Abstract
Rationale
Stimuli that are paired with opioid withdrawal can themselves produce effects similar to withdrawal that might promote relapse.
Objective
This study compared precipitated and conditioned withdrawal, and tested whether withdrawal is modified by clonidine or morphine.
Methods
Morphine-treated rats (10 mg/kg/12 h) received naloxone (3.2 mg/kg) in a novel environment (conditioned stimuli=CS). Other rats received naloxone in the absence of the CS. Body weight and observable signs were used to measure withdrawal.
Results
Naloxone produced weight loss and withdrawal signs in morphine-treated rats. Following pairings of the CS and naloxone, the CS alone had effects similar to naloxone; conditioned withdrawal was greater after three naloxone/CS pairings, as compared to one, and with longer morphine treatment. Antagonist-precipitated withdrawal was greater in rats that previously were physically dependent on morphine, as compared to withdrawal in rats that were never dependent; however, conditioned withdrawal did not differ between groups. When administered concurrently with naloxone, clonidine (0.1 mg/kg) attenuated some precipitated withdrawal signs, although conditioned withdrawal was largely unchanged. Administration of 10 mg/kg of morphine before the CS alone attenuated all conditioned withdrawal signs, whereas 0.1 mg/kg of clonidine before the CS alone reduced some directly observable signs and not weight loss.
Conclusions
Conditioned withdrawal occurs rapidly and is greater with longer periods of morphine treatment or more pairings of naloxone and the CS; however, a history of physical dependence does not increase conditioned withdrawal. Modification of conditioned withdrawal by drugs might be a useful approach for treating relapse.
Keywords: morphine, conditioned withdrawal, naloxone, clonidine, rats
Opioid abuse remains a serious public health problem. From 1995 to 2005, admission rates for treatment of primary abuse of opioids in the United States increased (Treatment Episode Data Set 2006; http://www.oas.samhsa.gov). Once patients leave treatment, 60% use heroin again within 90 days (Gossop et al. 2002), possibly to avoid or alleviate withdrawal (Solomon and Corbit 1974; Koob et al. 1989). Anecdotal accounts from opioid abusers suggest that sights, sounds and smells previously associated with drug withdrawal can elicit withdrawal-like responses for months after discontinuation of opioids (Wikler 1948; O’Brien et al. 1976; McLellan et al. 1986). These responses can be classically conditioned in opioid-dependent animals; after repeated pairings of a neutral stimulus with antagonist-precipitated or spontaneous withdrawal, the stimulus presented alone can elicit withdrawal signs (Irwin and Seevers 1956; Goldberg and Schuster 1967; Wikler and Pescor 1967; O’Brien et al 1976). For example, morphine-dependent rats exhibit wet-dog shakes when placed in an environment where they previously experienced spontaneous withdrawal (Wikler and Pescor 1967). After lights or tones are paired with an opioid antagonist in morphine-treated rats, presentation of those stimuli alone disrupts food-maintained responding and produces ptosis and diarrhea -- signs of withdrawal (Schnur 1992; McNally and Akil 2001; Amitai et al. 2006). Conditioned withdrawal can persist for long periods. Conditioned effects are evident in monkeys for up to 4 months after morphine discontinuation (Goldberg and Schuster 1967) and formerly opioid-dependent individuals report withdrawal-like signs months after the last drug administration (McLellan et al. 1986). Because these conditioned effects last longer than the withdrawal syndrome itself, they might contribute to relapse long after drug use is terminated.
In the current study, withdrawal was precipitated by naloxone in morphine-treated rats and changes in body weight and directly observable withdrawal signs were assessed; naloxone was paired with peppermint odor and a novel, wire-mesh floor, and when these conditioned stimuli (CS) were later presented alone, effects similar to those produced by naloxone were observed. Withdrawal can be conditioned to the discontinuation of treatment (Parker et al. 1973). The current study used antagonist-precipitated withdrawal for conditioning because more robust withdrawal signs were observed following naloxone administration than following discontinuation of treatment and because acute withdrawal occurs reliably only when it is precipitated by an antagonist. Thus, withdrawal that is conditioned by acute or repeated intermittent injections of morphine can be compared to withdrawal that is conditioned by chronic, daily morphine treatment.
The current study extends previous results (Goldberg and Schuster 1967; Azar et al, 2003; Amitai et al. 2006) on conditioned withdrawal by assessing changes in body weight and directly observable withdrawal signs; many previous studies examined other withdrawal signs, including suppression of operant responding or place aversion conditioning (Goldberg and Schuster 1967; Azar et al, 2003; Amitai et al. 2006). The current study also increases the number of treatment conditions examined because larger doses of morphine and naloxone are generally required to elicit weight loss and directly observable withdrawal signs (Schulteis et al. 1998). Finally, this study tested for extinction of conditioned withdrawal. Although conditioned withdrawal can persist for many weeks (Stinus et al. 2000), the effects wane when the CS is presented repeatedly in the absence of the unconditioned stimulus. One possibility is that withdrawal signs are differentially resistant to extinction; the current study examined whether the time course of conditioned withdrawal is similar for different signs.
Initially, the effect of varying the number of conditioning trials or the duration of morphine treatment on conditioned withdrawal was studied. Although conditioned withdrawal can be observed after 1 pairing of naloxone and the CS (Azar et al. 2003), the magnitude of conditioned withdrawal increases as the number of pairings or the duration of morphine treatment increases. The current study examined precipitated and conditioned withdrawal in separate groups that received morphine before either 1 or each of 3 conditioning trials in which naloxone was paired with the CS; 3 trials have been shown to produce robust and persistent conditioned withdrawal (Stinus et al. 2000). Differences between repeated intermittent morphine treatment, in which rats received morphine only before each of 3 conditioning trials which were separated by 2 days, and chronic, daily morphine treatment on conditioned withdrawal were also evaluated.
