blood pressure, and albuminuria on survival in individuals with diabetes. It will end with an overview of evidence from trials examining the impact of multifactorial interventions on survival in diabetes.
Glycemic control – does it matter? How intensive?
Both the DCCT and the UKPDS were large-scale, well-designed and well-conducted randomized trials which showed convincingly that good glycemic control reduces the risk of microvascular complications in both type 1 (A1c of 7% vs. 9%) and type 2 diabetes (A1c of 7% vs 7.9%)51, 52. Since the publication of these initial results in the 1990s, long-term follow-up studies have been performed for both trials, recently demonstrating benefits of good glycemic control on cardiovascular events in both diabetes populations53, 54. There was a “legacy effect” observed in both cohorts, in which the long-term benefits of good glycemic control persisted in the “intensive” arms of each cohort despite regression to a higher A1c during the long follow-up period. It is important to note that what was formerly termed “intensive” control is now considered to be standard of care.
The next major question to be answered became whether tight glycemic control decreases risk for cardiovascular events. This question was addressed in 3 major trials: Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) and the Veterans Affairs Diabetes Trial (VADT). ACCORD was stopped prematurely because of an increased risk of mortality in the intervention group55. However, this increased risk of mortality with tight glycemic control was not observed in ADVANCE56 or VADT57, which were completed as planned. Neither ADVANCE nor VADT demonstrated a benefit for tight glycemic control (A1c of <7%) on cardiovascular endpoints compared with what was achieved in the “standard” control group, which reached an A1c of 7.3% in ADVANCE and 8.4% in VADT.
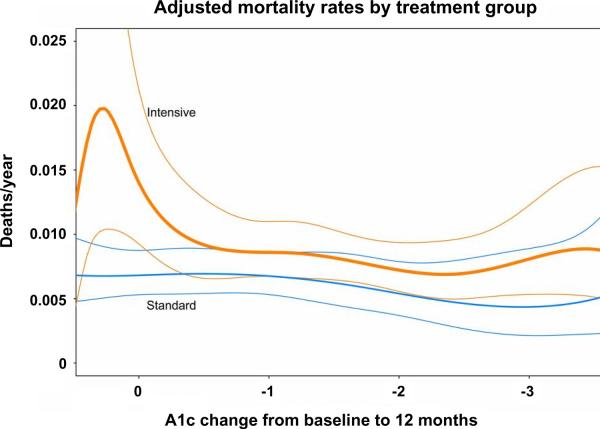
Since publication of these trial results, there has been much discussion of the unexpected increased total mortality observed in ACCORD and post-hoc analyses to understand these findings58–64. Although severe hypoglycemia requiring outside assistance was definitely a risk factor for death in both treatment arms59, it may have been a marker for underlying increased risk for mortality in the standard treatment arm while individuals in the intensive treatment were likely to develop severe hypoglycemia merely from the intensive titration regimen. Post-hoc analyses show that the excess mortality in the intensive treatment group cannot be accounted for by hypoglycemia alone, and the increased rate of mortality continued in these subjects after they were transferred to standard treatment62. Furthermore, the intensive group had a greater risk for mortality than the standard therapy group only in individuals with A1c greater than 7% and those with the least lowering of A1c during the initial phase of treatment61 (Fig. 2 – adapted from Figure 2, Riddle 2010). These findings suggest that factors related to resistance to intensification of glycemic control, or higher A1c, NOT lower A1c, were associated with the increased risk observed in the intensive treatment group61. Interestingly, individuals in the standard treatment group had a much great risk of severe hypoglycemia with every increment in A1c compared with the intensive treatment group, which suggests that the frequent mild hypoglycemia experienced by subjects in the intensive group may have somehow preconditioned the myocardium, protecting it against the effects of severe hypoglycemia59.
Figure 2.
Complex relationship between A1c and mortality in the ACCORD trial: increased mortality in the intensive-therapy group was concentrated among participants whose A1c decreased the least or did not decrease at all in the first year of intensification of glycemic therapy. The orange lines indicate the intensive treatment group with 95% confidence intervals; the blue lines indicate the standard treatment group with 95% confidence intervals
Adapted from Riddle -Diabetes Care 2010 (Fig. 2)
Compared to the ACCORD cohort, the ADVANCE cohort had shorter duration of diabetes, lower baseline A1c, markedly fewer individuals on insulin or thiazolidinedione therapy at baseline or at study end, but similar A1c levels reached in the intensive and standard groups. The VADT cohort had a slightly longer duration of diabetes than the ACCORD cohort, with higher baseline A1c (9.4 vs. 8.1%), and greater use of insulin at baseline and study end. Furthermore, the mean A1c levels reached in each group differed; both study groups in ACCORD reached lower mean A1c than in VADT (ACCORD vs. VADT intensive therapy: 6.4 vs. 6.9%, standard therapy: 7.5 vs. 8.4%). However, these differences have not definitively been shown to account for the differences seen in these trial results.
