Abstract
The present study was designed to estimate the ability of chlorophyllin (CHL) to interact with two acridine mutagens, quinacrine mustard (QM) and acridine orange (AO), and with the antitumor anthracycline doxorubicin (Dox). To this end, aqueous solutions of QM, AO or Dox during titration with CHL were subjected to spectrophotometry and spectrofluorimetry to detect possible interactions between these reagents. The data indicate that CHL forms complexes with AO, QM or Dox in these solutions. The presence of the complexes was manifested by a bathochromic shift of the absorption spectra, as well as by strong quenching of the fluorescence of each of these mutagens in the presence of CHL. CHL, thus, may serve as an interceptor of these mutagenic acridines in different in vivo or in vitro applications. Its ability to interact with Dox may potentially be utilized to detoxify patients overdosed with this or similar drugs.
Keywords: Chlorophyllin, Acridine orange, Quinacrine mustard, Doxorubicin, Absorption, Fluorescence spectroscopy
1. Introduction
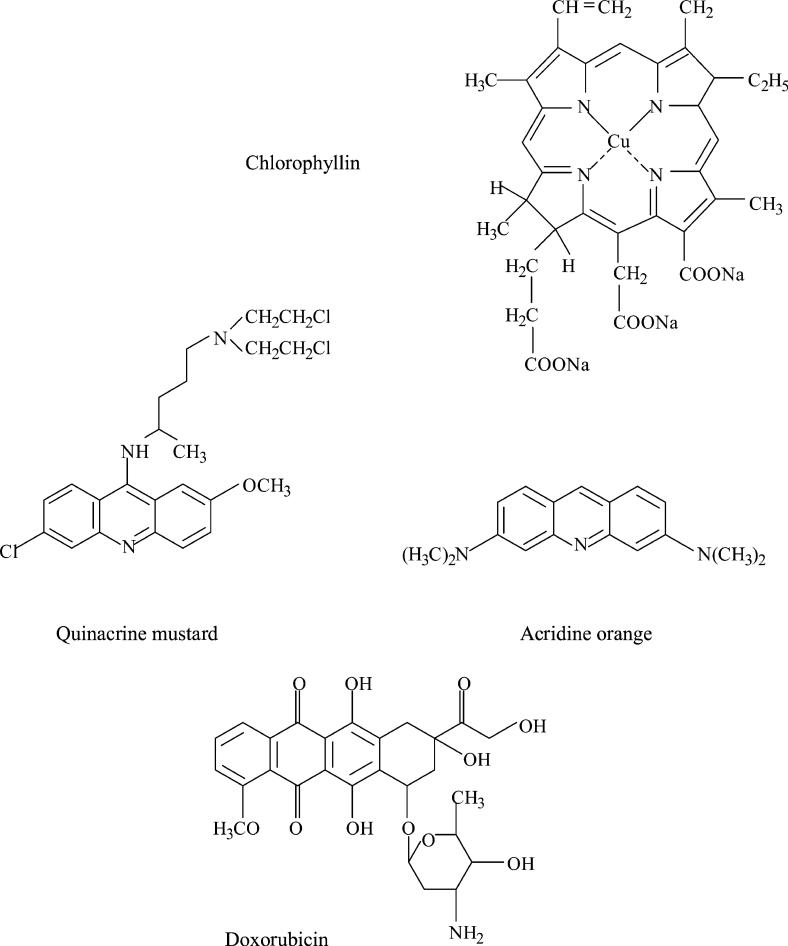
Chlorophyllin (CHL) is the water-soluble sodium and copper salt of chlorophyll (Fig. 1). It has frequently been reported during the past decade that CHL has strong anti-oxidative, anti-mutagenic and anti-carcinogenic properties [1]. Thus, the mutagenicity of such diverse agents as heterocyclic amines [2–5], aflatoxin B1 [6,7], benzo[a]pyrene [8–11], dibenzo[a,1]pyrene [12], doxorubicin [13], cyclophosphamide [14], reactive oxygen intermediates (ROIs) [15] or heavy metal ions [16–18], was shown to be reduced in the presence of CHL.
Fig. 1.
Chemical structure of the compounds studied.