This study also assessed whether precipitated or conditioned withdrawal is greater in rats that were previously dependent on morphine. Repeated experience with precipitated withdrawal in opioid-treated subjects can enhance sensitivity to withdrawal (Schulteis et al. 2003) which might increase conditioned withdrawal. Thus, rats received morphine chronically using treatment conditions that were shown to produce dependence, and conditioning trials began 8 days after discontinuation of treatment (i.e., after spontaneous withdrawal had dissipated); results were compared to those from rats that never received morphine chronically.
A final study examined pharmacological modification of precipitated and conditioned withdrawal. The α2 adrenergic agonist clonidine reduces some withdrawal signs (Sparber and Meyer 1978; Kleber at al. 1980; Rosen et al. 1996; Holtzman 1985). In morphine-treated rats, clonidine attenuates naloxone-precipitated disruptions in operant responding and conditioned place aversion and it can prevent the development of conditioned withdrawal (Schulteis et al. 1998). In the current study, precipitated and conditioned withdrawal were examined in rats that received clonidine with naloxone during conditioning trials. One strategy for reducing the impact of conditioned withdrawal on relapse might be to suppress withdrawal signs that emerge when conditioned stimuli are encountered. The ability to alter the expression of conditioned withdrawal was examined by administering either morphine or clonidine before tests for conditioned withdrawal.
Methods
Subjects
A total of 54 male Sprague Dawley rats (n=6/group; Harlan Inc., Indianapolis, IN, USA) were used. Rats weighed approximately 275 g at the start of the experiment and had free access to food and water except during daily 30-min test periods. Rats were singly housed (24 ± 1°C, 50 ± 10% relative humidity) under a 12-h light/dark cycle. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
Apparatus
During sessions, rats were placed into clear, polycarbonate chambers similar in appearance to the home cage. Absorbent pads were placed in the bottom of each chamber and a wire-mesh floor was placed on top of the absorbent pad. Three drops of commercially available peppermint oil (Sun Harvest Farms, San Antonio, TX, USA) were placed on the absorbent pad. The wire-mesh floor and peppermint oil served as the CS.
Procedure
These studies comprise 5 experiments conducted in 9 separate groups of rats as outlined in Table 1. Each group received morphine, although the duration of morphine treatment varied among groups. Preliminary studies showed that doses of morphine larger than 10 mg/kg produced untoward effects, although naloxone did not produce robust withdrawal in rats that received a single injection of 10 mg/kg of morphine. Consequently, Groups 1, 3 and 6 received 2 injections of 10 mg/kg of morphine 14 hr apart on 3 separate occasions; Group 2 received only 2 injections of 10 mg/kg of morphine 14 hr apart on 1 occasion (i.e., repeated intermittent). Other groups of rats received morphine twice daily for 14 (Group 6) or 21 (Groups 4, 5, 7, 8 and 9) days. Prior to repeated intermittent administration of morphine for conditioning, Group 6 received 10 mg/kg/12 hr of morphine for 14 days. A 14-day period of morphine treatment was chosen because, under these conditions, withdrawal emerged after discontinuation of morphine treatment (McNally and Akil 2001; Kalinichev and Holtzman 2003). Groups 4, 5, 7, 8 and 9 received 10 mg/kg/12 hr of morphine for 21 days; conditioning trials began on day 15 of morphine treatment with sessions conducted 2 hr after morphine administration; the last conditioning trial occurred on day 21 and treatment was discontinued the next day.
Table 1.
Summary of experimental groups
| Experiment | Group | Morphine treatmenta (duration) | Conditioning Trialsb | Tests for conditioned withdrawal | Figure |
|---|---|---|---|---|---|
| 1 | 1 | Repeated intermittent (3 days) | 3 trials, unpaired | Saline Days 1, 8, 15 and 22c |
1 |
| 2 | Repeated intermitten (1 day) t | 1 trial, paired | Saline Days 1, 8, 15 and 22 |
1 | |
| 3 | Repeated intermittent (3 days) | 3 trials, paired | Saline Days 1, 8, 15 and 22 |
1 | |
| 2 | 4 | Chronic (21 days) | 3 trials, unpaired | Saline Days 8, 15 and 22 |
1 |
| 5 | Chronic (21 days) | 3 trials, paired | Saline Days 8, 15 and 22 |
1 | |
| 3 | 6 | Repeated intermittent (3 days) Prior chronic (14 days) | 3 trials, paired | Saline Days 1, 8, 15 and 22 |
2 |
| 4 | 7 | Chronic (21 days) | 3 trials, paired, 0.1 mg/kg clonidine | Saline Days 8, 15 and 22 |
3 |
| 5 | 8 | Chronic (21 days) | 3 trials, paired | 10 mg/kg morphine Days 8, 15 and 22 |
4 |
| 9 | Chronic (21 days) | 3 trials, paired | 0.1 mg/kg clonidine Days 8, 15 and 22 |
4 |
Repeated intermittent treatment: 10 mg/kg of morphine given 16 and 2 hr before each conditioning trial. Chronic treatment: 10 mg/kg/12 hr of morphine
Unpaired: saline given before conditioning trials; 3.2 mg/kg of naloxone given on different days paired: 3.2 mg/kg of naloxone given before conditioning trials; saline given on different days
Days after the last conditioning trial when tests for conditioned withdrawal were conducted
During conditioning trials, rats received 3.2 mg/kg of naloxone and were immediately placed in the cage with the CS for 30 min. Body weights were taken immediately before and 30 min after administration of naloxone. Although 16 withdrawal signs were recorded, only 4 signs (ptosis; leaning, in which rats used the walls of the cage for support; freezing; and salivation) occurred reliably and are reported here. Observations were conducted by persons blinded to treatments and began 5 min after naloxone administration. Preliminary studies showed that withdrawal signs emerged 5 to 10 min after naloxone. Signs were recorded as present or absent every 30 sec for a 5-min period (maximum frequency score=10 for each sign). In preliminary studies, 3.2 mg/kg of naloxone produced robust withdrawal signs and had a rapid onset (5 min) and short duration of action, factors which might increase the salience of withdrawal during conditioning. With the exception of Group 2 which received only 1 conditioning trial, rats received 3 conditioning trials, regardless of morphine treatment. When 3 conditioning trials were used, trials were separated by 2 days during which rats received saline in the absence of the CS. Two control groups (unpaired; Groups 1 and 4; Table 1) received morphine and naloxone on 3 separate occasions, as described above, although they remained in the home cage after receiving drug; on 3 different days, those rats received saline before being placed in the cage with the CS. Body weight was obtained daily and withdrawal signs were measured only on conditioning days.