These trials comparing the effects of standard glycemic control and tight glycemic control do not show a benefit for further lowering of glycemic targets. There are exceptions to this general conclusion, since certain subgroups may benefit, particularly younger individuals with diabetes duration <10–15 years and without significant cardiovascular disease or nephropathy prior to intensifying glycemic control. However, other subgroups of patients may be harmed by glycemic control that is too intensive (“too little, too late”). Overall, a general goal A1c of 7% should be used for many, if not most patients with diabetes, but this should be individualized to a slightly lower or higher goal depending on other factors such as patient motivation and support systems, risk for hypoglycemia, duration of diabetes, and presence/severity of comorbidities and complications. This individualized approach is consistent with A1c recommendations set forth by the American Diabetes Association (ADA)65 and detailed comprehensively in a recently-published position statement from the ADA and the European Association for the Study of Diabetes (EASD) (Diabetes Care 2012 published ahead of print April 19, 2012; doi:10.2337/dc12-0413).
Cholesterol-lowering
Multiple trials have demonstrated that cholesterol-lowering therapy with an HMG-co-A-reductase inhibitor (statin) prevents cardiovascular events in individuals with diabetes, a few of which are outlined below. Secondary prevention of cardiovascular events in diabetes was demonstrated in a subgroup analysis of the 4S (Scandinavian Simvastatin Survival Study) trial66, while primary prevention of cardiovascular events by statin therapy in diabetes was confirmed in CARDS (Collaborative Atorvastatin Diabetes Study)67. CARDS was a multi-center trial conducted in the United Kingdom and Ireland which included 2,838 subjects without prior cardiovascular disease randomized to receive placebo versus atorvastatin 10 mg daily for a mean follow-up period of 3.9 years. The trial was terminated 2 years prematurely because of demonstration of benefit for statin therapy on cardiovascular events. Interestingly, a similar trial, the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) did not demonstrate benefit for lowering of LDL below contemporary targets68.
The Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) trial randomized 9,014 patients with diabetes or impaired fasting glucose, coronary heart disease and total cholesterol between 4.0 – 7.0 mmol/l to placebo vs. pravastatin therapy 40 mg per day for 6 years69. This trial demonstrated that secondary prevention with moderate dose statin therapy reduced the risk of any cardiovascular event from 52.7 to 45.2% (p<0.008) in patients with diabetes, preventing one major CHD event (CHD death or nonfatal MI) in 18 patients with diabetes.
The Medical Research Council/British Heart Foundation Heart Protection Study (HPS) examined the effect of cholesterol-lowering with simvastatin 40 mg daily vs. placebo for 5 years in 20,536 high risk individuals70. The subgroup of the HPS that included 5,963 adults with diabetes71 demonstrated that the net lowering of LDL-cholesterol of this simvastatin dose in this cohort was 1 mmol/l. There was a 22% reduction in major vascular events (20.2% in the simvastatin-treated group and 25.1% in the placebo group, p<0.0001). This benefit of LDL-lowering was seen in the 2,912 subjects without occlusive arterial disease at trial entry, and the 2,426 subjects with pretreatment LDL cholesterol <3.0 mmol/l. This lowering of CVD risk was independent of duration of diabetes, type of diabetes, control of diabetes, age >65 at entry, hypertension, and total cholesterol <5.0 mmol/l. The effectiveness of statin therapy was demonstrated for both primary and secondary prevention of cardiovascular events in this trial.
In summary, strong clinical trial evidence establishes that lowering cholesterol with a statin reduces cardiovascular risk in individuals with diabetes.