Several mechanisms can contribute to the observed antimutagenic properties of CHL. Because CHL is an antioxidant, its ability to scavenge ROIs may be responsible for the reduction in mutagenicity of at least those mutagens for which activity is directly or indirectly mediated by ROIs [19–22]. Another mechanism that has been advanced was based on the observation that CHL reacts directly with active groups of mutagens, resulting in their neutralization [19,22,23]. Still another mechanism to account for the antimutagenic property of CHL was explained by the formation of heterologous complexes with mutagens [3,4,9]. The latter mechanism was postulated based on the observation that CHL was most effective in reducing mutagenicity in the case of polycyclic compounds with flat aromatic structures [5,11,24]. According to this mechanism, the complexes are maintained by stacking interactions that involve π–π bonds between the porphyrin ring of CHL and aromatic rings of the mutagens [4]. The formation of the complexes in aqueous solution sequesters free mutagen molecules, leads to a reduction in their concentration in a monomer (active) form, and thereby lowers their accessibility to the cell that results in decreased mutagenic activity.
The present study was aimed to reveal whether CHL forms complexes with three different heterocyclic aromatic compounds: acridine orange (AO), quinacrine mustard (QM) and doxorubicin (Dox; Fig. 1). Each of these agents binds to DNA by intercalation and can be mutagenic [25–28]. QM, in addition, can alkylate nucleic acids. AO and QM are used in analytical cytology as DNA (AO, QM) and RNA (AO) fluorochromes [29]. Dox is an antitumor drug of the anthracyclin family that is widely used to treat different types of solid tumors and leukemias.
2. Experimental
2.1. Reagents
Chlorophyllin (sodium–copper salt), AO (CI 46005; 3,6-bis[dimethylamino]acridine hydrochloride), QM {2-methoxy-chloro-9-[4(β-chloroethyl) amino-1-methylbutylamino]acridine} and doxorubicin hydrochloride all were purchased from Sigma Chemical Co (St. Louis, MO, USA). Tris-(hydroxymethyl)-aminomethane was obtained from Fluka (Switzerland). HCl was provided by Polish Chemical Reagents Co (Gliwice, Poland). All reagents were used without additional purification.
2.2. Absorption measurements
Absorption spectral measurements were carried out on Cary 300 spectrophotometer (Varian, Australia). Solutions of individual mutagens were prepared in 30 mM Tris–HCl at pH 7.4. These solutions, maintained at constant mutagen concentration (AO, 3×10–6–5×10–6 M; QM, 4.5×10–6–7×10–6 M; Dox, 6×10–6–8×10–6 M) were titrated with CHL to obtain increasing final CHL concentration from 3×10–7 to 4×10–5 M. The solutions were freshly prepared before each measurement. Because of the photosensitivity of the reagents studied (particularly CHL) the solutions were protected from light. The association constants Ka and the absorption spectra of the complexes were numerically estimated using a linear regression fit according to Eq. (1). The least sums of squares of deviations between the measured and computed absorption spectra were used. The data indicated 1:1 stoichiometry of the mutagen to CHL within the complexes in the concentration ranges studied.
| (1) |
where ; A is the total absorption of the sample measured; , and are the absorption coefficients for the mutagen, CHL and of the complex, respectively; CM is the mutagen concentration; CCHL is the CHL concentration; Ka is the association constant; and l is the length of the light path.
2.3. Fluorescence measurement
Fluorescence measurements were carried out on LS 50B spectrophotometer (Perkin Elmer, UK), in 1-cm×1-cm cuvettes at 20 °C. Fluorescence was induced using an excitation wavelength at or near the maximum absorption of the individual mutagen, namely at 492, 474 and 422 nm, and with a 5- and 5-nm, 5- and 15-nm, and 5- and 8-nm excitation and emission spectral width for AO, Dox and QM, respectively. The solutions were maintained at constant mutagen concentration (AO, 5×10–7–3×10–6 M; QM, 4×10–6–7×10–6 M; Dox, 6×10–6–8×10–6 M) and were titrated with CHL to obtain an increasing final CHL concentration from 10–7 to 4×10–5 M. No inner filter effect was apparent at these concentrations [30]. The association constants Ka were estimated according to a non-linear regression [Eq. (2)]. This form of equation was used because within the range of excitation and emission used the mutagen–CHL complexes showed no detectable fluorescence. It was apparent that the formation of complexes led to quenching of the mutagen fluorescence.
| (2) |
where F is the total fluorescence intensity of the sample measured, F0 is the fluorescence intensity of the mutagen and the other symbols are as in Eq. (1).
