Abstract
Diabetes and congestive heart failure (HF) commonly coexist in the same patient, and the presence of diabetes in HF patients is associated with increased adverse events compared to patients without diabetes. Recent guidelines regarding glycemic control stress individualization of glycemic therapy based on patient comorbid conditions and potential adverse effects of medical therapy. This balance in glycemic control may be particularly relevant in patients with diabetes and HF. In this review, we address data regarding the influence that certain HF medications may have on glycemic control. Despite potential modest changes in glycemic control, clinical benefits of proven pharmacologic HF therapies extend to patients with diabetes and HF. In addition, we review potential benefits and challenges associated with commonly used glycemic medications in HF patients. Finally, recent data and controversies on optimal glycemic targets in HF patients are discussed. Given the large number of patients with diabetes and HF and the health burden of these conditions, much needed future work is necessary to define the optimal glycemic treatment in HF patients with diabetes.
Keywords: Diabetes, Heart Failure, Prognosis
Multiple epidemiologic studies have demonstrated that diabetes mellitus amplifies the risk for the development of heart failure (HF).1–3 The mechanisms contributing to the greater rates of HF in individuals with diabetes are likely multifactorial and include the shared and accelerated comorbid conditions of hypertension, coronary artery disease, obesity, renal insufficiency, and aortic stiffness. In addition, insulin resistance and hyperglycemia may contribute directly to cardiac dysfunction through mechanisms related to the direct and indirect effects of advanced glycation endproducts, abnormalities of cardiac metabolism, increased myocardial fibrosis, increased oxidative stress, abnormalities of autophagy, and increased local activation of the renin-angiotensin system.4–6
Increasing evidence also suggest that HF itself may be considered an insulin resistant state with increased risk for the development of diabetes in patients with established HF. In the Candesartan Heart Failure Assessment of Reduction (CHARM) Program7, the incidence of new diabetes in HF patients was approximately 28 cases per 1000 patient years of follow-up, an incidence that is higher than the incidence rates described in unselected US adults age 45–79 years (estimated incidence rates 12.4–13.5 per 1000 patient years).8 The potential reasons for this association between HF and greater rates of incident diabetes have not been well established, but include increased neurohormonal activation in HF patients promoting both skeletal and myocardial insulin resistance.9 More recent animal data have demonstrated that sympathetic activation in HF may also contribute to insulin resistance through upregulation of p53 expression in adipose tissue and associated adipose inflammation and lipolysis.10 Clinical predictors of incident diabetes in HF patients include elevated body mass index (BMI), elevated glucose or hemoglobin A1c (HbA1c), diuretic therapy, digoxin therapy, lower serum creatinine concentration, and more severe NYHA class.7,11–12