Blood pressure-lowering
The UKPDS demonstrated that lowering blood pressure from a mean of 160/94 mm Hg at study entry to 144/82 mm Hg reduces the incidence of deaths related to diabetes compared with a group with “less tight” blood pressure control, in which blood pressure was reduced to 154/87 mm Hg72. When the macrovascular disease deaths were combined (myocardial infarction, sudden death, stroke and peripheral vascular disease), the tight blood pressure control group experienced a 34% reduction in risk compared to the “less tight” blood pressure control group (p=0.019). These results confirmed results observed in the diabetes subgroup of the Systolic Hypertension in the Elderly Program73. Subsequent studies including ALLHAT74, subgroup analysis of the UKPDS72, and ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial)75 among others have examined comparative efficacy of different antihypertensive regimens. UKPDS also demonstrated a 56% reduction in risk of heart failure with tight blood pressure control (p=0.0043).
More recently, the ACCORD study group investigated whether further lowering of blood pressure resulted in additional reduction in a primary composite outcome of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes76. Four thousand seven hundred thirty-three subjects with type 2 diabetes were randomized to intensive therapy or standard therapy and followed for a mean of 4.7 years. The mean systolic BP decreased to 119.3 mm Hg in the intensive therapy group and 133.5 mm Hg in the standard therapy group. By the end of the follow-up period, there was no benefit demonstrated for further lowering systolic blood pressure below 120 mm Hg.
In summary, strong clinical trial evidence demonstrates the beneficial effects of blood pressure control in all individuals with diabetes. However, there is no evidence for aggressive lowering of systolic blood pressure below 120 mm Hg in individuals without albuminuria or other evident renal complications of diabetes.
Control of albuminuria
The presence of microalbuminuria in diabetes correlates with risk for progression to diabetic nephropathy as well as risk for cardiovascular events. However, the published trials were not designed to separate the effects of reducing albuminuria from improvements in glycemia or blood pressure. The LIFE study evaluated urinary albumin-to-creatinine ratio over 4.7 years in patients with diabetes, hypertension and left ventricular hypertrophy77. The urinary albumin-to-creatinine ratio (UACR) during treatment with atenolol or losartan was closely related to the risk of the primary composite endpoint of cardiovascular death, myocardial infarction, and stroke, with a 2-fold increased risk with the highest quartile of UACR compared with the lowest quartile.
A longitudinal study examining 216 patients with type 2 diabetes and microalbuminuria over 8 years showed that patients who achieved significant improvements in microalbuminuria had an adjusted risk of first renal or cardiovascular event of 0.41 (95% CI 0.15–0.96), with significantly lower cumulative incidence of death from and hospitalization for renal or cardiovascular events (p=0.0019)78. Improvement in microalbuminuria was defined as shift to normoalbuminemia or a 50% or greater reduction in microalbuminuria compared with baseline. The group who achieved improvement in microalbuminuria had lower systolic blood pressure and BMI when compared to the “nonreduction” group and also when compared to baseline, prior to any interventions. This study was not designed to determine which interventions were most effective in reducing microalbuminuria.
The Microalbuminuria, Cardiovascular and Renal Outcomes - Heart Outcomes Prevention Evaluation (MICRO-HOPE) trial was a substudy of the HOPE trial consisting of 3577 subjects with diabetes age 55 and older, with or without known cardiovascular disease, who were randomized to receive ramipril or placebo for 5 years79, 80. The HOPE trial itself included 9297 subjects with known cardiovascular disease or diabetes plus at least one other cardiovascular risk factor. The primary outcome was a composite of myocardial infarction, stroke, or death from cardiovascular causes. There were preplanned analyses to determine whether ramipril delayed or prevented microalbuminuria or overt nephropathy in participants with diabetes. HOPE demonstrated that the ramipril group had a relative risk of the primary outcome of 0.78, and relative risk of death from any cause 0.84. Of the total HOPE cohort, 1956 had microalbuminuria, making up approximately 21% of each treatment group. The effect of ramipril to decrease cardiovascular endpoints was demonstrated whether subjects did or did not have microalbuminuria. MICRO-HOPE showed similar results in the subgroup of subjects with diabetes, with a relative risk reduction of 25% for the primary outcome, and total mortality of 24% in the ramipril group. The rate of increase in urine albumin/creatinine ratio was significantly lower in the ramipril group, but since blood pressure was also affected by ramipril, it is unclear whether these effects on albuminuria were independent of improvement in blood pressure.
Physical activity
Increased physical activity is associated with reduced risk for cardiovascular disease in diabetes. In a long-term, prospective study of 3,708 individuals with diabetes followed for 18.7 years, moderate or high levels of physical activity at baseline were associated with decreased total and cardiovascular mortality81. The beneficial effects of physical activity were demonstrated regardless of BMI, blood pressure, total cholesterol and even smoking.