To examine extinction, conditioned withdrawal was examined repeatedly after the last conditioning trial. Rats were placed in the cage with the CS after receiving saline. For groups that received repeated intermittent morphine treatment, tests occurred 1, 8, 15 and 22 days after the last conditioning trial (Groups 1–3 and 6). For groups that received morphine chronically, tests occurred 8, 15 and 22 days after treatment (Groups 4, 5, 7, 8 and 9); tests were not conducted for the first 7 days after discontinuation of chronic morphine treatment to avoid discontinuation-induced withdrawal, which was monitored by measuring body weight and directly observable signs twice daily for 7 days.
Experiment 1: Compare conditioned withdrawal following 1 or 3 conditioning trials in rats receiving repeated intermittent morphine
Six rats received 10 mg/kg of morphine 16 and 2 hr before 1 (Group 2) or each of 3 (Group 3) conditioning trials (Table 1). A separate group of 6 rats received the same 3 pairings of morphine (two injections) and naloxone, but those pairings did not occur in the presence of the CS (Group 1). In both cases, morphine was administered no more frequently than every 3rd day and drug was not administered during the 2 intervening days.
Experiment 2. Compare conditioned withdrawal in rats receiving repeated intermittent or chronic morphine
To determine whether chronic treatment produces more robust conditioned withdrawal than repeated intermittent treatment, 2 groups of 6 rats received 10 mg/kg/12 hr of morphine for a total of 21 days with the first of 3 conditioning trials conducted on day 15. During conditioning trials (days 15, 18 and 21), naloxone was paired with the CS in Group 5 (Table 1) and not paired with the CS in Group 4. Morphine was discontinued on the day after the last conditioning trial; tests for conditioned withdrawal occurred 8, 15 and 22 days later. Conditioned withdrawal in rats treated chronically with morphine (Group 5) was compared to conditioned withdrawal in rats that received repeated intermittent morphine (Group 3).
Experiment 3. Determine whether a history of physical dependence alters conditioned withdrawal
Six rats received 10 mg/kg/12 hr of morphine for 14 days (Group 6; Table 1). Conditioning trials began 8 days after termination of treatment when withdrawal signs were no longer evident. The 3 conditioning trials were identical to those described above after repeated intermittent morphine. Precipitated and conditioned withdrawal in Group 6 were compared to withdrawal in rats that were never physically dependent (Group 3).
Experiment 4. Determine whether clonidine affects the development of conditioned withdrawal
Six rats received morphine chronically for 14 days; during conditioning trials, 0.1 mg/kg of clonidine was administered 10 min before conditioning with naloxone and the CS (Group 7; Table 1). This dose of clonidine attenuated antagonist-precipitated diarrhea and weight loss in morphine-dependent rats (Sparber and Meyer 1978; DiStefano and Brown 1985; Holtzman 1985). Precipitated and conditioned withdrawal were compared to withdrawal in rats that did not receive clonidine prior to naloxone (Group 5).
Experiment 5. Determine whether morphine or clonidine modifies the expression of conditioned withdrawal
Two separate groups of 6 rats received morphine chronically for 21 days and 3 conditioning trials. Eight days after discontinuation of treatment, rats in Group 8 (Table 1) received 10 mg/kg of morphine 5 min before the test for conditioned withdrawal, and those in Group 9 received 0.1 mg/kg of clonidine 10 min before the test. Saline was administered before tests for conditioned withdrawal conducted on days 15 and 22. Conditioned withdrawal was compared to withdrawal in rats that received saline on test days (Group 5).
Drugs
Compounds used in this study were morphine sulfate, naloxone hydrochloride (Research Technology Branch, National Institute on Drug Abuse, Rockville, MD, USA) and clonidine hydrochloride (Sigma, St. Louis, Missouri, USA). Drugs were dissolved in sterile saline and administered s.c. (morphine) or i.p. (naloxone, clonidine) in a volume of 0.1–1 ml.