Given this interrelationship between diabetes and HF, it is not surprising that these conditions commonly exist in the same individual. In studies of individuals with LV dysfunction, it is estimated that approximately 12–30% of individuals have known diabetes.13 The prevalence may be even greater when more systematic assessment for the presence of diabetes is performed in HF populations. In a cohort of outpatients with systolic HF who underwent systematic oral glucose tolerance testing, 18% of individuals without a prior diagnosis of diabetes were found to have newly diagnosed diabetes.14
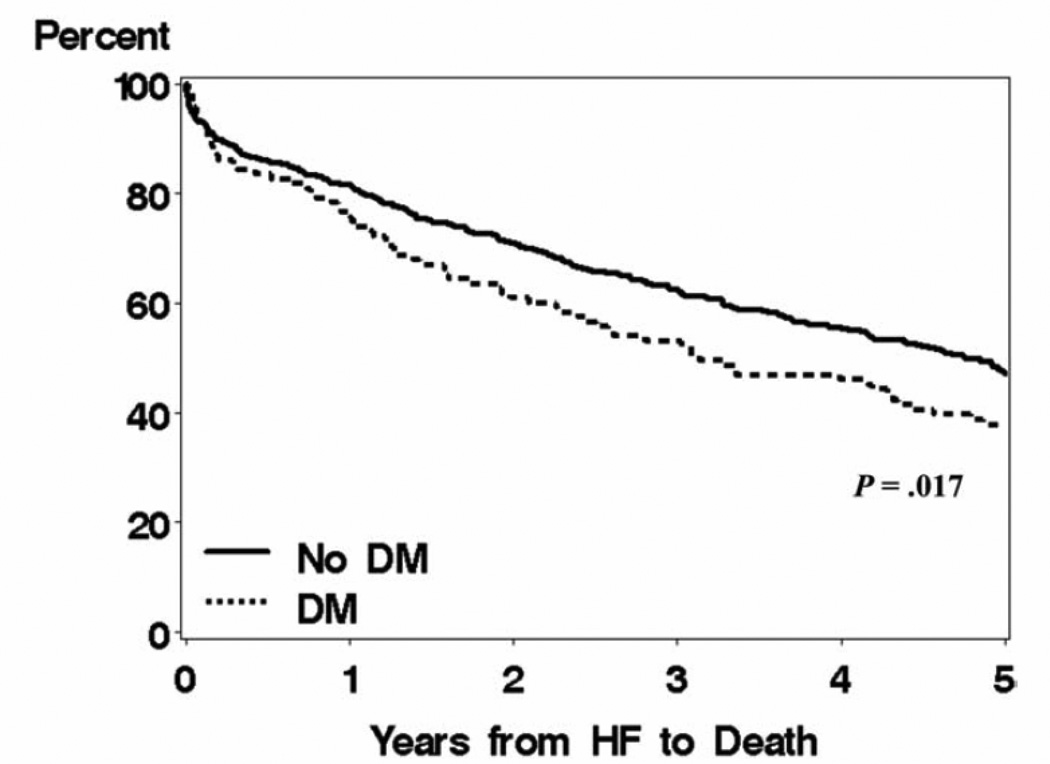
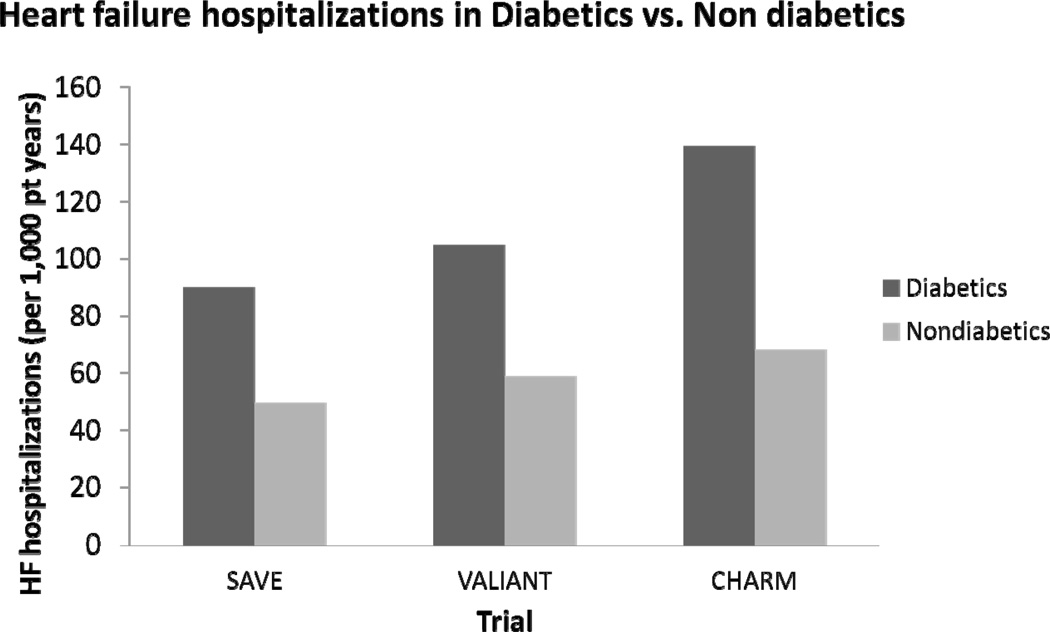
Importantly, the coexistence of diabetes and HF portends a poor prognosis. Both population studies15–16 and clinical trials17–20 have demonstrated that diabetes is associated with increased mortality in HF patients (Figure 1). This diabetes-associated increased risk of death persists after adjustment of clinically recognized potential confounders. Similarly, recurrent HF hospitalizations are markedly increased in individuals with diabetes (Figure 2). For example, following myocardial infarction complicated by HF, the rates of readmission for HF hospitalization in diabetic patients are nearly twice the rates than in those patients without diabetes.17,19,21 In the CHARM program of chronic HF, rates of HF hospitalization in patients with diabetes were also approximately twice the rates of those without diabetes.20 In CHARM, this increased rate of HF hospitalization was particularly elevated in those patients with preserved LVEF.20 Thus, the presence of diabetes in a HF patient is a marker of significantly increased morbidity and mortality.
Figure 1. Heart failure survival with and without diabetes.
Data from Olmstead county demonstrating survival in heart failure patients with and without diabetes after a mean follow-up of 5.0 ± 4.6 years. Reprinted with permission from Am J Med.15
Figure 2. Heart failure hospitalizations in HF patients with and without diabetes.
Rate of heart failure hospitalizations per 1000 patient years. SAVE= Survival and Ventricular Enlargement.19 VALIANT= Valsartan in Acute Myocardial Infarction Trial.21 CHARM= Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity. SOLVDTX = Studies of Left Ventricular Dysfunction Treatment arm.20
Given the health burden of diabetes in HF patients, it is important to understand the balance that exists between the treatments of both conditions. Specifically, pharmacologic therapies that are used to treat HF may affect glycemic levels and the future risk of diabetes. Nonetheless, despite potential modest glycemic effects of HF pharmacotherapy, clinical benefits of these agents on mortality and HF hospitalizations are preserved in patients with diabetes and HF. Similarly, it is important to understand the potential role and challenges in commonly used glycemic therapies that may be particularly relevant in HF patients. Finally, controversies and data regarding optimal glycemic targets in HF will be reviewed.