Multifactorial interventions
The Look AHEAD trial is designed to assess the long-term effects of an intensive lifestyle intervention in obese or overweight individuals with type 2 diabetes82. The intensive lifestyle intervention arm includes weight loss with decreased caloric intake and increased physical activity compared with a control group receiving diabetes support and education. A total of 5145 subjects with diabetes are participating in this ongoing study, and subjects have now been followed for over 10 years. The interim trial data demonstrate that an intensive lifestyle intervention resulting in a 5.3% weight loss, increased fitness, and improvements in blood pressure, HDL-cholesterol and triglycerides compared with placebo, can be sustained over 4 years83. Whether this improvement in multiple cardiovascular risk factors with intensive lifestyle intervention will translate to a reduction in cardiovascular events is still unknown.
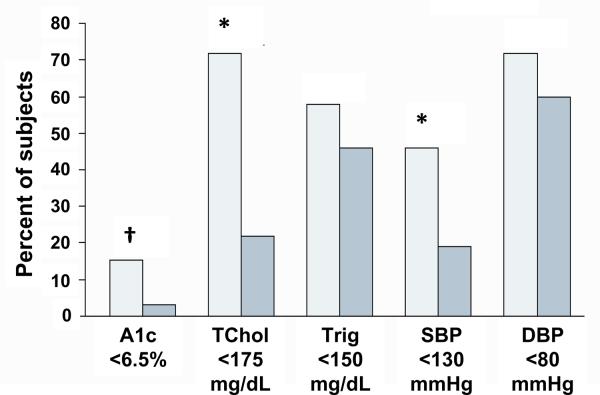
Subjects in the Steno-2 study had type 2 diabetes and microalbuminuria. The 160 subjects had a mean age of 55 years and relatively mild diabetes (a quarter were on diet alone, half were on oral agents, and 5–11% were on insulin) but the diabetes was not well-controlled (mean A1c 8.4% versus 8.8% in the intensive- versus conventional-therapy group). They were randomized to receive conventional treatment according to national guidelines, or intensive treatment that included dietary and lifestyle recommendations and targeting multiple risk factors of hyperglycemia, hypertension, dyslipidemia and microalbuminuria, in addition to secondary prevention of cardiovascular disease with aspirin84. Over a mean follow-up period of 7.8 years, the improvement in all targeted risk factors was more evident in the intensive-therapy group. Despite not achieving each individual risk factor target, the intensive-therapy group had markedly lower risk for cardiovascular events (hazard ratio 0.47, 95% CI 0.24–0.73) (Fig. 3 – adapted from Figure 2B, Gaede 2003).
Figure 3.
Targeting of multiple risk factors in Steno-2. The intensive-therapy group had better control of multiple risk factors in Steno-2 compared with the standard-therapy group, but a significant proportion of participants did not achieve target levels for risk factors, and only 1 individual achieved all of the targets. Despite this, there was a 20% risk reduction in cardiovascular events in the intensive-therapy group. Light bars indicate the intensive-therapy group; shaded bars represent the conventional-therapy group. Comparison of intensive- with conventional-therapy group: *p<0.05; †p<0.10. A1c, hemoglobin A1c. TChol, total cholesterol. Trig, triglycerides. SBP, systolic blood pressure. DBP, diastolic blood pressure
adapted from Gaede 2003 (Fig 2B)
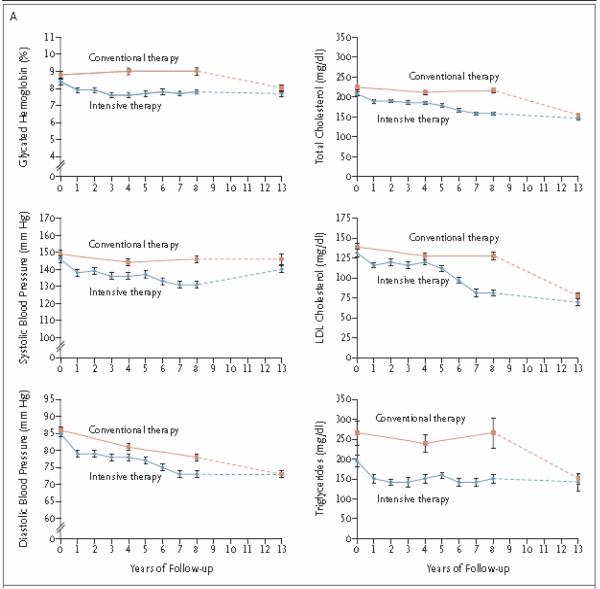
When Steno-2 was completed, all of the participants and their diabetes specialists were informed of the beneficial effects of targeting multiple risk factors, and intensive treatment was stopped in that group9. A follow-up visit was performed 5.5 years after Steno-2 stopped and showed that the end-of-study differences between groups in A1c, total cholesterol, LDL, triglycerides, blood pressure and urinary albumin excretion had narrowed between the 2 groups (Fig. 4 – Figure 2A from Gaede 2008). Surprisingly, only 30% of patients in the intensive-therapy group died compared to 50 % in the conventional-therapy group, an absolute risk reduction of 20% (p=0.02) (Fig. 5 – adapted from Figure 3A, Gaede 2008). This finding was consistent with what was observed in DCCT/EDIC and the UKPDS Follow-up Study of glycemic control. Interestingly, during the Steno-2 trial, A1c increased from a baseline of 8.8% to 9.0% in the conventional-therapy group and improved from 8.4 to 7.9% in the intensive-therapy group, while decreasing to 8.0% and 7.7% respectively during the follow-up period. These achieved levels of A1c are higher than those targeted in ACCORD, ADVANCE and VADT. Results from Steno-2 and the Steno-2 follow-up study suggest that achieving an A1c of 7.7–7.9% was sufficient to reduce cardiovascular endpoints and all-cause mortality in the context of multi-faceted therapy that includes lifestyle changes and targets other cardiovascular risk factors. Furthermore, although good glycemic control has been demonstrated to be an effective way to reduce cardiovascular morbidity, we do not fully understand the increased total mortality observed in the intensive treatment arm of ACCORD, and current methods for achieving tight glycemic control do not reduce cardiovascular events or mortality, whereas we have strong data (albeit from a small cohort) regarding the beneficial effects of a multifaceted, comprehensive approach to improving cardiovascular risk factors on cardiovascular endpoints and mortality.
