Abstract
There is evidence that major depressive disorder (MDD) is associated with increased peripheral markers of oxidative stress. To explore oxidation and antioxidant response in MDD, we assayed human dermal fibroblast cultures derived from skin biopsies of age-, race-, and sex-matched individuals in depressed and normal control groups (n=16 each group), cultured in glucose and galactose conditions, for relative protein carbonylation (a measure of oxidative stress), glutathione reductase (GR) expression, and total glutathione concentration. In control-group fibroblasts, galactose induced a significant increase from the glucose condition in both protein carbonylation and GR. The cells from the MDD group showed total protein carbonylation and GR expression in the glucose condition that was significantly higher than control cells in glucose and equivalent to controls in galactose. There was a small decrease in protein carbonylation in MDD cells from glucose to galactose and no significant change in GR. There was no difference in total glutathione among any of the groups. Increased protein carbonylation and GR expression, cellular responses to oxidative stress induced by galactose in control fibroblasts, are present in fibroblasts derived from MDD patients and are not explainable by reduced GR or total glutathione in the depressed patients. These studies support the role of oxidative stress in the pathophysiology of MDD. Further confirmation of these findings could lead to the development of novel antioxidant approaches for the treatment of depression.
Keywords: human dermal fibroblasts, oxidative stress, glutathione, glutathione reductase, protein carbonylation, major depression
Introduction
Major depressive disorder (MDD) is a chronic, highly disabling condition, and both short and longer-term treatment outcome is not optimal (Kessler et al., 2005; Schmidt et al., 2011; Shelton & Tomarken, 2001). Research has linked the pathophysiology of MDD to a wide range of molecular biomarkers in blood and other peripheral tissues, demonstrating the complexity of the disorder (Schmidt et al., 2011). One important finding is a reduction in certain signal transduction proteins in MDD, particularly protein kinases A (PKA) and C (PKC; Dwivedi et al. 2004; Perera et al., 2001; Perez et al., 2002; Shelton et al., 2009 A; Pandey et al., 1998; Pandey et al., 1997). Although the mechanism for alteration of these signal transduction proteins remains obscure, there is evidence for redox regulation (Humphries et al., 2005; Humphries et al., 2007; Giorgi et al., 2010), and a growing number of studies link MDD with an increased state of oxidative stress (Ng et al., 2008).
Reactive oxygen species (ROS) are formed in the human body through a variety of processes, including normal cell metabolism, especially the mitochondrial electron transport chain (Halliwell, 1991), as well as ultraviolet light (Heck et al., 2003) and heat stress (Cho et al., 2009). ROS are buffered through antioxidant defenses, mostly thiol compounds that act as substrates for oxidation, and oxidative damage to cells occurs when the balance between ROS produced and concentration of protective antioxidants favors ROS (Halliwell, 1991). Therefore, increasing levels of oxidation could be explained by disrupted function of a major antioxidant mechanism, such as the production and redox efficiency of glutathione.
Glutathione is the most abundant thiol compound in cells of all organs and plays an important protective role against oxidative stress in the brain (Dringen et al., 2000; Dringen, 2000; Gawryluk et al., 2011). Glutathione reductase (GR) is the enzyme responsible for recycling oxidized glutathione to the reduced, antioxidant form and has been shown to be up-regulated in response to oxidative stress (Gawryluk et al., 2011; Schuliga et al., 2002). For example, GR was seen to increase in activity with increasing oxidation in human keratinocytes and fibroblasts exposed to increasing concentrations of arsenic (Schuliga et al., 2002). The role of oxidative stress, glutathione, and GR has been implicated in the pathogenesis of both psychiatric and non-psychiatric diseases including Leber's Hereditary Optic Neuropathy (LHON; Floreani et al., 2005), Type 2 Diabetes mellitus (Calabrese et al., 2011), Huntington's Disease (del Hoyo et al., 2006), schizophrenia (Gysin et al., 2009; Mahadik & Mukherjee, 1996), bipolar disorder (BD; Andreazza et al., 2008; Yumru et al., 2009), autism and ADHD (Ng et al., 2008).
Although work has been done regarding oxidation and glutathione response in MDD using serum (Kodydková et al., 2009) and post-mortem brain tissue (Gawryluk et al., 2011), little has been tested using human cell culture. Dermal fibroblasts are not only easy to establish, control, reproduce, and eliminate outside factors, but they have proven to be a relevant study system for signal transduction pathways in MDD (Akin et al., 2004; Manier et al., 2000; Shelton et al., 1996). In addition, fibroblasts have been used to study glutathione, GR, and oxidative stress with success in other diseases such as Leber's hereditary optic neuropathy (LHON) (Floreani et al., 2005), Type 2 Diabetes (Calabrese et al., 2011), and schizophrenia (Gysin et al., 2009; Gysin et al., 2007).