All data were analyzed using computer program PRISM 2 (Graph Pad Software Inc, USA)
3. Results
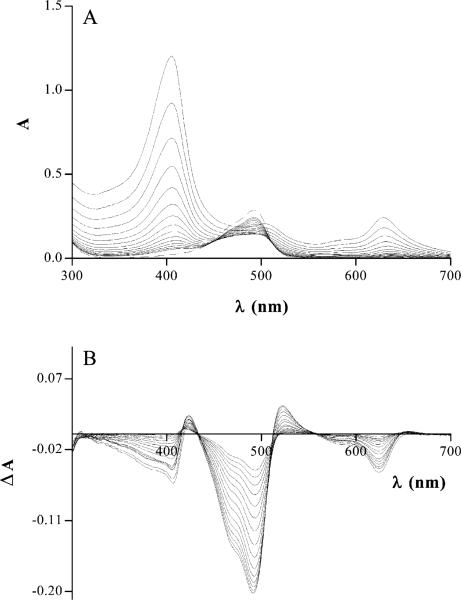
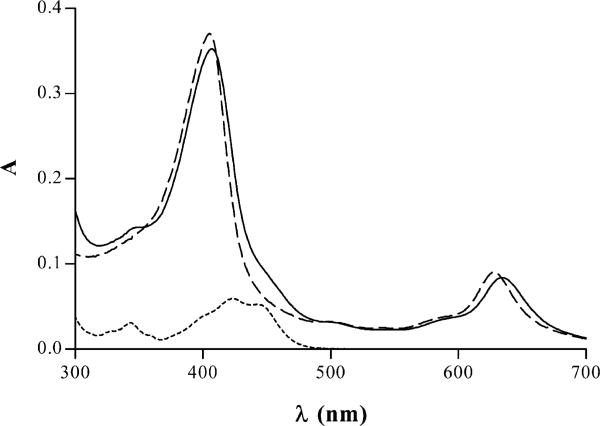
Fig. 2 provides an example illustrating the characteristic changes in absorption spectra occurring during titration of AO with CHL. Also presented are the differential spectra of this sample. Similar changes were observed during titration of QM and Dox (data not shown). It was quite evident that the absorption spectra of the solutions containing mixtures of CHL with AO, QM or Dox were distinctly different from the sums of spectra of the same reagents when analyzed individually at the same concentrations as in the mixture. The absorption bands of the solutions containing mutagen–CHL were shifted to longer wavelength compared to the absorption band for CHL alone. This was particularly evident in the case of the second absorption band of CHL, at 628 nm (Fig. 3).
Fig. 2.
(a) Solid lines, absorption spectra of AO (5×10–6 M) admixed during titration with different concentrations (1.06×10–6–4×10–5 M) of CHL; dashed line, absorption spectrum of AO alone (5×10–6 M). (b) Differential spectra as in (a).
Fig. 3.
Absorption spectra of: CHL (10–5 M), dashed line; QM (2×10–6 M), dotted line, and mixture of CHL and QM at concentrations as above, solid line.