Relationship between HF therapy and Glycemic Control
Beta-Blockers
Despite initial concerns that beta-blockers may worsen glycemic control and mask symptoms of hypoglycemia, multiple studies have demonstrated that beta-blockers are associated with improved survival in individuals with diabetes and HF.22–23 In a meta-analysis of randomized controlled trials, beta-blocker therapy compared with placebo was associated with a 16% reduction in mortality (RR 0.84, 95% CI 0.73–0.96).22 In addition, beta-blockers have been shown to reduce HF hospitalization in patients with diabetes and HF to a similar extent as those patients without diabetes.24–25
Some studies have demonstrates that beta-blockers with potential vasodilator properties (non-selective beta-blockers) may be associated with improved measures of glycemia relative to beta-blockers without these properties. In a study of 1235 patients with DM and hypertension (without HF), carvedilol was associated with modest improvements of glycemia compared to metoprolol tartrate.26 In a retrospective analysis of the Carvedilol Or Metoprolol European Trial (COMET), the use of carvedilol in HF patients was associated with a reduced incidence of new-onset diabetes when compared with metoprolol tartrate.11 Whether these modest benefits in relative glycemia or reduction in new diabetes with specific beta-blockers are associated with improved outcomes has not been determined.
ACEI/ARB
Randomized clinical trials have clearly established improved survival with the use of angiotensin-converting enzyme inhibitors (ACEI) in diabetic patients with HF.23 Similarly, in the low ejection fraction arms of the CHARM program, the benefit of candesartan, an angiotensin-receptor blocker (ARB), on cardiovascular death or HF hospitalization compared to placebo was similar in patients with and without diabetes.27 In addition to beneficial effects on clinical outcomes, some studies have demonstrated associations between ACEI or ARBs and reduced incidence of new diabetes in HF patients. Data from the Studies of Left Ventricular Dysfunction (SOLVD)28 and CHARM Program29 have demonstrated reduced incidence of new-diabetes in patients treated with enalapril and candesartan, respectively, when compared with placebo. The mechanisms for the reduced incidence of diabetes in patients with ACEI or ARBs is not fully understood, but include improved insulin sensitivity by increase of peripheral blood flow to skeletal muscle through suppression of angiotensin II or by elevating bradykinin levels.30–31 Other non-HF studies have also demonstrated potential reductions in the incidence of diabetes with ACEI or ARB, but the beneficial findings have not been consistent.32–33
Aldosterone Antagonists
Similar to patients without diabetes and HF, aldosterone antagonists are associated with improved outcomes in patients with diabetes and HF.34–36 The reported effects of aldosterone antagonists on glycemic control are mixed. Some studies have demonstrated modest increases in HbA1c in patients with type 2 diabetes and nephropathy or hypertension treated with spironolactone,37–38 while other studies have shown that spironolactone may improve insulin resistance in patients with non-alcoholic fatty liver disease.39 Limited data exist for the glycemic effects of eplerenone; in one study of patients with mild HF, eplerenone was not associated with changes in HbA1c.40 Given the clinical efficacy demonstrated with aldosterone antagonists in reducing adverse outcomes in diabetic patients with HF, the clinical meaning of potential modest changes in glycemia with these agents is uncertain.
Relationship between Hyperglycemic Therapies and Outcomes in HF Patients
Recent guidelines for the treatment of hyperglycemia in patients with type 2 diabetes stress the importance of individualization of therapy based on patient needs, comorbid conditions, and potential adverse effects of hyperglycemic medications.41 The diabetic patient with HF presents particular challenges with the pharmacologic treatment of diabetes, and it is important to understand the potential benefits and risks of commonly used medications that may be particularly relevant in HF patients.
Metformin
Metformin is currently recommended as first-line therapy in patients with type 2 diabetes in the absence of contraindications.41–42 Metformin was previously contraindicated in individuals with HF due to potential concerns of lactic acidosis. More recent observational studies have demonstrated that metformin may not only be safe, but may be associated with improved survival in patients with diabetes and HF.43–47 In further support of a potential benefit, recent animal studies in HF models have demonstrated cardioprotective effects of metformin therapy on progression of HF via activation of AMP-activated protein kinase pathways,4,48–49 inhibition of cardiac fibrosis,49–50 and modulation of cardiomyocyte autophagy.4 In this setting, the contraindication to metformin use in HF patients was recently removed.51 Metformin remains contraindicated in patients with advanced renal insufficiency and should be used cautiously (or avoided) in patients at risk for worsening renal dysfunction (such as acute decompensated HF). Further prospective human studies are necessary to assess the potential benefits of metformin in patients with diabetes and HF.
Sulfonylurea
Sulfonylureas are frequently utilized in diabetic patients with HF. In a study of more than 16,000 Medicare recipients who had been discharged with a diagnosis of HF, approximately half of the patients were treated with sulfonylureas.46 In that observational study, sulfonylurea was not associated with increased mortality (hazard ratio [HR] 0.99; 95 CI 0.91–1.08). Other observational studies have suggested improved survival with metformin when compared with sulfonylurea.44,47 Sulfonylureas are associated with hypoglycemia and weight gain, and these potential effects need to be monitored in patients with diabetes and HF.