Our study explores levels of protein oxidation, glutathione concentration, and GR expression in dermal fibroblasts extracted from skin biopsies of a population of patients diagnosed with MDD [n=16] compared to sex-, age-, and race-matched healthy controls [n=16] and grown in glucose and galactose conditions in order to identify differences in oxidative damage and antioxidant capacity associated with MDD in peripheral tissue.
Materials and Methods
Population
The Vanderbilt University Institutional review board (IRB) approved the study and written informed consent was obtained from all study participants before any procedures were conducted. The study population was composed of 16 human patients diagnosed with MDD and 16 age, race, and sex-matched healthy control participants (Table 1). Recruitment and diagnosis procedures for patients and controls have been described in previous publications (Shelton et al., 1996; Manier et al., 1996). In brief, all participants were assessed using the Structured Clinical Interview for DSM-IV (SCID; First et al., 2009). Exclusion criteria included other primary DSM-IV diagnosis (American Psychiatric Association, 1994), a history of bipolar disorder or non-mood psychotic disorder, or any medical condition that would preclude the biopsy (e.g., hemophilia). The groups included 8 males and 24 females, ages ranging from 20 to 53 years (Table 1).
Table 1. Study Population.
The age, race, sex, and current medical conditions and medications of study participants, both healthy controls and those diagnosed with MDDD. Controls from the left were matched for relative comparisons with members of the MDD group in the same row.
| Control | MDD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Race | Sex | Medical Conditions | Current Medications | Age | Race | Sex | **Medical Conditions | Current Medications |
| 36 | W | Male | - | - | 33 | W | Mala | Diabetes mellitus type 1 | Escitalopram 10 mg., Exenatide 20 mcg |
| 34 | AA | Male | - | - | 33 | AA | Male | - | Venlafaxine 75 mg. |
| 30 | W | Male | Gastroesopahgeal reflux disease | Omeprazole 40 mg. | 29 | W | Male | - | |
| 48 | W | Male | - | - | 46 | W | Male | - | - |
| 20 | W | Female | - | - | 22 | W | Female | - | Escitalopram 15 mg. |
| 27 | W | Female | - | Estradiol 24 mg. | 27 | W | Female | - | Amitriptyline 25 mg. |
| 22 | W | Female | - | - | 22 | W | Female | - | Fluoxetine 40 mg. |
| 51 | AA | Female | - | - | 51 | AA | Female | Gastroesophageal reflux disease | Escitalopram 30 mg. |
| 23 | AA | Female | - | - | 23 | AA | Female | - | - |
| 52 | W | Female | - | Alprazolam 1 mg. | 53 | W | Female | C4-C5 hemiated disc, hypertension | Escitalopram 20 mg. Ziprasidone 160 mg., Lisinopril 40 mg. |
| 44 | W | Female | Intersticial cystitis | Topiramate 100 mg. | 43 | W | Female | hypertension | Escitalopram 10 mg., Lisinopril 20 mg. Verapamil 240 mg. |
| 27 | AA | Female | Cervical dysplasia | 26 | AA | Female | |||
| 22 | W | Female | - | drospirenone/ethinyl estradiol | 23 | W | Female | - | Fluoxetine 10mg |
| 49 | W | Female | - | - | 52 | W | Female | - | Escitalopram 20 mg. Ziprasidone 20 mg. |
| 35 | W | Female | C5-C6 herniated disc, hypertension | Triamterine/Hydrochlorthiazide | 34 | W | Female | ||
| 40 | W | Female | - | - | 37 | W | Female | - | Escitalopram 10 mg. Topiramate 100 mg.. |
*Abbreviations: W, white; AA, African American
Non-psychiatric
Italicized medications are psychiatric medications
Collection and processing of skin biopsies and establishment of fibroblast cultures
Specimens for fibroblast cultures were obtained by skin punch biopsies (1×2 mm) taken from the upper arm on participants using the method previously described in detail (Shelton et al., 1996; Manier et al., 1996). Skin biopsies were placed in plain DMEM medium and processed the same day as previously described (Shelton et al., 1996; Manier et al., 1996). The medium was changed three times a week. In about 2-3 weeks, the fibroblasts divided and became confluent. All fibroblast samples obtained for this study were able to propagate. The fibroblasts were subcultured using 0.5% Trypsin-EDTA (Invitrogen) as previously published (Akin et al., 2004) and expanded for freezing in a liquid nitrogen cell repository.