Figure 4.
Steno-2 follow-up study. During the follow-up period, control of multiple cardiovascular risk factors converged between the intensive- and conventional-treatment groups.
Gaede 2008, Figure 2A
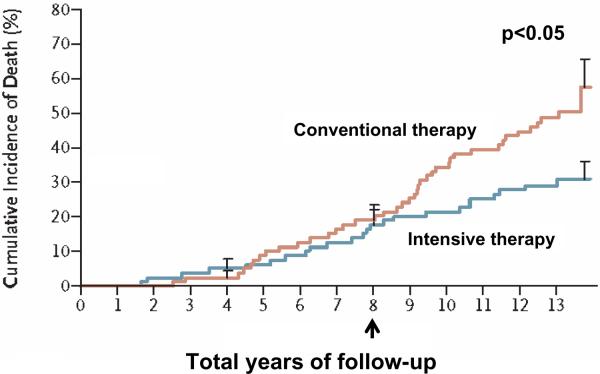
Figure 5.
Long-term effect of intensive control of multiple risk factors in the Steno-2 follow-up study. Despite a narrowing of differences in A1c, total cholesterol, LDL, triglycerides, blood pressure and urinary albumin excretion between treatment groups during the 5.5-year follow-up period, the intensive-therapy group had a markedly decreased cumulative risk of cardiovascular mortality compared with the conventional group, highlighting the importance of targeting multiple cardiovascular risk factors to increase long-term survival among individuals with diabetes. Arrow indicates timepoint for completion of Steno-2 study, and beginning of Steno-2 follow-up study. P<0.05 for comparison of cumulative incidence of death between groups
adapted from Gaede 2008 (Fig 3A)
CONCLUSION
While epidemiological studies have demonstrated associations between glycemic control and the development of CV disease, a number of confounding variables could account for the association between poor glycemic control and CVD such as blood pressure control, dyslipidemia (increased LDL cholesterol, serum triglyceride level, VLDL and IDL and decreased HDL cholesterol levels) and weight gain, among others. As discussed in other manuscripts in this supplement, although sulfonylureas have been traditionally used as first-line pharmacological therapy for type 2 diabetic patients, different classes of hypoglycemic agents, metformin, thiazolidinediones (TZD's), dipeptidyl peptidase-4 (DPP-4) agonists, glucagon-like peptide-1 (GLP-1) agonists, and insulin can have beneficial effects on CV risk factors. Clinical trial evidence supports good glycemic control, LDL-cholesterol-lowering, blood pressure control, ACE-inhibition, anti-platelet therapy, and increased physical activity to decrease the risk of cardiovascular events and mortality in patients with diabetes. Effective multi-faceted approaches targeting cardiovascular risk factors - dyslipidemia, elevated blood pressure, sedentary lifestyle, high saturated- and trans-fat diet, tobacco use - and emphasizing lifestyle changes such as increasing physical activity are urgently needed to improve long-term survival in our patients with diabetes. The cardiologist's role in improving glucose control and global CV risk reduction in patients with type 2 diabetes mellitus is significant. As the population with type 2 diabetes is increasing, diabetes is becoming increasingly relevant to cardiologists due to the accentuated CV risk inherent with type 2 diabetes mellitus. Achieving global risk reduction through glycemic control as well as CV risk reduction strategies will require continued efforts. This is crucial for patients with type 2 diabetes in whom CVD is a major cause of mortality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997 Apr;20(4):537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- (3).Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Collins VR, Hamman RF. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997 Apr;46(4):701–10. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Knowler WC, Narayan KM, Hanson RL, Nelson RG, Bennett PH, Tuomilehto J, Schersten B, Pettitt DJ. Preventing non-insulin-dependent diabetes. Diabetes. 1995 May;44(5):483–8. doi: 10.2337/diab.44.5.483. [DOI] [PubMed] [Google Scholar]
- (5).Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007 Jun 11;167(11):1145–51. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- (6).Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005 Sep;28(9):2130–5. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- (7).Barnett KN, Ogston SA, McMurdo ME, Morris AD, Evans JM. A 12-year follow-up study of all-cause and cardiovascular mortality among 10,532 people newly diagnosed with Type 2 diabetes in Tayside, Scotland. Diabet Med. 2010 Oct;27(10):1124–9. doi: 10.1111/j.1464-5491.2010.03075.x. [DOI] [PubMed] [Google Scholar]