Thiazolidinediones
Thiazolidinediones (TZDs), agonists for the nuclear transcription factor peroxisome receptor γ system, are insulin-sensitizing agents that have been used for the treatment of type 2 diabetes. TZDs have been associated with fluid retention and increased rates of HF in randomized controlled trials.52–55 Although the exact mechanisms of the increased HF events with TZDs are not known, the predominant proposed mechanism relates to TZD-associated volume expansion due to increased renal sodium reabsorption56 rather than a direct effect on myocardial structure and function.57 Nonetheless, prospective randomized controlled studies in patients with established HF have demonstrated increased rates of edema, need for increased HF medications, and increased HF hospitalization in patients treated TZDs compared with placebo or sulfonylurea.58–59 Therefore, caution is urged for the use of TZDs in all patients with signs and symptoms suggestive of HF. TZDs are contraindicated in patients with New York Heart Association (NYHA) class III/IV HF.52
Incretin Based Therapies
Incretin peptides are a group of gastrointestinal proteins that regulate glucose metabolism through multiple mechanisms, and incretin-based therapies have been developed to treat type 2 diabetes.60 These agents include glucagon-like peptide-1 (GLP-1) agonists (exenatide and liraglutide) and dipeptidyl peptidase-IV (DPP-IV) inhibitors (sitagliptin, saxagliptin, and linagliptin). The side effect profile of these agents (less risk of hypoglycemia when used as monotherapy and potential modest weight loss) represent potential benefits in patients with HF, but neither GLP-1 agonists nor DPP-IV inhibitors have been studied extensively in HF patients.
Preclinical work has demonstrated a potential role for GLP-1 based therapies in HF. In a canine model of rapid pacing-induced HF, a 48-hour continuous GLP-1 infusion was associated with improvements in myocardial glucose uptake and improvements in LV and systemic hemodynamics in conscious dogs with dilated cardiomyopathy.61–62 Other rat models of HF, such the spontaneously hypertensive HF model63 or post-MI HF,64 have also demonstrated potential benefits of GLP-1 based therapies on left ventricular (LV) structure and function.
Human studies of GLP-1 based therapies in HF patients have been very limited and have demonstrated mixed results. In a “proof of concept”, open label study of 10 survivors of acute MI complicated by severe LV dysfunction, a 72-hour continuous infusion of GLP-1 was associated with significant improvements in LV ejection fraction.65 Similarly, in a non-randomized study of 12 patients with NYHA class III/IV HF, a continuous subcutaneous infusion of GLP-1 for 12 weeks improved LV ejection fraction, VO2 max, and 6 minute walk compared to 9 HF patients on standard therapy.66 Another randomized, double-blind cross-over design study of 20 non-diabetic patients with NYHA II-III systolic HF failed to show improvement in non-invasive measures of cardiac function with a 48-hour continuous infusion of GLP-1.67 Finally, in a more recent study of 20 diabetic patients with NYHA III/IV HF with an LV ejection fraction of ≤ 35%, a 6-hour infusion of intravenous exenatide was associated with increased cardiac index, predominantly the result of increased heart rate with exenatide.68 Clearly, further studies are required to assess the safety and efficacy of these agents in patients with diabetes and HF.
Insulin
Many patients with diabetes and HF require insulin either as monotherapy or in combination with other glycemic agents in order to achieve adequate blood glucose control. The evidence regarding the effect of insulin on mortality in HF is conflicting, and no large, randomized controlled trials have been performed examining the effects of insulin on clinical outcomes. For example, diabetic patients with HF enrolled in CHARM who were treated with insulin had a greater risk of death than those who were not treated with insulin.18 It is likely that insulin use is a maker for longer duration and severity of diabetes rather than a direct contributor to adverse outcomes. In a cohort of 16,000 Medicare patients with diabetes recently discharged with HF, insulin was not an independent predictor of mortality.46
Glycemic Targets in HF Patients
Another important issue that remains unresolved is the optimal glycemic targets in patients with diabetes and HF. Prospective, randomized controlled trials addressing the optimal glycemic targets specifically in HF patients with diabetes have not been performed. Recent randomized controlled clinical trials which have compared strategies targeting more intensive glucose control (HbA1c <6–6.5%) compared with standard care in patients with established type 2 diabetes and either cardiovascular (CV) disease or high-risk for CV disease have failed to demonstrate significant improvements in the reduction of major CV events with more intensive glycemic control.69–71 In the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, a strategy of intensive glycemic control targeting a HbA1c level of < 6% was associated with an unexpected increased mortality compared to standard therapy (targeting a HbA1c level from 7.0 to 7.9%).70 The reason for the increased mortality in ACCORD remains unexplained, and increased mortality with strategies of more intensive glycemic control was not observed in either the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial71 or the VADT (Veterans Affairs Diabetes Trial) trial.69
It is important to note that few diabetic patients with HF were enrolled in these studies which targeted more intensive glycemic control. Individuals with NYHA class III–IV HF were excluded in VADT 72, and the subset of individuals who may have had more mild HF were not reported in the main study results.69 Similarly, HF as a baseline characteristic was not reported in the main results of the ADVANCE trial.71 Approximately 5% (n=494) of individuals enrolled in ACCORD had a previous diagnosis of HF.70,73 In ACCORD, those diabetic individuals with previous HF had a similar increased mortality associated with a strategy of intensive glycemic control as those individuals without prior HF (interaction p-value =0.71).