Data analyses
Precipitated withdrawal signs were analyzed using one-factor analysis of variance (ANOVA) when more than 2 treatment groups were compared or t-test when 2 groups were compared; a post hoc Student-Newman-Keuls test was used to further evaluate differences among means (GraphPad Prism version 5.01 for Windows; GraphPad Software, San Diego, CA, USA). Conditioned withdrawal signs were analyzed using two-factor repeated measures ANOVA with groups as the between-subjects factor and test day as the within-subjects factor, followed by Bonferroni post hoc tests. Signs observed during the first test for conditioned withdrawal were compared with those observed during precipitated withdrawal using a paired t-test. Data were considered significantly different when p<0.05.
Results
Experiment 1: Compare conditioned withdrawal following 1 or 3 conditioning trials in rats receiving repeated intermittent morphine
Naloxone precipitated withdrawal in rats that received morphine on 1 or 3 occasions (Groups 2 and 3, points above PW, left panels, Figure 1). Withdrawal signs were not evident when rats received saline before they were placed in the cage with the CS (Group 1, Figure 1). Naloxone significantly decreased body weight and increased ptosis, leaning and freezing but not salivation (F[2,15]≥4.19, p≤0.036; F[2,15]=3.07, NS, respectively). Body weight, ptosis and leaning were not significantly different between Groups 2 and 3.
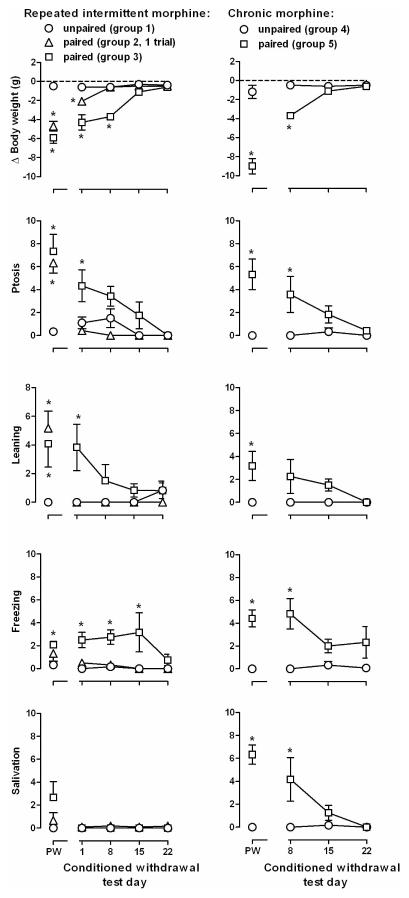
Figure 1.
Effect of number of conditioning trials and length of morphine treatment on precipitated or conditioned withdrawal. Ordinates: top panels, change in body weight (g) that occurred during the 30 min session; bottom 4 rows, frequency of directly observable signs. Abscissa: PW = precipitated withdrawal (the only [Group 2] or last of 3 [Groups 1 and 3] conditioning session when naloxone [Groups 2 and 3] or saline [Group 1] was administered prior to placement in the CS environment; Conditioned Withdrawal Test Day. * = P<0.05 for paired versus unpaired groups (Groups 1 versus 2, 1 versus 3, and 4 versus 5).
Conditioned withdrawal was evident during the first test which occurred the day after the last conditioning trial, although some conditioned withdrawal signs were not as robust as those precipitated by naloxone (compare points above 1 and PW, left panels, Figure 1). For example, on the first test for conditioned withdrawal, body weight and ptosis were significantly different from naloxone-precipitated withdrawal in Groups 2 and 3 (t[5]≥2.64, p<0.05); leaning was significantly increased in rats that received 1 conditioning trial (Group 2; t[5]≥4.32, p≤0.008) and freezing and salivation were not different between Groups 2 and 3 (t[5]≤1.94, NS). During conditioned withdrawal, there was a group x time interaction for body weight and ptosis (F[6,45] ≥2.33, p<0.05) and not for other signs (F[6,45]≤2.27, NS). There was a main effect of group for leaning and freezing (F[2,45]>6.66, p≤0.01), and no main effects for salivation (F[2,45]≤1. 97, NS). During the first test for conditioned withdrawal, body weight, ptosis, leaning and freezing were significantly different in rats that received 3 conditioning trials (Group 3), as compared to rats that received 1 conditioning trial (Group 2; left panels, Figure 1). These conditioned effects decreased over time, although weight loss and freezing were still evident during the second test for conditioned withdrawal.
Experiment 2. Compare conditioned withdrawal in rats receiving repeated intermittent or chronic morphine
Dependence developed after 14 days of treatment with 10 mg/kg/12 hr of morphine, as evidenced by the emergence of withdrawal following discontinuation of treatment (data not shown). Body weights were decreased for 1–2 days and recovered by the fourth day. Directly observable withdrawal signs, including ptosis and leaning, were increased 1 day after discontinuation of morphine and recovered within 6 days. Thus, at the time when tests for conditioned withdrawal occurred (i.e., 8 days after discontinuation of morphine), withdrawal signs from the discontinuation of treatment were no longer present.