All fibroblasts used for experiments were cultured between passages 5 and 10 in order to minimize any effect from exposure in vivo to factors such as hormones, neurotransmitters, cytokines, or drugs (Akin et al., 2004). All fibroblasts were taken from liquid nitrogen storage and maintained in Dulbecco's modified Eagle's medium™ (DMEM; Mediatech, Manassas, VA, USA) containing 25 mM glucose and 1 mM sodium pyruvate supplemented with 2mM L-glutamine (Mediatech), 10% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT, USA), and antibiotic/antimycotic solution (A/A; Invitrogen) at 37°C and 5% CO2. For exposure to galactose, cells were cultured in DMEM deprived of glucose (Mediatech) supplemented with 10mM galactose, 2mM L-glutamine, 10% FBS, 1mM, A/A, and sodium pyruvate (Sigma Aldrich). The cells from matching pairs of control and MDD subjects were cultured simultaneously under same conditions until reaching confluence, trypsinized, and counted using a Bright-Line Hemacytometer (Hausser Scientific, Horsham, PA). Cells, 0.5×106/plate were grown in two 60mm dishes (Sarstedt, Newton, NC, USA) in glucose-containing medium. After 24 hours, once fibroblasts adhered, the medium in one plate was changed into fresh glucose medium and the other to galactose medium. Cell growth and proliferation were checked and were not affected by the galactose condition in either group (data not shown). After another 24 hours, the cells were washed in 1X PBS twice on ice and collected with a cell scraper in cooled 1X PBS (Mediatech). The cell suspension was pelleted by centrifugation at 280g for 10 min at +4°C. Excess PBS was removed, and cells were resuspended in NP-40 lysis buffer (150 mM NaCl, 50mM Tris-Cl [pH 7.4], 5mM EDTA, 0.5% NP-40, 0.5% sodium deoxycholate) supplemented with PMSF, protease inhibitor cocktail (Sigma Aldrich) and phosphatase inhibitor cocktail (Sigma Aldrich). After 30 min of lysis on ice, cell lysate was cleared by centrifugation at 14,000Xg for 5 min at +4°C. Protein concentration w as determined using the Dc protein assay (Bio-Rad, Hercules, CA). Twenty μg of protein lysate was used immediately for determining protein carbonylation.
Protein carbonylation determined by OxyBlot™ Protein Detection kit
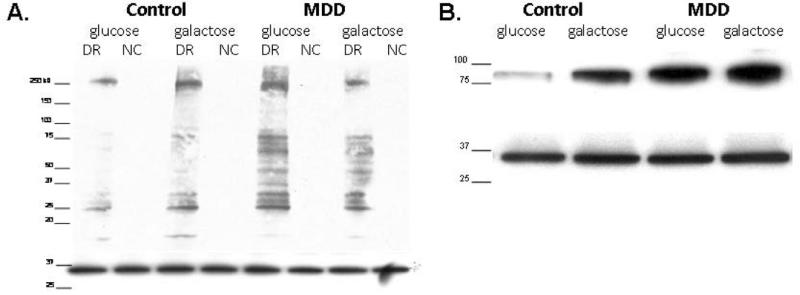
Protein carbonyl levels were measured using protocol found in the commercially available OxyBlot™ Protein Detection kit (Millipore, Billerica, MA, USA). For each sample, two reactions were prepared: derivatized and negative control. Carbonyl groups in the proteins were derivatized to 2,4-dinitrophenylhydrazine (DNPH). Equal loading was assessed by restaining PDVF membranes with an antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling, Danvers, MA, USA). All blots were analyzed and normalized by measuring the pixel density of bands appearing in the final blots using UN-SCAN-IT (Silk Scientific Corporation, Orem, UT, USA) as previously described (Shelton et al., 2009 B). Refer to Figure 1A for a representative blot.
Figure 1.
A) Representative DNPH blot. Western blot probed with DNPH antibody (top) and re-probed with GAPDH antibody (bottom). Blot shows one control-MDD matched pair, derivatized reaction (DR) and negative control (NC) for each sample with molecular weight marker; from left to right lanes read: control in glucose medium, control in galactose medium for 24 hours, MDD in glucose medium, and MDD in galactose medium for 24 hours. Directly below the DNPH blot is the GAPDH restain of the same PVDF membrane displaying equal protein loading. B) Representative GR blot. Western blot probed with Glutathione Reductase antibody (top) and re-probed with GAPDH antibody (bottom). Blot shows one control-MDD matched pair with molecular weight marker; from left to right lanes read: control in glucose medium, control in galactose medium, MDD in glucose medium, and MDD in galactose medium. Directly below the GR blot is the GAPDH restain of the same PVDF membrane displaying equal protein loading.