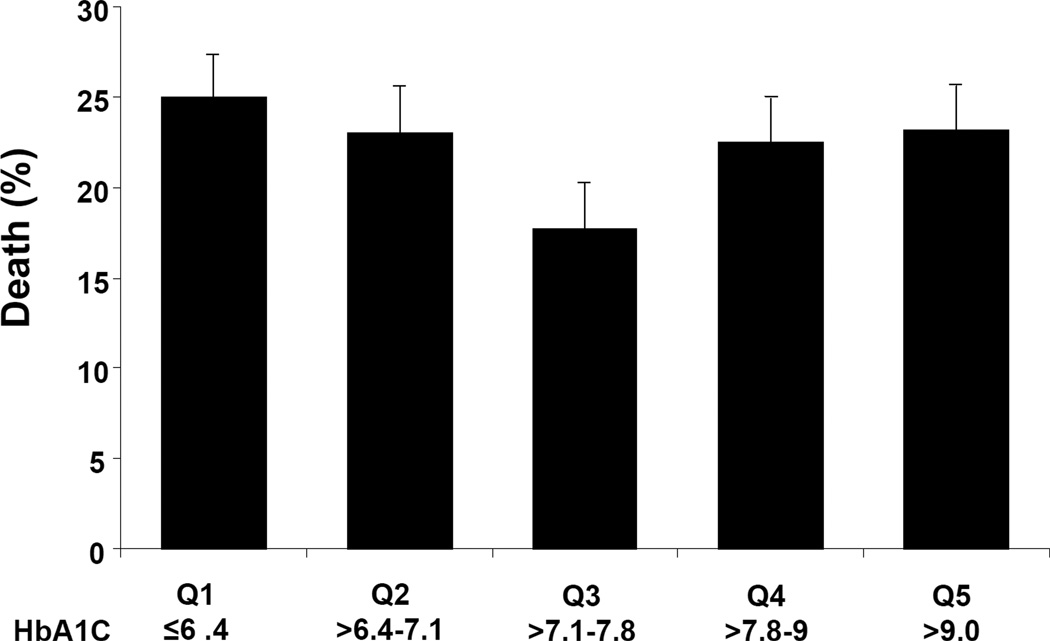
In the absence of prospective randomized controlled trials, several observational studies have assessed the relationship between HbA1c levels and outcomes in patients with established HF.74–78 In contrast to epidemiologic evidence that has demonstrated a near linear relationship between elevated HbA1c levels and worse CV outcomes in people free of CV disease or HF at baseline,79–80 the relationship between HbA1c and outcomes appears more complex in patients with diabetes and established HF with some studies demonstrating a potential “U-shaped” or an inverse relationship between HbA1c and mortality.74–75,78 In a study of 5815 ambulatory HF patients receiving medical treatment for diabetes, individuals with modest glycemic control (HbA1c >7.1–7.8%) had lower mortality compared with HbA1c levels that were either higher or lower (Figure 3).74 In smaller cohorts of diabetic individuals with more advanced systolic HF treated at a single academic medical center, higher HbA1c levels were paradoxically associated with improved outcomes in patients with diabetes.75,78 It is important to note, that as observational studies, significant differences exist in the baseline characteristics beyond levels of glycemia in these cohorts. Multivariable models have been used to adjust for differences in baseline characteristics, but residual confounding may persist. Also, it is possible that lower HbA1c levels may be a marker of more severe disease or cardiac cachexia.
Figure 3. Proportion of Patients Who Died at 2-year follow-up by quintiles of HbA1c.
Proportion of patients who died at 2-year follow-up by quintiles (Q) of glycosylated hemoglobin (HBA1c). Global chi-square p=0.001. Error bars indicate the 95% confidence intervals. Reprinted permission from J Am Coll Cardiol.74
In addition, not all studies have demonstrated a paradoxical relationship between HbA1c and increased mortality in HF patients; in 2,412 participants (of which 907 participants had known diabetes) enrolled in CHARM study, increasing levels of HbA1c was associated with increased risk of total mortality, HF hospitalization, and a composite outcome of CV death or HF hospitalization.76 Of note, the graded relationship between HbA1c and mortality was more pronounced in the nondiabetic patients enrolled CHARM and did not reach statistical significance for the outcomes of CV death (p for heterogeneity= 0.04) and total mortality in the cohort of HF patient with diabetes (p for heterogeneity= 0.008). Other studies have also suggested that elevated HbA1c levels may be a more important prognostic marker of increased risk in HF patients without previously diagnosed diabetes.77
Current guidelines recommend an HbA1c goal of 7% as a general goal for most adults but allow for individualization based on patient characteristics and comorbid conditions (Table 1).42 Perhaps particularly relevant to patients with advanced HF, the American Diabetes Association recommends that less stringent goals (HbA1c<8%) may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, extensive comorbid conditions, and advanced macrovascular/microvascular disease.42 Observational data from patients with diabetes and HF would suggest that these goals may be appropriate, but more prospective data is needed to help define the optimal targets in HF patients.