- (8).Li CL, Chang HY, Shyu YI. The excess mortality risk of diabetes associated with functional decline in older adults: results from a 7-year follow-up of a nationwide cohort in Taiwan. BMC Public Health. 2011;11:953. doi: 10.1186/1471-2458-11-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008 Feb 7;358(6):580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- (10).Redmon JB, Bertoni AG, Connelly S, Feeney PA, Glasser SP, Glick H, Greenway F, Hesson LA, Lawlor MS, Montez M, Montgomery B. Effect of the look AHEAD study intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care. 2010 Jun;33(6):1153–8. doi: 10.2337/dc09-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, Zhang Q. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012 Mar 29;366(13):1209–17. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D'Agostino RB., Sr Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care. 2008 Aug;31(8):1582–4. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002 Jul;25(7):1129–34. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]
- (14).Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993 Feb;16(2):434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- (15).Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998 Jul 23;339(4):229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- (16).Kengne AP, Patel A, Marre M, Travert F, Lievre M, Zoungas S, Chalmers J, Colagiuri S, Grobbee DE, Hamet P, Heller S, Neal B, Woodward M. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. Eur J Cardiovasc Prev Rehabil. 2011 Jun;18(3):393–8. doi: 10.1177/1741826710394270. [DOI] [PubMed] [Google Scholar]
- (17).Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008 Feb 7;358(6):580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- (18).Sprafka JM, Burke GL, Folsom AR, McGovern PG, Hahn LP. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care. 1991 Jul;14(7):537–43. doi: 10.2337/diacare.14.7.537. [DOI] [PubMed] [Google Scholar]
- (19).Kuusisto J, Mykkanen L, Pyorala K, Laakso M. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes. 1994 Aug;43(8):960–7. doi: 10.2337/diab.43.8.960. [DOI] [PubMed] [Google Scholar]
- (20).Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001 Sep;24(9):1614–9. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- (21).Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000 Aug 29;102(9):1014–9. doi: 10.1161/01.cir.102.9.1014. [DOI] [PubMed] [Google Scholar]
- (22).Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001 Aug;38(2):421–8. doi: 10.1016/s0735-1097(01)01408-5. [DOI] [PubMed] [Google Scholar]
- (23).Reusch JE, Wang CC. Cardiovascular disease in diabetes: where does glucose fit in? J Clin Endocrinol Metab. 2011 Aug;96(8):2367–76. doi: 10.1210/jc.2010-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Reusch JE, Draznin BB. Atherosclerosis in diabetes and insulin resistance. Diabetes Obes Metab. 2007 Jul;9(4):455–63. doi: 10.1111/j.1463-1326.2006.00620.x. [DOI] [PubMed] [Google Scholar]
- (25).Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004 Nov;53(11):2735–40. doi: 10.2337/diabetes.53.11.2735. [DOI] [PubMed] [Google Scholar]
- (26).Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P, Camici P, Picano E, Cortigiani L, Bevilacqua M, Milazzo L, Cusi D, Barlassina C, Sarzi-Puttini P, Turiel M. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010 Oct;9(12):830–4. doi: 10.1016/j.autrev.2010.07.016. [DOI] [PubMed] [Google Scholar]
- (27).Otsuka A, Azuma K, Iesaki T, Sato F, Hirose T, Shimizu T, Tanaka Y, Daida H, Kawamori R, Watada H. Temporary hyperglycaemia provokes monocyte adhesion to endothelial cells in rat thoracic aorta. Diabetologia. 2005 Dec;48(12):2667–74. doi: 10.1007/s00125-005-0005-6. [DOI] [PubMed] [Google Scholar]
- (28).Pandolfi A, Giaccari A, Cilli C, Alberta MM, Morviducci L, De Filippis EA, Buongiorno A, Pellegrini G, Capani F, Consoli A. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol. 2001;38(2):71–6. doi: 10.1007/s005920170016. [DOI] [PubMed] [Google Scholar]
- (29).Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005 Oct;99(4):1471–6. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]
- (30).Velusamy T, Jain SK. Effects of high glucose and ketosis (acetoacetate, ss-hydroxybutyrate) on PAI-1 secretion in human umbilical vascular endothelial cells. Clin Appl Thromb Hemost. 2011 Jun;17(3):288–92. doi: 10.1177/1076029610366434. [DOI] [PubMed] [Google Scholar]
