SUMMARY
A major cause of hyperglycemia in diabetic patients is inappropriate hepatic gluconeogenesis. PGC-1α is a master regulator of gluconeogenesis, and its activity is controlled by various post-translational modifications. A small portion of glucose metabolizes through the hexosamine biosynthetic pathway, which leads to O-linked β-N-acetylglucosamine (O-GlcNAc) modification of cytoplasmic and nuclear proteins. Using a proteomic approach, we identified a broad variety of proteins associated with O-GlcNAc transferase (OGT), among which host cell factor C1 (HCF-1) is highly abundant. HCF-1 recruits OGT to O-GlcNAcylate PGC-1α and O-GlcNAcylation facilitates the binding of the deubiquitinase BAP1, thus protecting PGC-1α from degradation and promoting gluconeogenesis. Glucose availability modulates gluconeogenesis through the regulation of PGC-1α O-GlcNAcylation and stability by the OGT/HCF1 complex. Hepatic knockdown of OGT and HCF-1 improves glucose homeostasis in diabetic mice. These findings define the OGT/HCF-1 complex as a glucose sensor and key regulator of gluconeogenesis, shedding light on new strategies for treating diabetes.
INTRODUCTION
Glucose flux through the hexosamine biosynthetic pathway (HBP) leads to the post-translational modification of cytoplasmic, nuclear and mitochondrial proteins by O-linked β-N-acetylglucosamine (O-GlcNAc), termed O-GlcNAcylation (Hart et al., 2007; Torres and Hart, 1984). O-GlcNAcylation is emerging as a key regulator of diverse cellular processes, such as signal transduction, transcriptional regulation, and proteasomal degradation (Yang et al., 2002; Zachara and Hart, 2004, 2006). Aberrant O-GlcNAcylation has been linked to a plethora of human diseases, including diabetes, cancer, and neuronal diseases (Lazarus et al., 2009; Ngoh et al., 2010; Slawson et al., 2010).
UDP-GlcNAc, the donor substrate, and O-GlcNAcylation levels within the cell are modulated by the availability of glucose, fatty acids, amino acids and nucleotides. Therefore, O-GlcNAcylation is proposed as a nutrient sensor and metabolic regulator (Butkinaree et al., 2010; Hanover et al., 2012). Overexpression of the rate-limiting enzyme of the HBP, glutamine fructose-6-phosphate transaminase (GFAT), leads to peripheral insulin resistance (Hebert et al., 1996; Veerababu et al., 2000). Transgenic mice overexpressing O-GlcNAc transferase (OGT) in skeletal muscle and fat exhibit elevated circulating insulin levels and insulin resistance (McClain et al., 2002). Key components of insulin signaling can be O-GlcNAcylated (Whelan et al., 2010), and O-GlcNAcylation has been shown to be a negative regulator of insulin signaling (Yang et al., 2008). Hyperglycemia is also associated with O-GlcNAcylation of transcription factors and cofactors. O-GlcNAcylation of FOXO1, CRTC2 and PGC-1α modulate expression of gluconeogenic genes (Dentin et al., 2008; Housley et al., 2008; Housley et al., 2009; Kuo et al., 2008). Chronic increases in the levels of PDX1 and NeuroD1 O-GlcNAcylation may contribute to hyperinsulinemia in Type 2 diabetes (Andrali et al., 2007; Gao et al., 2003). Thus, O-GlcNAc signaling is believed to serve as a nexus between nutrient flux, insulin resistance and diabetes.
Unlike the presence of hundreds of protein kinases and phosphatases in the human genome, O-GlcNAc cycling is modified only by one O-GlcNAc transferase (OGT) and one O-GlcNAcase (OGA). It is largely unknown how the substrate specificity of OGT and OGA is achieved. It has been proposed that OGT recognizes substrates primarily though the tandem tetratricopeptide repeats (TPRs). Indeed, different OGT isoforms with various lengths in TPRs show different substrate specificities. Another possibility is that OGT forms dynamic holoenzymes with various protein partners that facilitate substrate recognition (Butkinaree et al., 2010; Chikanishi et al., 2010). For instance, interaction of OGT and p38MAPK activates O-GlcNAcylation of neurofilament H (Cheung and Hart, 2008). We hypothesize that OGT recognizes its substrates by association with a hierarchy of highly conserved adaptor proteins, analogous to the ubiquitin system in which dual E1 enzymes interact with dozens of E2 and hundreds of E3 ligases for substrate recognition.