Table 1.
Glycemic (HbA1c) goals in adults. Adapted from Standards of Medical Care in Diabetes-201242
| American Diabetes Association guidelines on glycemic control | |
|---|---|
| Goal of A1C <7.0% is reasonable for most non-pregnant adults based on potential microvascular benefits and potential macrovascular benefits if implemented soon after the diagnosis of diabetes. | |
| Stringent A1C goals (such as < 6.5%) | In patients with short duration of diabetes, long life expectance, and no significant CVD, and if goals can be achieved without significant hypoglycemia. |
| Less Stringent A1C goals (such as < 8%) | In patients with history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications. |
Conclusion
The development of HF is a common complication in patients with diabetes, and the co-existence of diabetes and HF portends increased morbidity and mortality. It is important to understand the balance that exists in the pharmacologic treatment for both conditions. Current guidelines recommend individualization of hyperglycemic therapy for treatment of type 2 diabetes based on patient needs, comorbid conditions, and potential adverse effects of medications.41 This recommendation may be particular important in patients with diabetes and HF. Much needed work is needed to further clarify the optimal treatment of glycemia in patients with diabetes and HF.
Acknowledgments
We thank Novo Nordisk for their funding development for the supplement, and we acknowledge the assistance of Ruth Kleinpell, PhD, RN, and Mary Lou Briglio in the preparation of this supplement.
Disclosure: Dr. Aguilar has received consulting fees from Amylin and Sanofi-Aventis. Dr. Aguilar is a research investigator in the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial and Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL) trial and is supported by an NHLBI Mentored Career Development award (5K01-HL092585-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 2.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42:1446–1453. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 4.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murarka S, Movahed MR. Diabetic cardiomyopathy. J Card Fail. 2010;16:971–979. doi: 10.1016/j.cardfail.2010.07.249. [DOI] [PubMed] [Google Scholar]
- 6.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Preiss D, Zetterstrand S, McMurray JJ, Ostergren J, Michelson EL, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Gerstein HC, Sattar N. Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes Care. 2009;32:915–920. doi: 10.2337/dc08-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevention. CfDCa. Centers for Disease Control and Prevention. Diabetes Data and Trends [Google Scholar]
- 9.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R, Komuro I, Kobayashi Y, Minamino T. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 2012;15:51–64. doi: 10.1016/j.cmet.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Torp-Pedersen C, Metra M, Charlesworth A, Spark P, Lukas MA, Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Remme WJ, Scherhag A. Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: data from the Carvedilol Or Metoprolol European Trial (COMET) Heart. 2007;93:968–973. doi: 10.1136/hrt.2006.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenenbaum A, Motro M, Fisman EZ, Leor J, Freimark D, Boyko V, Mandelzweig L, Adler Y, Sherer Y, Behar S. Functional class in patients with heart failure is associated with the development of diabetes. Am J Med. 2003;114:271–275. doi: 10.1016/s0002-9343(02)01530-9. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald MR, Petrie MC, Hawkins NM, Petrie JR, Fisher M, McKelvie R, Aguilar D, Krum H, McMurray JJ. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J. 2008;29:1224–1240. doi: 10.1093/eurheartj/ehn156. [DOI] [PubMed] [Google Scholar]
- 14.Egstrup M, Schou M, Gustafsson I, Kistorp CN, Hildebrandt PR, Tuxen CD. Oral glucose tolerance testing in an outpatient heart failure clinic reveals a high proportion of undiagnosed diabetic patients with an adverse prognosis. Eur J Heart Fail. 2011;13:319–326. doi: 10.1093/eurjhf/hfq216. [DOI] [PubMed] [Google Scholar]
- 15.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, Grobbee DE. The prognosis of heart failure in the general population: The Rotterdam Study. Eur Heart J. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar D, Solomon SD, Kober L, Rouleau JL, Skali H, McMurray JJ, Francis GS, Henis M, O'Connor CM, Diaz R, Belenkov YN, Varshavsky S, Leimberger JD, Velazquez EJ, Califf RM, Pfeffer MA. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation. 2004;110:1572–1578. doi: 10.1161/01.CIR.0000142047.28024.F2. [DOI] [PubMed] [Google Scholar]
- 18.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 19.Murcia AM, Hennekens CH, Lamas GA, Jimenez-Navarro M, Rouleau JL, Flaker GC, Goldman S, Skali H, Braunwald E, Pfeffer MA. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med. 2004;164:2273–2279. doi: 10.1001/archinte.164.20.2273. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 21.Shah AM, Uno H, Kober L, Velazquez EJ, Maggioni AP, MacDonald MR, Petrie MC, McMurray JJ, Califf RM, Pfeffer MA, Solomon SD. The inter-relationship of diabetes and left ventricular systolic function on outcome after high-risk myocardial infarction. Eur J Heart Fail. 2010;12:1229–1237. doi: 10.1093/eurjhf/hfq179. [DOI] [PubMed] [Google Scholar]