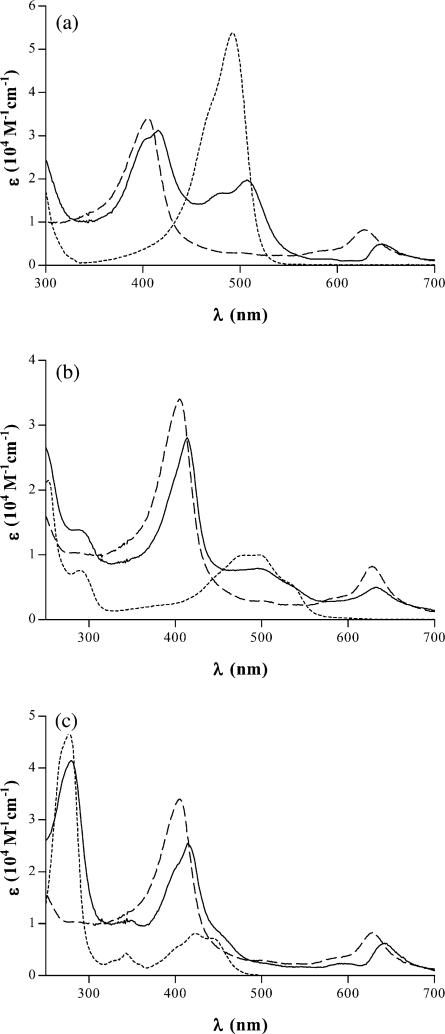
The spectral changes observed indicated the formation of complexes in the mixtures of each of the mutagens studied with CHL. Indeed, the computed absorption spectra for each of the complexes, which were distinctly red-shifted compared to the first and second absorption bands of CHL (Fig. 4), were consistent with the presence of such complexes.
Fig. 4.
Absorption spectra of CHL (dashed line), each of the mutagens studied alone (dotted lines) and computed spectra of (a) AO-CHL, (b) Dox-CHL and (c) QM-CHL (solid lines).
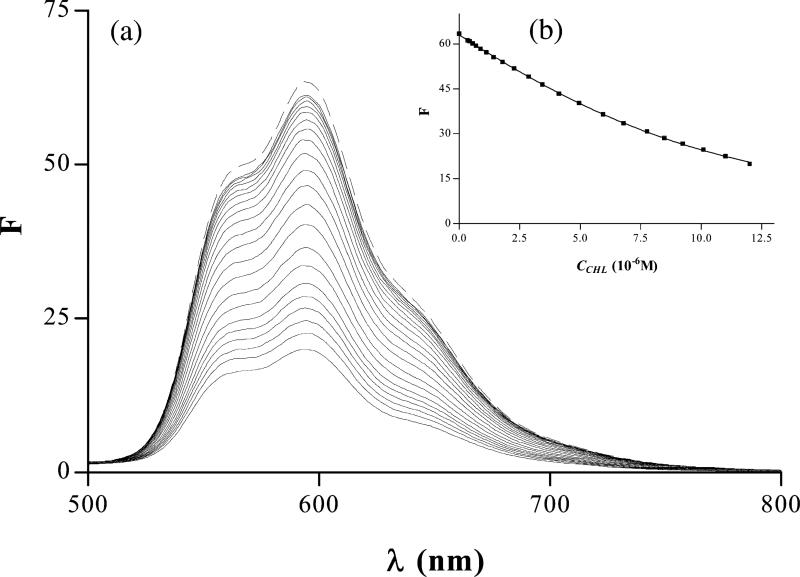
Also consistent with the formation of these complexes were changes (quenching) in the fluorescence of AO, QM and Dox after the addition of CHL. Similar changes in fluorescence spectra as those shown in the case of Dox (Fig. 5) were also observed during titration of AO and QM (data not shown). Strong quenching of the mutagen fluorescence was observed for each of the mutagens studied, with neither a demonstrable change in shape of the spectrum nor in the spectral shift for maximum fluorescence. Nearly complete quenching was apparent when CHL was in molar excess compared with the mutagen concentration. It appears, thus, that in the wavelength range studied the CHL–mutagen complexes were not fluorescent.
Fig. 5.
(a) Fluorescence spectra of Dox (6×10–6 M) alone (dashed line) and admixed during titration with different concentration (3.8×10–6–1.2×10–5 M, from top to bottom) of CHL (solid lines). (b) Non-linear regression calculated according to Eq. (2) at a wavelength of 492 nm.
4. Discussion
The present data indicate that in aqueous solution CHL forms complexes with AO, QM and Dox. The presence of these complexes was manifested by a bathochromic shift of the absorption spectra and strong quenching of the fluorescence of each of these mutagens observed in the presence of CHL. The estimated association constants are relatively high and appear to be within a similar range to the case of CHL complexes with aflatoxin B1 (Ka = 7.2×105 M–1), aflatoxin B2 (Ka = 5.3×105 M–1) [27] and dibenzo[a,1]pyrene (Ka = 6.3×105 M–1) [12]. It should be noted, however, that the association constants of CHL with aromatic amines, in the range 103–104 M–1 [4,31], are one or two orders of magnitudes lower.