- (31).Inoguchi T, Yu HY, Imamura M, Kakimoto M, Kuroki T, Maruyama T, Nawata H. Altered gap junction activity in cardiovascular tissues of diabetes. Med Electron Microsc. 2001 Jun;34(2):86–91. doi: 10.1007/s007950170002. [DOI] [PubMed] [Google Scholar]
- (32).Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol. 2008 Jul;295(1):C221–C230. doi: 10.1152/ajpcell.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008 Sep 29;205(10):2409–17. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Meng L, Park J, Cai Q, Lanting L, Reddy MA, Natarajan R. Diabetic conditions promote binding of monocytes to vascular smooth muscle cells and their subsequent differentiation. Am J Physiol Heart Circ Physiol. 2010 Mar;298(3):H736–H745. doi: 10.1152/ajpheart.00935.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Tanaka K, Nagata D, Hirata Y, Tabata Y, Nagai R, Sata M. Augmented angiogenesis in adventitia promotes growth of atherosclerotic plaque in apolipoprotein E-deficient mice. Atherosclerosis. 2011 Apr;215(2):366–73. doi: 10.1016/j.atherosclerosis.2011.01.016. [DOI] [PubMed] [Google Scholar]
- (36).Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004 Dec 21;44(12):2293–300. doi: 10.1016/j.jacc.2004.07.060. [DOI] [PubMed] [Google Scholar]
- (37).Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195(1–2):73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012 Mar;302(5):E481–E495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- (39).Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996 May 22;275(20):1557–62. [PubMed] [Google Scholar]
- (40).de SG, Devereux RB, Chinali M, Lee ET, Galloway JM, Barac A, Panza JA, Howard BV. Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J Hypertens. 2010 Feb;28(2):353–60. doi: 10.1097/HJH.0b013e3283331169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Shah AM, Uno H, Kober L, Velazquez EJ, Maggioni AP, MacDonald MR, Petrie MC, McMurray JJ, Califf RM, Pfeffer MA, Solomon SD. The inter-relationship of diabetes and left ventricular systolic function on outcome after high-risk myocardial infarction. Eur J Heart Fail. 2010 Nov;12(11):1229–37. doi: 10.1093/eurjhf/hfq179. [DOI] [PubMed] [Google Scholar]
- (42).Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003 Jan 28;107(3):448–54. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- (43).Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ, Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004 Apr 21;43(8):1432–8. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- (44).Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000 May 16;101(19):2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- (45).Dhingra R, Vasan RS. Diabetes and the risk of heart failure. Heart Fail Clin. 2012 Jan;8(1):125–33. doi: 10.1016/j.hfc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harmancey R, Taegtmeyer H. The complexities of diabetic cardiomyopathy: lessons from patients and animal models. Curr Diab Rep. 2008 Jun;8(3):243–8. doi: 10.1007/s11892-008-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Burgmaier M, Sen S, Philip F, Wilson CR, Miller CC, III, Young ME, Taegtmeyer H. Metabolic adaptation follows contractile dysfunction in the heart of obese Zucker rats fed a high-fat “Western” diet. Obesity (Silver Spring) 2010 Oct;18(10):1895–901. doi: 10.1038/oby.2009.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010 Feb;95(2):513–21. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009 Oct;94(10):3687–95. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Blacher J, Protogerou AD, Safar ME. Large artery stiffness and antihypertensive agents. Curr Pharm Des. 2005;11(25):3317–26. doi: 10.2174/138161205774424654. [DOI] [PubMed] [Google Scholar]
- (51).The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- (52).UKPDS Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–53. [PubMed] [Google Scholar]