Measurements of Glutathione and Glutathione Reductase
Total glutathione levels were assayed using the published enzymatic method (Rahman, 2006) and normalized to total protein amount as determined by Dc protein assay (Bio-Rad Laboratories, Hercules, CA).
Glutathione Reductase (GR) protein expression was assayed by western blot using 20 μg total protein from the same total lysate used for DNPH derivitization, normalized to GAPDH as above, and again analyzed using UN-SCAN-IT. Membranes were stained according to manufacturer's recommendations with anti-GR antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and with anti-mouse IgG secondary antibody (Sigma). Refer to Figure 1B for example blot.
Statistical Analyses
In the 16 controls and 16 MDD, both protein carbonylation and GR were analyzed based on intensity of individual protein bands in the final western blot in fibroblasts from all participants relative to the corresponding pair. Data was then grouped into control and MDD plus the condition, glucose or galactose, for final comparison and analysis. Differences in treatments and controls were analyzed using means, standard error, and between-group comparisons conducted with Student's t-tests. Statistical significance was defined as p ≤ 0.05.
Results
Increased protein carbonylation in cultured MDD human fibroblasts
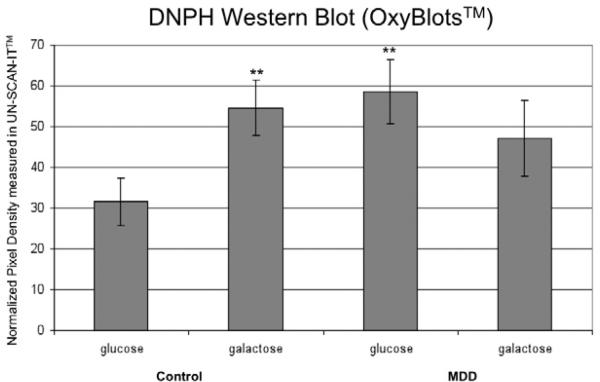
Protein carbonylation was assessed in cultured fibroblasts established from control and MDD participants. The majority of DNPH detectable protein products, as illustrated in Figure 1A, span molecular weight species from ~ 20 to ~ 250kDa. Control cells grown in glucose showed a very low number of DNPH protein products – markedly different from MDD cells under the same condition. Quantification of western blots showed that the sum of DNPH products was 1.85 times higher in MDD than in controls in the glucose condition (p≤0.01) (Figure 2).
Figure 2. Protein Carbonylation.
Normalized pixel density measurements from stained western blots for each experimental condition. Condition and control or subject are as labeled.
Bars represent standard error.
* = significantly different from the control in glucose condition, p-value ≤ 0.05
** = significantly different from the control in glucose condition, p-value ≤ 0.01
Cultured fibroblasts were exposed to galactose in the place of glucose in the culture medium in order to induce oxidative stress (Marroquin et al., 2007). Under these conditions, we observed significant increase in protein carbonylation in control fibroblasts from the glucose condition; quantification of western blots showed that the sum of DNPH products was 1.73 times higher in the galactose than in glucose-containing medium (p≤0.01) (Figure 2). We saw an opposite response in MDD cells when exposed to galactose. There was a small but significant decrease in protein carbonylation in the MDD cells when grown in the galactose condition compared to the glucose; the sum DNPH products in the galactose condition was 0.81 times the measured in the glucose condition (p≤0.01). These results indicate that the cells from participants with MDD have increased protein carbonylation relative to controls in the glucose condition, and under conditions of increased oxidative stress, the MDD fibroblasts do not respond with an increase in protein oxidation as do control fibroblasts.
Increased Glutathione Reductase protein expression in MDD fibroblasts without changes in total glutathione
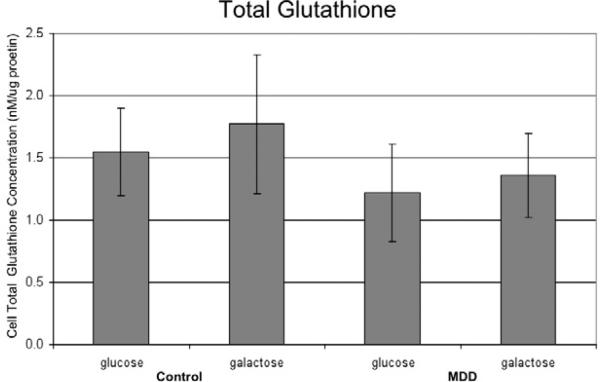
To determine if increased protein carbonylation in MDD fibroblasts could be the result of diminished antioxidant capacity, we measured total glutathione concentration in the cells. We found no significant difference in the level of total glutathione among any of the groups (Figure 3). This result indicates that total the level of glutathione in fibroblasts is not significantly different between control and MDD, and levels are not significantly altered by the presence of galactose in either group.