In this study, we show OGT and its interacting protein host cell factor C1 (HCF-1) cooperatively up-regulate gluconeogenesis by stabilizing PGC-1α. O-GlcNAcylation of PGC-1α decreases its ubiquitination by recruiting the de-ubiquitinase BAP1. Glucose homeostasis in diabetic animals can be improved by knocking down OGT and HCF-1 in liver. Hence, OGT and HCF-1 may serve as potential targets for treating diabetes.
RESULTS
Proteome-wide analysis identifies HCF-1 and PGC-1α as OGT-interacting proteins
To identify candidate adaptor proteins that mediate substrate recognition of OGT on a proteome-wide level, we performed tandem affinity purification of OGT-binding proteins in HEK 293T cells (Figure S1A). Purified proteins were then identified by Multidimensional Protein Identification Technology (MudPIT) (Washburn et al., 2001), and subjected to pathway analysis using MetaCore software (Figure 1A). 853 putative OGT-interacting proteins involved in a wide range of biological processes were identified (Supplementary Table 1). Strikingly, a large majority of these proteins participate in signal transduction and metabolism, supporting the notion that O-GlcNAcylation is a sensor and regulator of metabolic homeostasis.
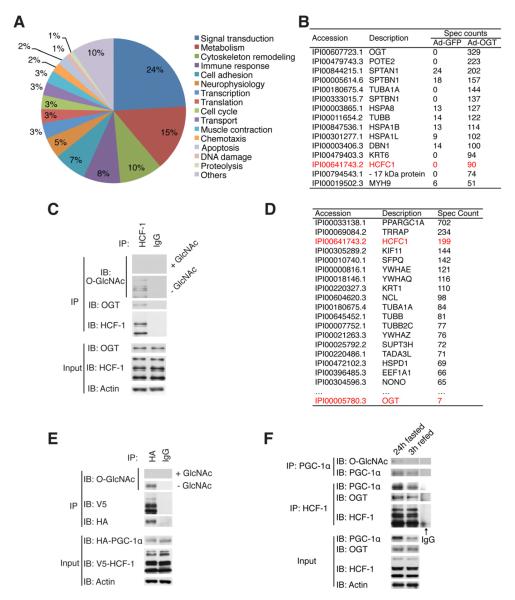
Figure 1. Identification of OGT/HCF-1/PGC-1α protein complex.
(A) Pie chart of functional distribution of identified putative OGT-binding proteins. (B) The top of the list of putative OGT-binding proteins. Spectrum counts in GFP and OGT samples were shown. (C) The interaction between OGT and HCF-1 was confirmed by co-immunoprecipitation of endogenous proteins from hepatoma FAO cells. 0.1 M of free GlcNAc was added to control the specificity of O-GlcNAc antibody. (D) List of top proteins identified in PGC-1α complex purification. (E) HEK 293T cells were co-transfected with HA-tagged PGC-1α and V5-tagged HCF-1, and their interaction and PGC-1α O-GlcNAcylation were determined. (F) Interactions of HCF-1 with OGT and PGC-1α (middle) and the O-GlcNAcylation of PGC-1α (top) in the livers from 24h fasted and 3h refed mice are shown.
One of the highly abundant proteins co-purified with OGT is HCF-1 (Figure 1B). HCF-1 is an essential transcriptional cofactor, and has been shown to be required for herpes virus gene expression, cell cycle regulation, and stem cell growth (Dejosez et al., 2010; Julien and Herr, 2003; Peng et al., 2010). Consistent with previous reports (Capotosti et al., 2011; Daou et al., 2011), co-immunoprecipitation analysis of endogenous proteins confirms the interaction of OGT and HCF-1, and shows that HCF-1 is highly O-GlcNAcylated (Figure 1C, S1B). HCF-1 exists as a complex of N- and C-terminal fragments that result from proteolytic processing of the precursor protein (Wilson et al., 1993). We found that both the N- and C-terminal fragments of HCF-1 interact with OGT and are O-GlcNAcylated (Figure S1C, D).