The association constants of CHL–Dox and CHL–QM estimated based on absorption spectra were concordant with the estimates based on fluorescence changes (Table 1). This was not the case, however, with Ka of CHL–AO complexes, which varied by over 35%. The formation of AO dimers and higher aggregates that is observed in aqueous solution [32] may be responsible for this discrepancy.
Table 1.
The association constants Ka of the CHL–AO, CHL–QM and CHL–Dox complexes estimated by spectrophotometry and spectro-fluorimetry, respectively
| Compound | Absorption |
Fluorescence |
||
|---|---|---|---|---|
| Ka (M–1) | S.D. (M–1) | Ka (M–1) | S.D. (M–1) | |
| Acridine orange | 5.6×105 | 0.3×105 | 8.4×105 | 2.1×105 |
| Quinacrine mustard | 3.1×105 | 0.5×105 | 3.3×105 | 0.3×105 |
| Doxorubicin | 3.7×105 | 0.2×105 | 2.9×105 | 0.3×105 |
It should be mentioned, however, that the estimates of affinity binding and other parameters of CHL, in general, may be affected by the fact that commercially available CHL preparations contain certain impurities, such as chlorins and other green pigments, as well as variable combinations of ions [1,10,29]. Furthermore, because CHL is sensitive to light and easily undergoes oxidation, the conditions of its storage and preparation of solutions may also affect its chemistry.
It should also be noted that even though chlorophyll in plants fluoresces w33,34], the preparation of CHL itself had no detectable fluorescence at the wavelengths studied. Since CHL is in fact a chlorophyll chelated with copper, it is likely that chlorophyll chelation with this ion quenches its fluorescence. The lack of CHL fluorescence allowed us to measure the fluorescence of the carcinogens in the mixture and in complexes with CHL without interference from the latter.
The present measurements were carried out at pH 7.4, considered to represent a ‘physiological’ concentration of hydrogen ions. At this pH value one of the mutagens studied, AO, which has pKa ≈ 10.5, is expected to be predominantly in ionic form. Therefore, electrostatic interactions could contribute to its binding to CHL. In its uncharged form, thus, the AO association constant with CHL may be different compared to that estimated in the present study.
The high affinity of CHL to form complexes with QM observed here may explain the propensity of CHL to protect the cells from the cytostatic and cytotoxic activity of QM recently reported by us [35]. CHL, thus, may find an application as an interceptor of mutagens [36] that have aromatic structure similar to that of QM or AO. It should be noted that the interceptor affinity of CHL to aromatic carcinogens is comparable to that of substances isolated from green and black tea [37], and appears to be stronger than that of caffeine [26]. Because CHL alone, even at high concentration/doses, is non-toxic to normal cells and organisms, it may be considered as an attractive antimutagenic agent that can be applied in vivo or in vitro. There is an ongoing search for components of natural products that can protect normal cells from mutagens and possibly from the side effects of chemotherapy [38]. CHL, which can be inexpensively obtained in large quantities from green parts of plants or phytoplankton, is one of the leading products that holds promise for use in these applications.
The ability of CHL to form complexes with Dox should be taken into consideration during treatment of patients with Dox or other drugs of similar structure. Namely, since CHL is the major component of green vegetables and thus may be consumed in large quantity, it has the potential to interact with drugs, especially if taken orally, lowering their effective concentration in free, monomeric form. This propensity of CHL, however, may be exploited for the purpose of detoxifying patients mistakenly administered with an excess of the drug by dialyzing their blood against CHL, e.g. immobilized on insoluble salt-like material, such as CHL–chitosan [39]. Experiments are under way to detect whether carcinogens or Dox that are already bound to their targets in live cells can be removed from the cells by co-incubation with CHL, similar to behavior previously observed with caffeine [40].
Acknowledgments
Supported by BST 0706-802 and by RO1 28 704 grant from NCI (ZD).