Figure 3. Total Glutathione.
Total glutathione concentrations in total cell lysate measured using the enzymatic method and expressed in nM/μg protein. Graph labeling same as in Figure 2. No significant difference was found between any of the groups.
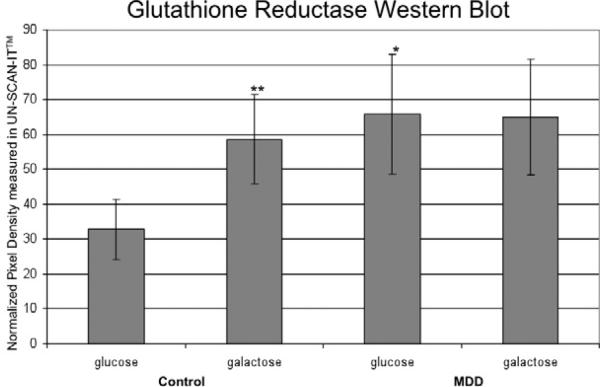
Levels of reduced glutathione in the cytosol are maintained by GR19. Therefore we compared the protein levels of this enzyme in control and MDD fibroblasts under glucose and galactose condtions via western blot (Figure 1B). In control cells, we found GR levels to increase in the galactose condition; controls in the galactose condition showed 1.78 times more GR than controls in glucose condition (p≤0.01) (Figure 4). MDD cells in the glucose condition showed 1.99 times the level of GR of control cells in glucose (p≤0.05) (Figure 4). There was no significant difference in GR between MDD cells in the glucose and galactose conditions; our data shows GR in MDD cells in the galactose condition to be 0.96 times the glucose condition (p=0.81). These results indicate that GR expression is up-regulated in MDD in the glucose condition as it is in control cells when stressed with galactose with no accompanying changes in glutathione.
Figure 4. GR Expression.
Normalized pixel density measurements from western blots stained with anti-GR antibody for each experimental condition. Graph labeling same as Figure 2.
Discussion
To our knowledge, this is the first clear demonstration of increased oxidative stress in actual living tissue culture derived from persons with MDD. Under normal glucose culture conditions the amount of total protein carbonylation, a measure of protein modification in response to oxidative stress (Stadtman, 1993), was significantly higher in MDD fibroblasts than in those derived from control subjects (Figure 2) with no significant difference in total glutathione concentration (Figure 3). In addition, our study shows GR expression to be significantly higher in MDD cells when compared to controls in the glucose condition (Figure 4), another cellular response to the presence of oxidation (Gawryluk et al., 2011; Schuliga et al., 2002). Other studies have confirmed the involvement of oxidative stress in MDD, but our findings regarding glutathione and glutathione reductase differ from some previously published work. In a 2011 study, Gawryluk et. al found reduced levels of total glutathione and no change in GR in MDD in post-mortem, pre-frontal cortex brain tissue (Gawryluk 2011). Furthermore, depleted levels of glutathione and reduced GR activity have been found in a rodent depression model rodents (Zafir 2009). In the current study, total glutathione levels did not differ between groups. As well, GR showed an increase in the glucose condition, consistent with compensation for increased oxidative stress. Therefore, our findings do not support a role for either glutathione or GR in increased oxidative stress in MDD.
Removing glucose and adding galactose to cell culture medium is a commonly used practice to induce oxidative stress. Galactose metabolism doubles O2 consumption and increases cell susceptibility to mitochondrial toxicants by shifting metabolism toward oxidative phosphorylation (OXPHOS; Marroquin et al., 2007). In our study, the galactose condition increased protein carbonylation in control fibroblasts but did not have the same effect in MDD cells. In fact, there was a slight decrease in overall protein carbonylation in MDD in the galactose condition when compared to the glucose (Figure 2). Furthermore, while GR was increased in the galactose condition in control cells, it was not significantly changed in MDD (Figure 4). We speculate that GR is maximally expressed in the glucose condition in MDD, and when galactose is introduced, there is no further increase in oxidative stress. Further exploration of the OXPHOS pathway in MDD is necessary for a more clear understanding of these results and could lead to important implications of the role of oxidative stress in the disease.
The current study was done in peripheral tissue in culture, and the results may or may not be relevant for brain (Teyssier et al., 2011). However, human fibroblasts have been used as a study system for neurological disorders, and they share most signal transduction enzymes and receptors with neurons (Manier et al., 2000). Assuming the connection can be made, the results of this study have important implications for the role of the oxidant anti-oxidant balance in MDD. The human brain metabolizes 20% of the oxygen in the body while only accounting for 2% of the body weight (Dringen et al., 2000; Dringen, 2000). As a result, ROS are generated at high rates, and glutathione is the organ's main protective antioxidant (Dringen et al., 2000; Dringen, 2000).