PGC-1α is a key transcriptional coactivator that regulates mitochondrial biogenesis and hepatic gluconeogenesis (Fernandez-Marcos and Auwerx, 2011). PGC-1α harbors a HCF-1 binding motif (HBM) (Lin et al., 2002). A proteomic approach to identify PGC-1α-interacting proteins shows that HCF-1 and OGT are among them (Figure 1D, Figure S1E). Co-immunoprecipitation analysis confirms the interaction between PGC-1α and HCF-1 overexpressed in HEK293T cells and demonstrates O-GlcNAcylation of PGC-1α (Figure 1E). It is known that PGC-1α is strongly induced in liver during fasting (Puigserver et al., 2003). To test the endogenous interactions between these proteins, HCF-1 was immunoprecipitated from the liver of mice after 24-hour fasting. The results show that both OGT and PGC-1α are enriched in the HCF-1 immunoprecipitate (Figure 1F). After 3-hour refeeding, the PGC-1α level is largely reduced, which is coincident with a decreased interaction between HCF-1 and OGT (Figure 1F). O-GlcNAcylation of PGC-1α is also evident in fasted mouse liver (Figure 1F). Taken together, these observations suggest that OGT/HCF-1 complex formation and O-GlcNAcylation of PGC-1α are sensitive to food availability.
OGT and HCF-1 cooperatively regulate gluconeogenesis
Next, we examined the functional consequence of the OGT/HCF-1 complex on PGC-1α. The presence of HCF-1 increases the transcriptional activity of PGC-1α, which is further augmented by OGT (Figure 2A). It has been known that PGC-1α co-activates FOXO1 via the insulin response sequence (IRS) to induce expression of the gluconeogenic genes such as glucose-6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase (Pck1) (Puigserver et al., 2003). We show that OGT and HCF-1 synergistically up-regulate FOXO1 activity on both the G6pc promoter (Figure 2B) and the synthetic IRS DNA binding element (Figure S2A, B). Such synergistic effect on FOXO1 activity is abolished by PGC-1α knockdown (Figure 2C). Overexpression of either the N- or C-terminal fragment of HCF-1 has an effect similar to the precursor HCF-1 (Figure S2C, D). We further show that the synergistic induction of G6pc promoter activity by OGT and HCF-1 is more dependent on FOXO1 than other factors such as CREB/CRTC2, despite the fact that OGT can promote CRTC2 activity (Dentin et al., 2008) (Figure S2E-G). PGC-1α also co-activates HNF4α to regulate gluconeogenic genes (Yoon et al., 2001). We found that OGT and HCF-1 co-expression can promote the activity of the HNF4α responsive reporter as well (Figure S2H, I), suggesting a general effect of OGT/HCF-1 on PGC-1α regulation.
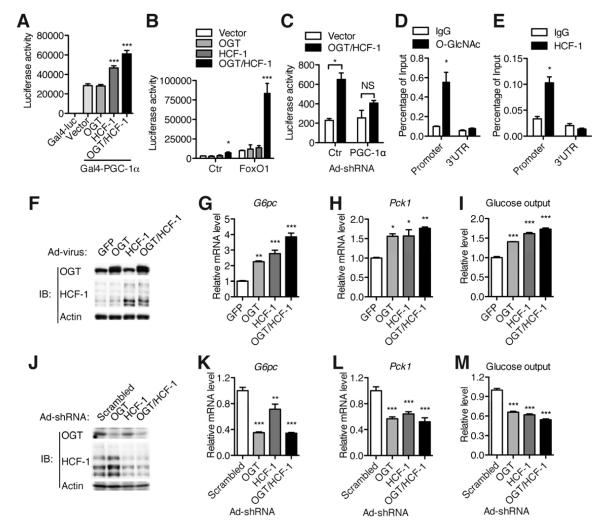
Figure 2. OGT and HCF-1 cooperatively up-regulate gluconeogenesis.