References
- 1.Dashwood RH. The important of using pure chemicals in (anti)mutagenicity studies: chlorophyllin as a case in point. Mutat. Res. 1997;381:283–286. doi: 10.1016/s0027-5107(97)00221-2. [DOI] [PubMed] [Google Scholar]
- 2.Dashwood RH, Liew C. Chlorophyllin-enhanced excretion of urinary and fecal mutagens in rats given 2-amino-3-methylimidazo[4,5-f]quinoline. Environ. Mol. Mutagen. 1992;20:199–205. doi: 10.1002/em.2850200308. [DOI] [PubMed] [Google Scholar]
- 3.Dashwood RH, Guo D. Antimutagenic potency of chlorophyllin in the Salmonella assay and its correlation with binding constants of mutagen–inhibitor complexes. Environ. Mol. Mutagen. 1993;22:164–171. doi: 10.1002/em.2850220309. [DOI] [PubMed] [Google Scholar]
- 4.Dashwood RH, Yamane S, Larsen R. Study of the forces stabilizing complexes between chlorophylls and heterocyclic amine mutagens. Environ. Mol. Mutagen. 1996;27:211–218. doi: 10.1002/(SICI)1098-2280(1996)27:3<211::AID-EM6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Hayatsu H, Sugiyama C, Arimoto-Kobayashi S, Negishi T. Porphyrins as possible prevents of heterocyclic amine carcinogenesis. Cancer Lett. 1999;143:185–187. doi: 10.1016/s0304-3835(99)00122-6. [DOI] [PubMed] [Google Scholar]
- 6.Breinholt V, Hendricks J, Pereira C, Arbogast D, Bailey G. Dietary chlorophyllin is a potent inhibitor of aflatoxin B1 hepatocarcinogenesis in rainbow trout. Cancer Res. 1995;55:57–62. [PubMed] [Google Scholar]
- 7.Dashwod RH, Negishi T, Hayatsu H, Breinholt V, Hendricks J, Bailey G. Chemopreventive properties of chlorophylls towards aflatoxin B1: a review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat. Res. 1998;399:245–253. doi: 10.1016/s0027-5107(97)00259-5. [DOI] [PubMed] [Google Scholar]
- 8.Terwel L, van der Hoeven JCM. Antimutagenic activity of some naturally occurring compounds towards cigarette-smoke condensate and benzo[a]pyrene in the Salmonella/microsome assay. Mutat. Res. 1985;152:1–4. doi: 10.1016/0027-5107(85)90039-9. [DOI] [PubMed] [Google Scholar]
- 9.Tachino N, Guo D, Dashwood WM, Yamane S, Larsen R, Dashwood RH. Mechanisms of the in vitro antimutagenic action of chlorophyllin against benzo[a]pyrene: studies of enzyme inhibition, molecular complex formation and degradation of the ultimate carcinogen. Mutat. Res. 1994;308:191–203. doi: 10.1016/0027-5107(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 10.Arimoto S, Kan-yama K, Rai H, Hayatsu H. Inhibitory effect of hemin, chlorophyllin and related pyrrole pigments on the mutagenicity of benzo[a]pyrene and its metabolites. Mutat. Res. 1995;345:127–135. doi: 10.1016/0165-1218(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 11.Park KK, Surh YJ. Chemopreventive activity of chlorophyllin against mouse skin carcinogenesis by benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. Cancer Lett. 1996;102:143–149. doi: 10.1016/0304-3835(96)04173-0. [DOI] [PubMed] [Google Scholar]
- 12.Reddy AP, Harttig U, Barth MC, et al. Inhibition of dibenzo[a,l]pyrene-induced multi-organ carcinogenesis by dietary chlorophyllin in rainbow trout. Carcinogenesis. 1999;20:1919–1926. doi: 10.1093/carcin/20.10.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amara-Mokrane YA, Lehucher-Michel MP, Balansard G, Duménil G, Botta A. Protective effects of α-hedrin, chlorophyllin and ascorbic acid towards the induction of micronuclei by doxorubicin in cultured human lymphocytes. Mutagenesis. 1996;11:161–167. doi: 10.1093/mutage/11.2.161. [DOI] [PubMed] [Google Scholar]