- 22.Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J. 2003;146:848–853. doi: 10.1016/S0002-8703(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 23.Shekelle PG, Rich MW, Morton SC, Atkinson CS, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Warner Stevenson L. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–1538. doi: 10.1016/s0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 24.Deedwania PC, Giles TD, Klibaner M, Ghali JK, Herlitz J, Hildebrandt P, Kjekshus J, Spinar J, Vitovec J, Stanbrook H, Wikstrand J. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT-HF. Am Heart J. 2005;149:159–167. doi: 10.1016/j.ahj.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 25.Domanski M, Krause-Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, Haffner S, Katz R, Lindenfeld J, Lowes BD, Martin W, McGrew F, Bristow MR. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol. 2003;42:914–922. doi: 10.1016/s0735-1097(03)00856-8. [DOI] [PubMed] [Google Scholar]
- 26.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT, Jr, Oakes R, Lukas MA, Anderson KM, Bell DS. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 27.Young JB, Dunlap ME, Pfeffer MA, Probstfield JL, Cohen-Solal A, Dietz R, Granger CB, Hradec J, Kuch J, McKelvie RS, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Held P, Solomon SD, Yusuf S, Swedberg K. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110:2618–2626. doi: 10.1161/01.CIR.0000146819.43235.A9. [DOI] [PubMed] [Google Scholar]
- 28.Vermes E, Ducharme A, Bourassa MG, Lessard M, White M, Tardif JC. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) Circulation. 2003;107:1291–1296. doi: 10.1161/01.cir.0000054611.89228.92. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Ostergren JB, Gerstein HC, Pfeffer MA, Swedberg K, Granger CB, Olofsson B, Probstfield J, McMurray JV. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation. 2005;112:48–53. doi: 10.1161/CIRCULATIONAHA.104.528166. [DOI] [PubMed] [Google Scholar]
- 30.Kaihara M, Nakamura Y, Makino H. [Effect of angiotensin II receptor blocker on insulin signaling in skeletal muscle cells] Nihon rinsho Japanese journal of clinical medicine. 2002;60:1945–1948. [PubMed] [Google Scholar]
- 31.Aguilar D, Solomon SD. ACE inhibitors and angiotensin receptor antagonists and the incidence of new-onset diabetes mellitus: an emerging theme. Drugs. 2006;66:1169–1177. doi: 10.2165/00003495-200666090-00001. [DOI] [PubMed] [Google Scholar]
- 32.Al-Mallah M, Khawaja O, Sinno M, Alzohaili O, Samra AB. Do angiotensin converting enzyme inhibitors or angiotensin receptor blockers prevent diabetes mellitus? A meta-analysis. Cardiol J. 2010;17:448–456. [PubMed] [Google Scholar]
- 33.Geng DF, Jin DM, Wu W, Xu Y, Wang JF. Angiotensin receptor blockers for prevention of new-onset type 2 diabetes: a meta-analysis of 59,862 patients. Int J Cardiol. 2012;155:236–242. doi: 10.1016/j.ijcard.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez HM, Leipzig RM. Spironolactone in patients with heart failure. N Engl J Med. 2000;342:132. doi: 10.1056/NEJM200001133420213. author reply 133–134. [DOI] [PubMed] [Google Scholar]
- 35.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 36.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 37.Matsumoto S, Takebayashi K, Aso Y. The effect of spironolactone on circulating adipocytokines in patients with type 2 diabetes mellitus complicated by diabetic nephropathy. Metabolism. 2006;55:1645–1652. doi: 10.1016/j.metabol.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Swaminathan K, Davies J, George J, Rajendra NS, Morris AD, Struthers AD. Spironolactone for poorly controlled hypertension in type 2 diabetes: conflicting effects on blood pressure, endothelial function, glycaemic control and hormonal profiles. Diabetologia. 2008;51:762–768. doi: 10.1007/s00125-008-0972-5. [DOI] [PubMed] [Google Scholar]
- 39.Polyzos SA, Kountouras J, Zafeiriadou E, Patsiaoura K, Katsiki E, Deretzi G, Zavos C, Tsarouchas G, Rakitzi P, Slavakis A. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. J Renin Angiotensin Aldosterone Syst. 2011;12:498–503. doi: 10.1177/1470320311402110. [DOI] [PubMed] [Google Scholar]
- 40.Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A(c) levels in patients with chronic heart failure. Am Heart J. 2010;160:915–921. doi: 10.1016/j.ahj.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 41.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach: Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012 doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 42.Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin Use and Mortality in Ambulatory Patients with Diabetes and Heart Failure. Circ Heart Fail. 2010 doi: 10.1161/CIRCHEARTFAILURE.110.952556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–2351. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 45.MacDonald MR, Eurich DT, Majumdar SR, Lewsey JD, Bhagra S, Jhund PS, Petrie MC, McMurray JJ, Petrie JR, McAlister FA. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. General Practice Research Database. Diabetes Care. 2010;33:1213–1218. doi: 10.2337/dc09-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 47.Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jorgensen CH, Lange T, Abildstrom SZ, Schramm TK, Vaag A, Kober L, Torp-Pedersen C, Gislason GH. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia. 2010;53:2546–2553. doi: 10.1007/s00125-010-1906-6. [DOI] [PubMed] [Google Scholar]