(A, B) Luciferase assays were performed in HepG2 cells; luciferase activity was normalized to co-transfected β-Gal activity. (A) Gal4-PGC-1α transactivation assay (n = 3), (B) G6pc-luciferase assay in the absence or presence of FOXO1 (n = 3). (C) G6pc-luciferase assay following infection of FOXO1-transfected FAO cells with adenovirus encoding PGC-1α shRNA or scrambled control shRNA (n = 3). (D, E) Chromatin immunoprecipitation assays of primary hepatocytes using O-GlcANc (D) and HCF-1 (E) antibodies (n = 3). IgG served as a negative control. Real time PCR was performed with primers flanking FOXO1/HNF4α binding region or 3′UTR (a negative control) of the G6pc gene. (F-M) FAO cells were infected with overexpression (F-I) or knockdown (J-M) adenoviruses as indicated, then protein expression (F, J), G6pc (G, K) and Pck1 (H, L) gene expression and glucose output (I, M) were determined (n = 3). All values represent mean ± SEM of data from three independent experiments. *, P < 0.05 **, P < 0.01; ***, P < 0.001 by ANOVA with a Bonferroni’s post hoc test compared with the vector control (A-B, G-I, K-M). *, P < 0.05 by student’s t-test (C-E).
Chromatin immunoprecipitation was performed to detect O-GlcNAc and HCF-1 at the promoter of the G6pc gene. Both O-GlcNAc and HCF-1 are highly enriched in the FOXO1/HNF4α binding region of the G6pc promoter (Figure 2D, E). To test the effects of OGT and HCF-1 on expression of endogenous gluconeogenic genes, adenoviruses encoding OGT and HCF-1 were used to infect hepatoma FAO cells (Figure 2F). Overexpression of either OGT or HCF-1 stimulates endogenous G6pc and Pck1 expression and co-expression of the two proteins has a cooperative effect (Figure 2G, H). By measuring glucose secretion to the culture medium, we also observed a cooperative effect of OGT and HCF-1 on glucose output (Figure 2I). On the other hand, knockdown of OGT and HCF-1 in FAO cells by adenoviruses encoding shRNAs (Figure 2J) decreases gluconeogenic gene expression (Figure 2K, L) and glucose production (Figure 2M). Treatment of FAO cells with PUGNAc, a potent OGA inhibitor that increases global O-GlcNAcylation levels, also significantly increases gluconeogenic gene expression and glucose output (Figure S2J, K). Collectively, these results indicate that the OGT/HCF-1 complex promotes glucose production in hepatocytes.
O-GlcNAcylation and HCF-1 interaction stabilize PGC-1α
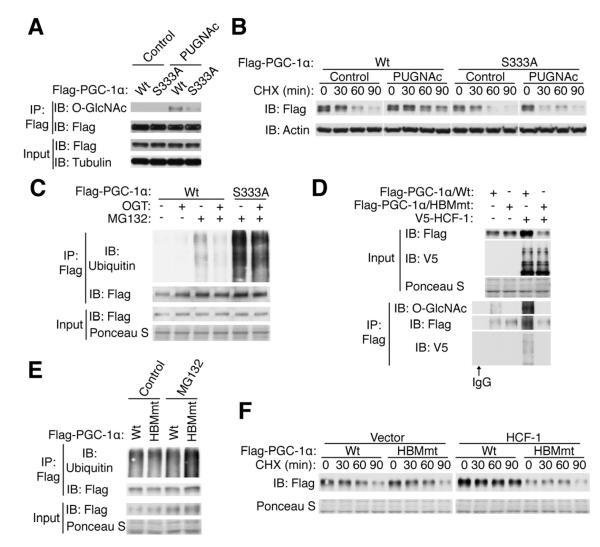
An O-GlcNAcylation site of PGC-1α (Ser 333) was previously identified, however, the functional role of this site was not fully determined (Housley et al., 2009). Here we show that PUGNAc treatment increases PGC-1α O-GlcNAcylation, and mutation of Ser 333 to Ala (S333A) substantially decreases PGC-1α O-GlcNAcylation (Figure 3A). Although the S333A mutation does not affect the transcriptional activity, phosphorylation or subcellular localization of PGC-1α (Figure S3A-C) (Roth et al., 2009), elevating the O-GlcNAc level by PUGNAc increases PGC-1α stability. In contrast, the S333A mutant shows slightly decreased stability and is refractory to PUGNAc treatment (Figure 3B, Figure S3D). PGC-1α can be targeted for ubiquitin-mediated proteolysis (Olson et al., 2008; Trausch-Azar et al., 2010). We found that OGT overexpression, which increases PGC-1α O-GlcNAcylation (Figure S3E), decreases ubiquitination of PGC-1α. While the S333A mutant has a dramatic increase in ubiquitination compared with the wildtype (Figure 3C), OGT overexpression still slightly decreases ubiquitination of the S333A mutant, suggesting the existence of other potential O-GlcNAcylation sites (also see Figure 3A). Moreover, the S333A mutant has less ability to induce G6pc and Pck1 transcription compared with the wildtype (Figure S3F). Taken together, these data indicate that O-GlcNAcylation protects PGC-1α from proteasomal degradation.