- 14.Te C, Gentile JM, Baguley BC, Pearson AE, Gregory T, Ferguson LR. In vivo effects of chlorophyllin on the antitumour agent cycliphosphamid. Int. J. Cancer. 1997;70:84–89. doi: 10.1002/(sici)1097-0215(19970106)70:1<84::aid-ijc13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Kamat JP, Boloor KK, Devasagayam TPA. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Bochim. Biophys. Acta. 2000;1487:113–127. doi: 10.1016/s1388-1981(00)00088-3. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh AK, Sen S, Sharma A, Talukder G. Effect of chlorophyllin on mercuric chloride-induced clastogenicity in mice. Fd. Chem. Toxic. 1991;29:777–779. doi: 10.1016/0278-6915(91)90187-c. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh AK, Sen S, Palit S, Ghosh A, Sharma A, Talukder G. Comparative efficacy of chlorophyllin in reducing cytotoxicity of some heavy metals. Biol. Met. 1991;4:158–161. doi: 10.1007/BF01141307. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar D, Sharma A, Talukder G. Differential protection of chlorophyllin against clastogenic effects of chromium and chlordane in mouse bone marrow in vivo. Mutat. Res. 1993;301:33–38. doi: 10.1016/0165-7992(93)90053-x. [DOI] [PubMed] [Google Scholar]
- 19.Ong TM, Whong WZ, Stewart J, Brockman HE. Chlorophyllin: a potent antimutagen against environmental and dietary complex mixtures. Mutat. Res. 1986;173:111–115. doi: 10.1016/0165-7992(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 20.Park KK, Surh YJ, Miller JA. Chemoprotective properties of chlorophyllin against vinyl carbamate, p-nitrophenyl vinyl ether and their electrophilic epoxides. Cancer Lett. 1995;94:33–40. doi: 10.1016/0304-3835(95)03822-e. [DOI] [PubMed] [Google Scholar]
- 21.Kumar SS, Chaubey RC, Devasagayam TP, Priyadarsini KI, Chauhan PS. Inhibition of radiation-induced DNA damage in plasmid pBR322 by chlorophyllin and possible mechanism(s) of action. Mutat. Res. 1999;425:71–79. doi: 10.1016/s0027-5107(98)00250-4. [DOI] [PubMed] [Google Scholar]
- 22.Olvera O, Arceo C, Zimmering S. Chlorophyllin [CHLN] and the mutagenicity of monofunctional alkylating agents in Drosophila: the action of CHLN need not include an influence on metabolic activation. Mutat. Res. 2000;467:113–117. doi: 10.1016/s1383-5718(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 23.Chung WY, Lee J, Lee W, Surh Y, Park K. Protective effects of hemin and tetrakis(4-benzoic acid)porphyrin on bacterial mutagenesis and mouse skin carcinogenesis induced by 7,12-dimethylbenz[a]anthracene. Mutat. Res. 2000;472:139–145. doi: 10.1016/s1383-5718(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 24.Arimoto S, Fukuoka S, Itome C, Nakano H, Rai H, Hayatsu H. Binding of polycyclic planar mutagens to chlorophyllin resulting in inhibition of the mutagenic activity. Mutat. Res. 1993;287:293–305. doi: 10.1016/0027-5107(93)90022-8. [DOI] [PubMed] [Google Scholar]
- 25.Waring MJ. DNA modification and cancer. Annu. Rev. Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- 26.Larsen RW, Jasuja R, Heltzer RK, Muraoka PT, Andrada VG, Jameson DM. Spectroscopic and molecular modeling study of caffeine complexes with DNA intercalators. Biophys. J. 1996;70:443–452. doi: 10.1016/S0006-3495(96)79587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi T, Schimerlik M, Bailey G. Mechanisms of chlorophyllin anticarcinogenesis: dose-responsive inhibition of aflatoxin uptake and biodistribution following oral co-administration in rainbow trout. Toxicol. Appl. Pharmacol. 1999;158:132–140. doi: 10.1006/taap.1999.8695. [DOI] [PubMed] [Google Scholar]