During chronic morphine treatment, 3.2 mg/kg of naloxone precipitated withdrawal, as evidenced by changes in body weight and directly observable withdrawal signs (t[10] ≥2.57, p<0.05; points above PW, right panels, Figure 1). Weight loss, freezing and salivation were significantly different in rats receiving morphine chronically, as compared to rats that received repeated intermittent morphine (t[10]≥2.94, p≤0.015); Groups 3 and 5, Figure 1), although the duration of morphine treatment did not alter ptosis or leaning (t[10]≤0.37, NS). Conditioned withdrawal was evident during the first test in rats that received morphine chronically. Only body weight was significantly different during conditioned withdrawal, as compared to withdrawal precipitated by naloxone (t[5]=4.36, p=0.007; other 4 signs: t[5]≤1.26, NS). Conditioned withdrawal signs decreased over time since the last conditioning trial. There was a group x time interaction for body weight and salivation (F[2,20]≥3.76, p<0.05) and a main effect of group for ptosis, leaning and freezing (F[2,20]≥5.75, p<0.05). Weight loss, ptosis, freezing and salivation were greater in tests for conditioned withdrawal when naloxone was paired with the CS, as compared to the signs observed when the CS was not paired with naloxone (Groups 4 and 5, Figure 1), and leaning was not different in these 2 groups. Conditioned withdrawal signs were not significantly different between rats treated chronically or those treated less frequently with morphine (t[10]≤2.14, NS; Groups 3 and 5). When tested 15 days after the last conditioning trial, withdrawal signs were not significantly different between groups in which naloxone was paired with the CS and groups in which it was not.
Experiment 3. Determine whether a history of physical dependence alters conditioned withdrawal
Naloxone-precipitated withdrawal was significantly greater in rats with a history of physical dependence (Group 6) as compared with rats that were never dependent (Group 3). Significant differences were detected for weight loss, ptosis, freezing and salivation (F[2,15]≥15.79, p≤0.0002) and not for leaning (F[2,15]=3.62, p=0.052; points above PW, Figure 2). Post hoc tests revealed that weight loss, freezing and salivation occurred more frequently in rats that were previously dependent, as compared with rats that were never dependent. Although a history of physical dependence appeared to enhance precipitated withdrawal, it did not impact conditioned withdrawal (Figure 2). A group x time interaction was detected for body weight (F[6,45]=7.11, p≤0.0001), with a main effect of time for ptosis, leaning and freezing (F[3,45]≥4.03, p<0.05). No significant differences were detected for salivation (F[3,45]≤1.20, NS). One day after the last conditioning trial, weight loss, ptosis, leaning and freezing were observed in rats that received naloxone during conditioning trials, regardless of whether they had previously been physically dependent (Groups 3 and 6, Figure 2), and conditioned withdrawal signs were not significantly different in the 2 groups. During the second test for conditioned withdrawal, which occurred 8 days after the last conditioning trial, rats that never received morphine chronically lost significantly more weight than rats that had a history of physical dependence; there was no other difference between the 2 groups.
Figure 2.
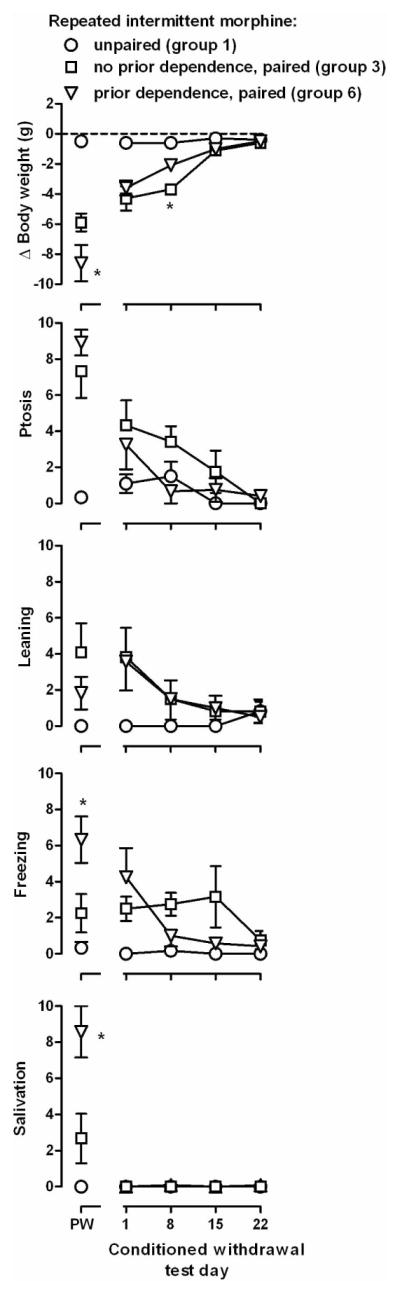
Effects of prior morphine treatment and physical dependence on precipitated and conditioned withdrawal. Data from Groups 1 and 3 are replotted on this figure. Ordinates: top panels, change in body weight (g) that occurred during the 30 min session; bottom 4 rows, frequency of directly observable signs. * = P<0.05 for no prior dependence versus prior dependence (Groups 3 versus 6). See Figure 1 for other details.
Experiment 4. Determine whether clonidine affects the development of conditioned withdrawal
When 0.1 mg/kg of clonidine was administered in combination with naloxone before the first conditioning trial, fewer withdrawal signs were observed, as compared with the signs observed when naloxone was administered alone (data not shown); some signs emerged by the third conditioning trial, although other signs did not (diamonds above PW, Figure 3). Significant overall differences were observed across all dependent variables (F[2,15]≥3.72, p≤0.049). Post hoc tests revealed differences in body weight and freezing among Groups 4, 5, and 7, whereas ptosis and salivation in rats receiving naloxone in combination with clonidine were significantly different from those signs in rats receiving naloxone alone and not from those signs in rats in which naloxone was not paired with the CS. There was no difference in leaning in Groups 5 and 7.
Figure 3.