A future research objective is to identify MDD-relevant proteins that may be consistent targets for modification in the presence of increased oxidative stress. Protein carbonylation most often occurs at specific amino acids proline, threonine, lysine, and arginine (Stadtman, 1993) and can significantly alter or destroy protein activity and function (Oliver, 1987). GR has been shown to be susceptible to oxidation in the presence of oxidative stress (Tbatabaie & Floyd, 1994), and it is important to note that although the results of our study indicate that that GR production is up-regulated in response to oxidative stress in MDD, they do not conclude that the enzymatic function of GR is unaltered. Further studies are needed to make definitive conclusions on the functionality of glutathione and GR in the presence of oxidative stress in MDD fibroblasts as well as the activity of other enzymes such as glutathione s-transferase and glutathione peroxidase.
Other proteins shown to be susceptible to oxidation include PKA and PKC (Humphries et al., 2005; Humphries et al., 2007; Giorgi et al., 2010), important components of signaling pathways that previous studies found to be reduced in MDD fibroblasts (Dwivedi et al. 2004; Perera et al., 2001; Perez et al., 2002; Shelton et al., 2009 A; Pandey et al., 1998; Pandey et al., 1997). The catalytic subunit of PKA is inactivated by thiol oxidation of cysteine 199 in the activation loop of the kinase, particularly in conditions of high oxidation stress (Humphries et al., 2007). PKA typically exists as an inactive heterotetramer, with two regulatory and two catalytic subunits (Beave et al., 1974 A; Beavo et al., 1974 B; Meinkoth et al., 1993). It is activated by the binding of two cyclic AMP molecules to each regulatory subunit, which releases the catalytic subunits leading to phosphorylation of protein substrates, including cyclic AMP response element binding protein (CREB; Meinkoth et al., 1993; Taylor et al., 1992). This PKA activation continues until cyclic AMP is hydrolyzed by phosphodiesterases (Jeon et al., 2005) or the catalytic subunit is inactivated by protein kinase inhibitor (Fantozzi et al., 1994; Wen et al., 1994). The catalytic PKA subunit is susceptible to oxidation primarily in the free, activated state in the cytosol (Humphries et al., 2002).
Looking at MDD through the perspective of oxidative damage and glutathione response could lead to a new approach to treatment. Previous studies have shown an increase in oxidative stress markers and decrease in antioxidants in serum to be correlated with the severity of depression (Yanik et al., 2004; Cumurcu et al., 2009; Sarandol et al., 2007). This correlation poses the possibility of reversing the oxidation by increasing natural antioxidant systems or administering antioxidant drugs to decrease the symptoms of depression. A number of stimuli can induce or inhibit glutathione (Soltaninassab et al., 2000), and some selective serotonin reuptake inhibitors (SSRIs) have been shown to behave as antioxidants (Khanzode et al., 2003; Zafir et al., 2009). Standard SSRIs fluoxetine and citalopram, for example, were shown to reverse effects of oxidative damage in serum of MDD subjects (Khanzode et al., 2003). In addition, administering fluoxetine as well as imipramine and venlafaxine, two other SSRIs, to rats with depressed phenotypes was shown to significantly reverse the biochemical effects of oxidation, including protein carbonylation, in brain tissue (Zafir et al., 2009).
Although the increased oxidation has been observed in a number of studies, using biological markers of oxidative stress primarily in blood plasma or serum samples (reviewed by Ng et al., 2008), demonstration of increased oxidation in our cell culture model opens a new area of research. In this cell culture model we will be able to assess the underlying mechanism and specific pathway involved and screen for potential remedies. Further exploration of carbonylated proteins in MDD may lead to the identification of diagnostic (pre-symptomatic) biomarker for oxidative damage and establishment of an effective antioxidant therapy, offering novel approaches to anti-depressant treatment.
Acknowledgments
This work was supported by grant from the Brain & Behavior Research Foundation (NARSAD) and by Vanderbilt Kennedy Center for Research on Human Development.