- 28.Kapuscinski J, Ardelt B, Piosik J, Zdunek M, Darzynkiewicz Z. The modulation of the DNA-damaging effect of polycyclic aromatic agents by xanthines. Part I. Reduction of cytostatic effects of quinacrine mustard by caffeine. Biochem. Pharmacol. 2002;63:625–634. doi: 10.1016/s0006-2952(01)00904-2. [DOI] [PubMed] [Google Scholar]
- 29.Darzynkiewicz Z. Simultaneous analysis of RNA and DNA content. Methods Cell. Biol. 1994;41:401–420. doi: 10.1016/s0091-679x(08)61731-8. [DOI] [PubMed] [Google Scholar]
- 30.Wieczorek Z, Stepinski J, Darzynkiewicz E, Lönnberg H. Association of nucleosides and their 5′-monophosphates with a tryptophan-containing tripeptide, Trp–Leu–Glu, by fluorescence spectroscopy. Biophys. Chem. 1993;47:233–240. doi: 10.1016/0301-4622(93)80048-n. [DOI] [PubMed] [Google Scholar]
- 31.Dashwood RH, Guo D. Inhibition of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)–DNA binding by chlorophyllin: studies of enzyme inhibition and molecular complex formation. Carcinogenesis. 1992;13:1121–1126. doi: 10.1093/carcin/13.7.1121. [DOI] [PubMed] [Google Scholar]
- 32.Kapuscinski J, Kimmel M. Thermodynamical model of mixed aggregation of intercalators with caffeine in aqueous solutions. Biophys. Chem. 1993;35:46–53. doi: 10.1016/0301-4622(93)85022-a. [DOI] [PubMed] [Google Scholar]
- 33.Barrett J, Anderson JM. The P-700-chlorophyll alpha-protein complex and two major light-harvesting complexes of Acrocarpia paniculata and other brown seaweeds. Biochem Biophys Acta. 1980;590:309–323. doi: 10.1016/0005-2728(80)90202-9. [DOI] [PubMed] [Google Scholar]
- 34.Satoh K, Butler WL. Competition between 735-nm fluorescence and the photochemistry of photosystem I in chloroplasts at low temperature. Biochem Biophys Acta. 1978;502:103–110. doi: 10.1016/0005-2728(78)90135-4. [DOI] [PubMed] [Google Scholar]
- 35.Ardelt B, Kunicki J, Traganos F, Darzynkiewicz Z. Chlorphyllin protects cells from the cytostatic and cytotoxic effects of quinacrine mustard but not nitrogen mustard. Int. J. Oncol. 2001;18:849–853. doi: 10.3892/ijo.18.4.849. [DOI] [PubMed] [Google Scholar]
- 36.Hartman PE, Shankel D. Antimutagens and anticarcinogens: a survey of putative interceptor molecules. Environ. Mol. Mutagen. 1990;5:145–182. doi: 10.1002/em.2850150305. [DOI] [PubMed] [Google Scholar]
- 37.Hernaez J, Xu M, Dashwood RH. Effects of tea and chlorophyllin on the mutagenicity of N-hydroxy-IQ: studies of enzyme inhibition, molecular complex formation, and degradation/scavenging of the active metabolites. Environ. Mol. Mutagen. 1997;30:468–474. doi: 10.1002/(sici)1098-2280(1997)30:4<468::aid-em12>3.0.co;2-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitscher LA, Telikepalli H, McGhee E, Shankel DM. Natural antimutagenic agents. Mutat. Res. 1996;350:143–152. doi: 10.1016/0027-5107(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 39.Arimoto-Kobayashi S, Harada N, Tokunaga R, Odo J, Hayatsu H. Adsorption of mutagens to chlorophyllin–chitosan, an insoluble form of chlorophyllin. Mutat. Res. 1997;381:243–249. doi: 10.1016/s0027-5107(97)00188-7. [DOI] [PubMed] [Google Scholar]
- 40.Bedner E, Du L, Traganos F, Darzynkiewicz Z. Caffeine dissociates complexes between DNA and intercalating dyes: application for bleaching fluorochromestained cells for their subsequent restaining and analysis by laser scanning cytometry. Cytometry. 2001;43:38–45. [PubMed] [Google Scholar]