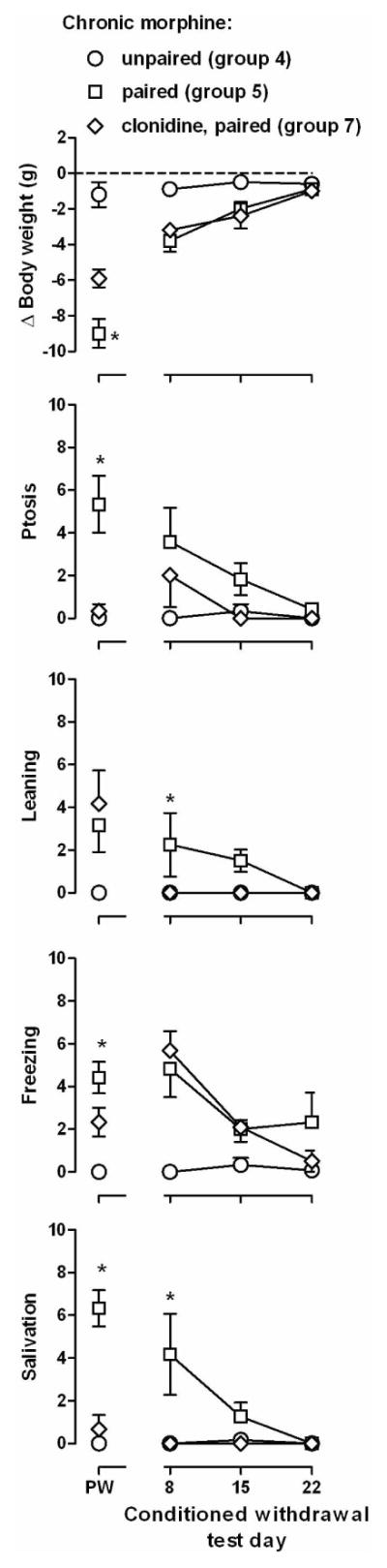
Effects of clonidine treatment on the establishment of conditioned withdrawal. Data from Groups 4 and 5 are replotted in this figure (circles and squares). Ordinates: top row, change in body weight (g) that occurred during the 30 min session; bottom 4 rows, frequency of directly observable signs. * = P<0.05 for naloxone only versus naloxone plus clonidine (Groups 5 versus 7). See Figure 1 for other details.
Administration of clonidine in combination with naloxone during conditioning affected only some conditioned withdrawal signs (Figure 3). There was a group x time interaction for freezing and salivation (F[4, 30]≥4.05, p≤0.01) and a main effect of group for body weight, ptosis and leaning (F[2,30]≥5.11, p≤0.020). Post hoc analyses indicated that leaning and salivation were significantly different in rats that received clonidine with naloxone before conditioning trials, as compared to rats that received naloxone alone before conditioning or rats in which naloxone was not paired with the CS. Body weight, ptosis and freezing were not different in rats that received clonidine with naloxone before conditioning trials (Group 7), as compared to rats that received naloxone alone before conditioning (Group 5).
Experiment 5. Determine whether morphine or clonidine modifies the expression of conditioned withdrawal
No significant differences in naloxone-precipitated withdrawal were detected between Groups 5 and 8 (t[10]≤1.23, NS) and only ptosis was significantly different between Groups 5 and 9 (t[10]=2.65, p=0.025; other 4 signs: t[10]≤2.15, NS; points above PW, Figure 4). Morphine prevented conditioned withdrawal-induced weight loss, ptosis, freezing, and salivation (t[10]=2.66, p<0.05); solid triangles, left panels, Figure 4). Leaning was not significantly different in rats that received morphine before the test for conditioned withdrawal and those that did not (t[10]=2.50,NS). When tested 1 week later in the absence of morphine, conditioned withdrawal signs were not different between rats that received morphine on test day 8 and those that did not (t[10]≤1.85, NS). In contrast to morphine, clonidine attenuated fewer conditioned withdrawal signs. Clonidine prevented the emergence of conditioned-withdrawal induced ptosis and salivation (t[10]≥2.64, p<0.05) and did not prevent weight loss, leaning or freezing (t[10]≤2.33, NS). Conditioned withdrawal signs observed 1 week later (test day 15) were not different between rats that received clonidine on test day 8 and those that did not (t[10]≤2.03, NS).
Figure 4.
Effects of morphine and clonidine on established conditioned withdrawal. Ordinates: top row, change in body weight (g) that occurred during the 30 min session; bottom 4 rows, frequency of directly observable signs. * = P<0.05 for saline versus morphine and saline versus clonidine (Groups 5 versus 8 and 5 versus 9). Filled symbols denote session preceded by a single injection of morphine (filled, inverted triangles) or clonidine (filled diamonds). See Figure 1 for other details.
Discussion
The abuse of opioids remains a serious concern and admissions to substance abuse treatment programs is increasing (Treatment Episode Data Set 2006; http://www.oas.samhsa.gov), although relapse rates remain high (Gossop et al. 2002). Avoidance of withdrawal when opioid treatment is discontinued might contribute to continued drug use; however, relapse sometime can occur long after the last drug use when withdrawal is not apparent. Stimuli previously associated with drug use (Siegel 1977; Weinstein et al. 1997) or drug withdrawal (Wikler 1948; O’Brien et al, 1976) might contribute to relapse months after discontinuation of drug use (Sell et al. 1995; Weinstein et al. 1997), suggesting that conditioned effects of both abused drugs and their absence might need to be managed therapeutically. These studies compared antagonist-precipitated and conditioned opioid withdrawal in rats and examined whether conditioned withdrawal can be modified pharmacologically.