Figure 3. O-GlcNAcylation and HCF-1 stabilize PGC1α.
HEK 293T cells were transfected and treated as indicated. (A) O-GlcNAcylation of Wt and S333A mutant PGC-1α. (B) Stability of PGC-1α was determined by treatment of cycloheximide (CHX), an inhibitor of protein synthesis. Relative half-lives are shown in Figure S3D. (C) Ubiquitination of Wt and S333A PGC-1α treated with proteasome inhibitor MG132. (D) O-GlcNAcylation and HCF-1 interaction of PGC-1αHBMmt protein. (E) Ubiquitination of PGC-1αHBMmt protein. (F) Stability of Wt and HBMmt PGC-1α in the presence or absence of HCF-1. Relative half-lives are shown in Figure S3G.
Because HCF-1 and OGT have a cooperative effect on PGC-1α activity and gluconeogenic gene expression (Figure 2), we hypothesized that HCF-1 is an adaptor protein for targeting OGT to PGC-1α. To test this, a Y385A mutation in the HCF-1 binding motif (HBM) of PGC-1α (PGC-1αHBMmt) was generated. The PGC-1αHBMmt protein shows no change in transcriptional activity, phosphorylation or localization (Figure S3A-C). Indeed, PGC-1αHBMmt does not bind to HCF-1, and strikingly it is devoid of O-GlcNAcylation (Figure 3D). Compared with the wildtype, PGC-1αHBMmt exhibits increased ubiquitination in the presence of MG132 (Figure 3E). HCF-1 overexpression increases the stability of wildtype PGC-1α, but has no effect on the PGC-1αHBMmt protein (Figure 3F, Figure S3G). These data argue that HCF-1 binding promotes O-GlcNAcylation and stability of PGC-1α.
OGT/HCF-1 complex mediates regulation of gluconeogenesis by glucose
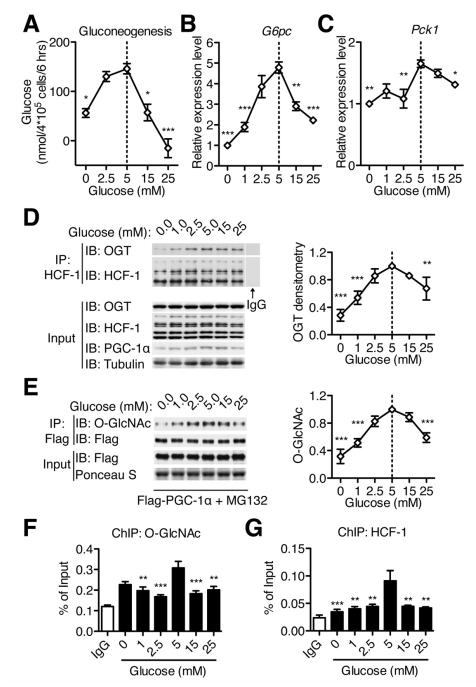
Hormones such as insulin, glucagon and glucocorticoids tightly control glucose homeostasis. Although insulin, forskolin (a cAMP agonist mimicking the action of glucagon), and dexamethasome (a synthetic glucocorticoid) are potent regulators of gluconeogenic gene expression (Figure S4A, B), our results show that they do not modulate the association between OGT and HCF-1 (Figure S4C). It has been known that glucose itself regulates gluconeogenesis (Rigoulet et al., 1987; Sacca et al., 1978; Seglen, 1974). However, the underlying mechanism is still obscure. Consistent with previous in vivo studies (Rigoulet et al., 1987), we show that glucose treatment can directly modulate gluconeogenesis in primary hepatocytes, with a maximal effect observed at 5 mM glucose (Figure 4A). Consistent with this observation, the expression of G6pc and Pck1 is also modulated by glucose availability, with the peak level at 5 mM glucose (Figure 4B, C). Since O-GlcNAc is a glucose sensor, we tested whether OGT/HCF-1 complex formation responds to glucose availability. Interestingly, OGT/HCF-1 interaction peaks under euglycemic conditions, although their expression levels are relatively constant at different glucose concentrations (Figure 4D). The levels of PGC-1α proteins also peak at 5 mM glucose (Figure 4D). Following treatment with MG132, which blocks protein degradation and normalizes PGC-1α levels, we observed that PGC-1α O-GlcNAcylation reaches its maximum level at 5 mM glucose (Figure 4E). Chromatin immunoprecipitation assays also show that O-GlcNAc and HCF-1 are most abundant at the G6pc promoter upon 5 mM glucose treatment (Figure 4F, G, and Figure SD, E). These findings suggest that the OGT/HCF-1 complex senses glucose availability, thereby regulating PGC-1α stability and glucose production.