- 48.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 50.Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, Shen Q, Zhu Y, Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res. 2010;87:504–513. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 51.Inzucchi SE, Masoudi FA, McGuire DK. Metformin in heart failure. Diabetes Care. 2007;30:e129. doi: 10.2337/dc07-1686. [DOI] [PubMed] [Google Scholar]
- 52.Kaul S, Bolger AF, Herrington D, Giugliano RP, Eckel RH. Thiazolidinedione drugs and cardiovascular risks: a science advisory from the American Heart Association and American College Of Cardiology Foundation. J Am Coll Cardiol. 2010;55:1885–1894. doi: 10.1016/j.jacc.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 54.Erdmann E, Charbonnel B, Wilcox RG, Skene AM, Massi-Benedetti M, Yates J, Tan M, Spanheimer R, Standl E, Dormandy JA. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08) Diabetes Care. 2007;30:2773–2778. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 55.Komajda M, McMurray JJ, Beck-Nielsen H, Gomis R, Hanefeld M, Pocock SJ, Curtis PS, Jones NP, Home PD. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31:824–831. doi: 10.1093/eurheartj/ehp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 57.Narang N, Armstead SI, Stream A, Abdullah SM, See R, Snell PG, McGavock J, Ayers CR, Gore MO, Khera A, de Lemos JA, McGuire DK. Assessment of cardiac structure and function in patients without and with peripheral oedema during rosiglitazone treatment. Diab Vasc Dis Res. 2011;8:101–108. doi: 10.1177/1479164111403334. [DOI] [PubMed] [Google Scholar]
- 58.Dargie HJ, Hildebrandt PR, Riegger GA, McMurray JJ, McMorn SO, Roberts JN, Zambanini A, Wilding JP. A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure. J Am Coll Cardiol. 2007;49:1696–1704. doi: 10.1016/j.jacc.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 59.Giles TD, Miller AB, Elkayam U, Bhattacharya M, Perez A. Pioglitazone and heart failure: results from a controlled study in patients with type 2 diabetes mellitus and systolic dysfunction. J Card Fail. 2008;14:445–452. doi: 10.1016/j.cardfail.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Addison D, Aguilar D. Diabetes and cardiovascular disease: the potential benefit of incretin-based therapies. Curr Atheroscler Rep. 2011;13:115–122. doi: 10.1007/s11883-010-0153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 62.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q, Anderson C, Broyde A, Polizzi C, Fernandez R, Baron A, Parkes DG. Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol. 2010;9:76. doi: 10.1186/1475-2840-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 66.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 67.Halbirk M, Norrelund H, Moller N, Holst JJ, Schmitz O, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, Botker HE, Wiggers H. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1096–H1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 68.Nathanson D, Ullman B, Lofstrom U, Hedman A, Frick M, Sjoholm A, Nystrom T. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia. 2012;55:926–935. doi: 10.1007/s00125-011-2440-x. [DOI] [PubMed] [Google Scholar]
- 69.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 70.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 72.Abraira C, Duckworth W, McCarren M, Emanuele N, Arca D, Reda D, Henderson W. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17:314–322. doi: 10.1016/s1056-8727(02)00277-5. [DOI] [PubMed] [Google Scholar]
- 73.Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, Bonds DE, Fonseca VA, Ismail-Beigi F, Banerji MA, Failor A, Hamilton B. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:721–727. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. 2009;54:422–428. doi: 10.1016/j.jacc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J. 2006;151:91. doi: 10.1016/j.ahj.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, Olofsson B, Pfeffer MA, Yusuf S. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168:1699–1704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 77.Goode KM, John J, Rigby AS, Kilpatrick ES, Atkin SL, Bragadeesh T, Clark AL, Cleland JG. Elevated glycated haemoglobin is a strong predictor of mortality in patients with left ventricular systolic dysfunction who are not receiving treatment for diabetes mellitus. Heart. 2009;95:917–923. doi: 10.1136/hrt.2008.156646. [DOI] [PubMed] [Google Scholar]
- 78.Tomova GS, Nimbal V, Horwich TB. Relation Between Hemoglobin A(1c) and Outcomes in Heart Failure Patients With and Without Diabetes Mellitus. Am J Cardiol. 2012 doi: 10.1016/j.amjcard.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, Selby JV. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 80.Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med. 2005;165:1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]