Conditions were identified that reliably produce conditioned withdrawal, including the number of pairings between the CS and withdrawal. In a conditioned place aversion procedure, conditioned withdrawal is evident in rats after a single pairing, although conditioned withdrawal is greater after 2 pairings (Azar et al. 2003). In the current study, a single pairing of the CS with naloxone-precipitated withdrawal also was sufficient to produce conditioned withdrawal-induced weight loss. In morphine-treated subjects, the potency of naloxone to precipitate withdrawal increases with repeated naloxone administration (Schulteis et al. 2003). Consequently, more conditioning trials might enhance sensitivity to naloxone, thereby increasing conditioned withdrawal; this possibility was supported by the increased frequency of observable signs with 3 conditioning trials.
The duration of morphine treatment might also impact precipitated and conditioned withdrawal. In the current study, a dose of 3.2 mg/kg of naloxone precipitated withdrawal following repeated intermittent or chronic morphine treatment, although some naloxone-precipitated withdrawal signs were greater in rats that were treated daily with morphine at the time of conditioning, as compared to rats that received morphine less frequently. Weight loss was greater after the second and third conditioning trials in rats receiving morphine chronically, as compared to rats receiving morphine less frequently. Although the frequency of ptosis and leaning was not different in rats receiving morphine across different dosing conditions, the occurrence of freezing and salivation was higher in rats receiving morphine chronically. Differences in precipitated withdrawal among groups did not predict differences in conditioned withdrawal. Thus, whether or not rats were physically dependent during or prior to conditioning did not significantly impact the magnitude of conditioned withdrawal. Because a large dose of naloxone was used to precipitate withdrawal, it is possible that a ceiling effect limited the magnitude of precipitated and conditioned withdrawal. Thus, current or past dependence on morphine might impact the potency, if not the maximum effectiveness, of naloxone in precipitating withdrawal and in producing conditioned withdrawal. In rats, conditioned opioid withdrawal can last for many weeks (e.g., place aversion). Moreover, conditioned withdrawal decreases when rats are repeatedly exposed to the CS in the absence of the unconditioned stimulus (Stinus et al. 2000). In the current study, repeated testing of the same rats might have facilitated extinction of conditioned withdrawal; rapid extinction among all rats precluded examination of whether withdrawal signs were differentially resistant to extinction among treatments.
Changes in withdrawal were also examined in non-dependent rats with a history of physical dependence. For weight loss, freezing and salivation, the magnitude of precipitated withdrawal was greater in rats that were previously physically dependent, as compared to rats that were never dependent. Despite differences in precipitated withdrawal, the magnitude of conditioned withdrawal was similar between groups, further supporting the view that the magnitude of conditioned withdrawal is not entirely predicted by the magnitude of precipitated withdrawal during conditioning.
To determine whether the development of conditioned withdrawal is modified pharmacologically, clonidine was administered with naloxone before conditioning sessions. Clonidine-attenuated naloxone-precipitated withdrawal with ptosis and salivation being most affected; clonidine prevented the emergence of these signs, which likely contributed to the significantly reduced frequency of conditioned ptosis and salivation. Clonidine attenuated naloxone-induced body weight decreases during the first 2 (data not shown) but not the third and final conditioning trial, perhaps because dependence might have increased over repeated injections of morphine (e.g., a larger dose of clonidine might be required with greater dependence). Conditioned withdrawal-induced weight loss was similar between rats that received clonidine with naloxone and those that received only naloxone, possibly related to the reduced effectiveness of clonidine in preventing naloxone-induced weight loss on the last conditioning trial. In another study clonidine prevented precipitated withdrawal and, subsequently, conditioned withdrawal (Schulteis et al. 1998). Together these results suggest that clonidine disrupts some aspects of conditioned withdrawal by blocking precipitated withdrawal signs.
Precipitated or discontinuation-induced withdrawal is attenuated by the treatment drug or by pharmacologically equivalent drugs; it is less clear which drugs alter conditioned withdrawal. In morphine-dependent monkeys a light, previously paired with an injection of the opioid antagonist nalorphine, increased morphine self-administration (Goldberg et al. 1969), suggesting that conditioned withdrawal contributes to drug-taking and might promote relapse. The current study extends those findings and shows that conditioned withdrawal is prevented by morphine (and reduced by clonidine). Although the effects of morphine on conditioned withdrawal-induced weight loss might be due to its constipating effects (Galligan and Burks 1983), the prevention of observable withdrawal signs indicates that morphine attenuates conditioned withdrawal. Clonidine prevented the expression of some conditioned withdrawal signs, consistent with a study in which opioid-dependent patients received either placebo or a different α2 adrenergic agonist, lofexidine. After discontinuation of opioid treatment, patients receiving lofexidine had reduced heart rates, attenuated drug cue- and stress-induced “craving” of opioids, higher abstinence rates, and improved relapse outcome (Sinha et al. 2007). In the current study, clonidine did not alter conditioned withdrawal-induced weight loss, leaning or freezing. Larger doses of clonidine might be needed to attenuate all conditioned withdrawal signs, although the dose of clonidine in this study (0.1 mg/kg) blocks withdrawal-induced diarrhea in morphine-dependent rats (Sparber and Meyer 1978; DiStefano and Brown 1985) and decreases locomotor activity and schedule-controlled responding in untreated rats (Schulteis et al., 1998). Alternatively, clonidine might selectively, or most effectively, prevent a subset of conditioned withdrawal effects, particularly those measured by suppression of operant responding or place aversion (Schulteis et al. 1998). Nevertheless, conditioned withdrawal can be reduced pharmacologically, perhaps providing a strategy for preventing relapse.