- (53).Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- (55).Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- (57).Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, the VADT, I Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009 Jan 8;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- (58).Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, Feinglos MN, Ismail-Beigi F, Largay JF, O'Connor PJ, Paul T, Savage PJ, Schubart UK, Sood A, Genuth S. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, Bonds DE, Fonseca VA, Ismail-Beigi F, Banerji MA, Failor A, Hamilton B. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010 Apr;33(4):721–7. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC, Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010 May;33(5):983–90. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byington RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011 Mar 3;364(9):818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Genuth S, Ismail-Beigi F. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab. 2012 Jan;97(1):41–8. doi: 10.1210/jc.2011-1679. [DOI] [PubMed] [Google Scholar]
- (64).Terry T, Raravikar K, Chokrungvaranon N, Reaven PD. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep. 2012 Feb;14(1):79–88. doi: 10.1007/s11886-011-0238-6. [DOI] [PubMed] [Google Scholar]
- (65).ADA Standards of medical care in diabetes--2012. Diabetes Care. 2012 Jan;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) Diabetes Care. 1997 Apr;20(4):614–20. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- (67).Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004 Aug 21;364(9435):685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- (68).Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006 Jul;29(7):1478–85. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- (69).Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, Hague W, Beller E, Arulchelvam M, Baker J, Tonkin A. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care. 2003 Oct;26(10):2713–21. doi: 10.2337/diacare.26.10.2713. [DOI] [PubMed] [Google Scholar]
- (70).Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002 Jul 6;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- (71).Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003 Jun 14;361(9374):2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- (72).UKPDS Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998 Sep 12;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- (73).Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, Camel G, Davis BR, Frost PH, Gonzalez N, Guthrie G, Oberman A, Rutan GH, Stamler J. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996 Dec 18;276(23):1886–92. [PubMed] [Google Scholar]
- (74).Barzilay JI, Davis BR, Bettencourt J, Margolis KL, Goff DC, Jr, Black H, Habib G, Ellsworth A, Force RW, Wiegmann T, Ciocon JO, Basile JN. Cardiovascular outcomes using doxazosin vs. chlorthalidone for the treatment of hypertension in older adults with and without glucose disorders: a report from the ALLHAT study. J Clin Hypertens (Greenwich ) 2004 Mar;6(3):116–25. doi: 10.1111/j.1524-6175.2004.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Ostergren J, Poulter NR, Sever PS, Dahlof B, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E. The Anglo-Scandinavian Cardiac Outcomes Trial: blood pressure-lowering limb: effects in patients with type II diabetes. J Hypertens. 2008 Nov;26(11):2103–11. doi: 10.1097/HJH.0b013e328310e0d9. [DOI] [PubMed] [Google Scholar]
- (76).Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010 Apr 29;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Snapinn SM, Wan Y, Lyle PA. Does albuminuria predict cardiovascular outcomes on treatment with losartan versus atenolol in patients with diabetes, hypertension, and left ventricular hypertrophy? The LIFE study. Diabetes Care. 2006 Mar;29(3):595–600. doi: 10.2337/diacare.29.03.06.dc05-1724. [DOI] [PubMed] [Google Scholar]
- (78).Araki S, Haneda M, Koya D, Hidaka H, Sugimoto T, Isono M, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes. 2007 Jun;56(6):1727–30. doi: 10.2337/db06-1646. [DOI] [PubMed] [Google Scholar]
- (79).Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000 Jan 20;342(3):145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- (80).HOPE Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000 Jan 22;355(9200):253–9. [PubMed] [Google Scholar]
- (81).Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005 Apr;28(4):799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- (82).Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003 Oct;24(5):610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- (83).Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010 Sep 27;170(17):1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003 Jan 30;348(5):383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]