This research was supported by the Vanderbilt Psychiatric Genotype/Phenotype Project and the Vanderbilt Institute for Clinical and Translational Research (1-UL1-RR024975 NCRR/NIH). We would also like to thank the Vanderbilt Kennedy Center and Drs. Beth Ann McLaughlin and Karoly Mirnics for their assistance in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric A . Diagnostic and Statistical Manual of Mental Disorders. fourth edition Washington, D.C.: 1994. [Google Scholar]
- Akin D, Manier DH, Sanders-Bush E, Shelton RC. Decreased serotonin 5-HT2A receptor-stimulated phosphoinositide signaling in fibroblasts from melancholic depressed patients. Neuropsychopharmacology. 2004;29:2081–7. doi: 10.1038/sj.npp.1300505. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kauer-Sant'anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, Yatham LN. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–44. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Bechtel PJ, Krebs EG. Activation of protein kinase by physiological concentrations of cyclic AMP. Proc Natl Acad Sci U S A. 1974a;71:3580–3. doi: 10.1073/pnas.71.9.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA, Bechtel PJ, Krebs EG. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974b;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Leso V, Trovato A, Ventimiglia B, Cavallaro M, Scuto M, Rizza S, Zanoli L, Neri S, Castellino P. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Cho S, Shin MH, Kim YK, Seo JE, Lee YM, Park CH, Chung JH. Effects of infrared radiation and heat on human skin aging in vivo. J Investig Dermatol Symp Proc. 2009;14:15–9. doi: 10.1038/jidsymp.2009.7. [DOI] [PubMed] [Google Scholar]
- Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63:639–45. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- del Hoyo P, García-Redondo A, de Bustos F, Molina JA, Sayed Y, Alonso-Navarro H, Caballero L, Arenas J, Jiménez-Jiménez FJ. Oxidative stress in skin fibroblasts cultures of patients with Huntington's disease. Neurochem Res. 2006;31:1103–9. doi: 10.1007/s11064-006-9110-2. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–71. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–6. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Shukla PK, Lyons J, Faludi G, Palkovits M, Sarosi A, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry. 2004;55:234–43. doi: 10.1016/j.biopsych.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Fantozzi DA, Harootunian AT, Wen W, Taylor SS, Feramisco JR, Tsien RY, Meinkoth JL. Thermostable inhibitor of cAMP-dependent protein kinase enhances the rate of export of the kinase catalytic subunit from the nucleus. J Biol Chem. 1994;269:2676–86. [PubMed] [Google Scholar]
- Floreani M, Napoli E, Martinuzzi A, Pantano G, De Riva V, Trevisan R, Bisetto E, Valente L, Carelli V, Dabbeni-Sala F. Antioxidant defences in cybrids harboring mtDNA mutations associated with Leber's hereditary optic neuropathy. FEBS J. 2005;272:1124–35. doi: 10.1111/j.1742-4658.2004.04542.x. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–30. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Agnoletto C, Baldini C, Bononi A, Bonora M, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A, Zavan B, Pinton P. Redox control of protein kinase C: cell- and disease-specific aspects. Antioxid Redox Signal. 2010;13:1051–85. doi: 10.1089/ars.2009.2825. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuénod M, Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–6. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R, Riederer IM, Cuénod M, Do KQ, Riederer BM. Skin fibroblast model to study an impaired glutathione synthesis: consequences of a genetic polymorphism on the proteome. Brain Res Bull. 2009;79:46–52. doi: 10.1016/j.brainresbull.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Heck DE, Vetrano AM, Mariano TM, Laskin JD. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem. 2003;278:22432–6. doi: 10.1074/jbc.C300048200. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Deal MS, Taylor SS. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J Biol Chem. 2005;280:2750–8. doi: 10.1074/jbc.M410242200. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Juliano C, Taylor SS. Regulation of cAMP-dependent protein kinase activity by glutathionylation. J Biol Chem. 2002;277:43505–11. doi: 10.1074/jbc.M207088200. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Pennypacker JK, Taylor SS. Redox regulation of cAMP-dependent protein kinase signaling: kinase versus phosphatase inactivation. J Biol Chem. 2007;282:22072–9. doi: 10.1074/jbc.M702582200. [DOI] [PubMed] [Google Scholar]
- Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, Cho JM. Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol Life Sci. 2005;62:1198–220. doi: 10.1007/s00018-005-4533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8:365–70. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- Kodydková J, Vávrová L, Zeman M, Jirák R, Macásek J, Stanková B, Tvrzická E, Zák A. Antioxidative enzymes and increased oxidative stress in depressive women. Clin Biochem. 2009;42:1368–74. doi: 10.1016/j.clinbiochem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Yanik M, Erel O, Kati M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatrica. 2004:200–203. doi: 10.1111/j.0924-2708.2004.00090.x. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophr Res. 1996;19:1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Manier DH, Eiring A, Shelton RC, Sulser F. Beta-adrenoceptor-linked protein kinase A (PKA) activity in human fibroblasts from normal subjects and from patients with major depression. Neuropsychopharmacology. 1996;15:555–61. doi: 10.1016/S0893-133X(96)00099-1. [DOI] [PubMed] [Google Scholar]
- Manier DH, Shelton RC, Ellis TC, Peterson CS, Eiring A, Sulser F. Human fibroblasts as a relevant model to study signal transduction in affective disorders. J Affect Disord. 2000;61:51–8. doi: 10.1016/s0165-0327(99)00190-1. [DOI] [PubMed] [Google Scholar]
- Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97:539–47. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition, with Psychotic Screen. (SCID-I/P W/Psy SCREEN) Biometrics Research, New York Psyhiatric Institute; New York, NY: 2009. [Google Scholar]
- Meinkoth JL, Alberts AS, Went W, Fantozzi D, Taylor SS, Hagiwara M, Montminy M, Feramisco JR. Signal transduction through the cAMP-dependent protein kinase. Mol Cell Biochem. 1993;127-128:179–86. doi: 10.1007/BF01076769. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Oliver CN. Inactivation of enzymes and oxidative modification of proteins by stimulated neutrophils. Arch Biochem Biophys. 1987;253:62–72. doi: 10.1016/0003-9861(87)90637-0. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Kumari R, Janicak PG. Protein kinase C in platelets of depressed patients. Biol Psychiatry. 1998;44:909–11. doi: 10.1016/s0006-3223(97)00535-0. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA. Protein kinase C in the postmortem brain of teenage suicide victims. Neurosci Lett. 1997;228:111–4. doi: 10.1016/s0304-3940(97)00378-9. [DOI] [PubMed] [Google Scholar]
- Perera T, Lisanby SH, Sackheim HA. Protein kinase a in major depression: the link between hypothalamic-pituitary-adrenal axis hyperactivity and neurogenesis. CNS Spectr. 2001;6:565–8. 571–2. doi: 10.1017/s1092852900002108. [DOI] [PubMed] [Google Scholar]
- Perez J, Tardito D, Racagni G, Smeraldi E, Zanardi R. cAMP signaling pathway in depressed patients with psychotic features. Mol Psychiatry. 2002;7:208–12. doi: 10.1038/sj.mp.4000969. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–65. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–94. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuliga M, Chouchane S, Snow ET. Upregulation of glutathione-related genes and enzyme activities in cultured human cells by sublethal concentrations of inorganic arsenic. Toxicol Sci. 2002;70:183–92. doi: 10.1093/toxsci/70.2.183. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Hal Manier D, Lewis DA. Protein kinases A and C in post-mortem prefrontal cortex from persons with major depression and normal controls. Int J Neuropsychopharmacol. 2009;12:1223–32. doi: 10.1017/S1461145709000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Mainer DH, Sulser F. cAMP-dependent protein kinase activity in major depression. Am J Psychiatry. 1996;153:1037–42. doi: 10.1176/ajp.153.8.1037. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–15. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatr Serv. 2001;52:1469–78. doi: 10.1176/appi.ps.52.11.1469. [DOI] [PubMed] [Google Scholar]
- Soltaninassab SR, Sekhar KR, Meredith MJ, Freeman ML. Multi-faceted regulation of gamma-glutamylcysteine synthetase. J Cell Physiol. 2000;182:163–70. doi: 10.1002/(SICI)1097-4652(200002)182:2<163::AID-JCP4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- Tabatabaie T, Floyd RA. Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Arch Biochem Biophys. 1994;314:112–9. doi: 10.1006/abbi.1994.1418. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Knighton DR, Zheng J, Ten Eyck LF, Sowadski JM. cAMP-dependent protein kinase and the protein kinase family. Faraday Discuss. 1992:143–52. doi: 10.1039/fd9929300143. [DOI] [PubMed] [Google Scholar]
- Teyssier JR, Ragot S, Chauvet-Gélinier JC, Trojak B, Bonin B. Expression of oxidative stress-response genes is not activated in the prefrontal cortex of patients with depressive disorder. Psychiatry Res. 2011;186:244–7. doi: 10.1016/j.psychres.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Wen W, Harootunian AT, Adams SR, Feramisco J, Tsien RY, Meinkoth JL, Taylor SS. Heat-stable inhibitors of cAMP-dependent protein kinase carry a nuclear export signal. J Biol Chem. 1994;269:32214–20. [PubMed] [Google Scholar]
- Yumru M, Savas HA, Kalenderoglu A, Bulut M, Celik H, Erel O. Oxidative imbalance in bipolar disorder subtypes: a comparative study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1070–4. doi: 10.1016/j.pnpbp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Zafir A, Ara A, Banu N. Invivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:220–8. doi: 10.1016/j.pnpbp.2008.11.010. [DOI] [PubMed] [Google Scholar]