In summary, conditioned withdrawal in rats increases as the number of conditioning trials or the duration of morphine treatment increases, and while a history of physical dependence enhances precipitated withdrawal for a fixed dose of antagonist, it does not further increase conditioned withdrawal. The development and expression of conditioned withdrawal can be modified pharmacologically; from a clinical perspective, focusing treatment on preventing the expression of conditioned withdrawal, rather than its development, might be more practical. Future studies might examine the therapeutic potential of other classes of drugs specifically for preventing the expression of conditioned withdrawal, perhaps by accelerating extinction of this learned response.
Acknowledgments
This work was supported by United States Public Health Service from the National Institute on Drug Abuse (DA 05018 and CPF is supported by a Senior Scientist Award [DA17918]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Amitai N, Liu J, Schulteis G. Discrete cues paired with naloxone-precipitated withdrawal from acute morphine dependence elicit conditioned withdrawal responses. Behav Pharmacol. 2006;17:213–222. doi: 10.1097/00008877-200605000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology. 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- DiStefano PS, Brown OM. Biochemical correlates of morphine withdrawal. 2. Effects of clonidine. J Pharmacol Exp Ther. 1985;233:339–344. [PubMed] [Google Scholar]
- Galligan JJ, Burks TF. Centrally mediated inhibition of small intestinal transit and motility by morphine in the rat. J Pharmacol Exp Ther. 1983;226:356–361. [PubMed] [Google Scholar]
- Goldberg SR, Schuster CR. Conditioned suppression by a stimulus associated with nalorphine in morphine-dependent monkeys. J Exp Anal Behav. 1967;10:235–242. doi: 10.1901/jeab.1967.10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Morphine: Conditioned increases in self-administration in rhesus monkeys. Science. 1969;166:1306–1307. doi: 10.1126/science.166.3910.1306. [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97:1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of morphine withdrawal in the dependent rat: suppression by opiate and nonopiate drugs. J Pharmacol Exp Ther. 1985;233:80–86. [PubMed] [Google Scholar]
- Irwin S, Seevers MH. Altered response to drugs in the post addict (Macaca mulatta) J Pharmacol Exp Ther. 1956;116:31–32. [Google Scholar]
- Kalinichev M, Holtzman SG. Changes in urination/defecation, auditory startle response, and startle-induced ultrasonic vocalizations in rats undergoing morphine withdrawal: similarities and difference between acute and chronic dependence. J Pharmacol Exp Ther. 2003;304:603–609. doi: 10.1124/jpet.102.044206. [DOI] [PubMed] [Google Scholar]
- Kleber HD, Gold MS, Riordan CE. The use of clonidine in detoxification from opiates. Bull Narcs. 1980;32:1–9. [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Childress AR, Ehrman R, O’Brien CP, Pashko S. Extinguishing conditioned responses during opiate dependence treatment: turning laboratory findings into clinical procedures. J Subst Abuse Treat. 1986;3:33–40. doi: 10.1016/0740-5472(86)90006-1. [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H. Effects of contextual or olfactory cues previously paired with morphine withdrawal on behavior and pain sensitivity in the rat. Psychopharmacology. 2001;156:381–387. doi: 10.1007/s002130100743. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Greenstein R. Conditioning in human opiate addicts. Pav J Biol Sci. 1976;11:195–202. doi: 10.1007/BF03000314. [DOI] [PubMed] [Google Scholar]
- Parker L, Failor A, Weidman K. Conditioned preferences in the rat with an unnatural need state: morphine withdrawal. J Comp Physiol Psychol. 1973;82:294–300. doi: 10.1037/h0033921. [DOI] [PubMed] [Google Scholar]
- Rosen MI, McMahon TJ, Hameedi FA, Pearsall HR, Woods SW, Kreek MJ, Kosten TR. Effect of clonidine pretreatment on naloxone-precipitated opiate withdrawal. J Pharmacol Exp Ther. 1996;276:1128–1135. [PubMed] [Google Scholar]
- Schnur P. Conditioned morphine withdrawal in the hamster. Psychopharmacology. 1992;107:517–522. doi: 10.1007/BF02245265. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Stinus L, Risbrough VB, Koob GF. Clonidine blocks acquisition but not expression of conditioned opiate withdrawal in rats. Neuropsychopharmacology. 1998;19:406–419. doi: 10.1016/S0893-133X(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J. Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav. 2003;76:493–503. doi: 10.1016/j.pbb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Sell LA, Cowen PJ, Robson PJ. Ondanesetron and opiate craving, a novel pharmacological approach to addiction. Br J Psychiatry. 1995;166:511–514. doi: 10.1192/bjp.166.4.511. [DOI] [PubMed] [Google Scholar]
- Siegal S. Learning and psychpharmacology. In: Jarvik ME, editor. Psychopharmacology in the practice of medicine. Appleton-Century-Crofts; New York: 1977. pp. 61–70. [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten T. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sparber SB, Meyer DR. Clonidine antagonizes naloxone-induced suppression of conditioned behavior and body weight loss in morphine-dependent rats. Pharmacol Biochem Behav. 1978;9:319–325. doi: 10.1016/0091-3057(78)90292-7. [DOI] [PubMed] [Google Scholar]
- Stinus L, Caille S, Koob GF. Opiate withdrawal-induced place aversion lasts up to 16 weeks. Psychopharmacology. 2000;149:115–120. doi: 10.1007/s002139900358. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Wilson S, Bailey J, Myles J, Nutt D. Imagery of craving in opiate addicts undergoing detoxification. Drug Alcohol Depend. 1997;48:25–31. doi: 10.1016/s0376-8716(97)00098-7. [DOI] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. Am J Psychiat. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and “relapse” in morphine-addicted rats. Psychopharmacologia. 1967;10:255–284. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]