Figure 4. Glucose availability regulates gluconeogenesis and OGT/HCF-1 complex.
(A) Primary hepatocytes from 24h fasted mice were treated with gluconeogenic medium containing 13C pyruvate, lactate and different levels of glucose. Newly synthesized glucose was calculated based on the distribution of glucose M+1 isotopomers (n = 3). (B-C) FAO cells were treated with medium containing different levels of glucose for 6 hours, and G6pc (B) and Pck1 (C) transcripts were determined by real time PCR (n = 3). (D) Immunoprecipitation of endogenous HCF-1 from protein lysates of FAO cells treated with different levels of glucose. Relative recovery of OGT in HCF-1 immunoprecipitation is shown at right. (E) FAO cells were infected with Flag/HA-PGC-1α adenovirus. MG132 was added during the glucose treatment to equalize PGC-1α expression. O-GlcNAcylation levels of PGC-1α were determined, with the densitometric values shown at right (n = 3). (F, G) Primary hepatocytes were treated with different levels of glucose for 6 h, and the association of O-GlcNAc (F) and HCF-1 (G) on the G6pc promoter was determined by chromatin immunoprecipitation (n = 3). All values represent mean ± SEM of data from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by ANOVA with a Bonferroni’s post hoc test compared with 5 mM glucose.
Knockdown of OGT and HCF-1 decreases gluconeogenesis
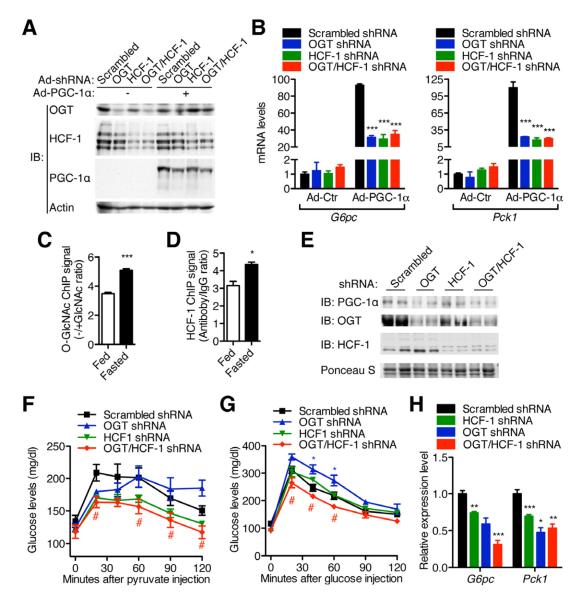
To determine whether OGT/HCF-1 regulates gluconeogenesis in a PGC-1α-dependent manner, primary hepatocytes from fed mice were infected with adenoviruses encoding shRNA targeting OGT and/or HCF-1. The efficacy of OGT and HCF-1 knockdown was validated by examining their mRNA and protein levels (Figure 5A, Figure S5A). Along with the observation that PGC-1α expression is not detectable in fed mice (Figure 5A), OGT and/or HCF-1 knockdown has no effect on expression of gluconeogenic genes G6pc and Pck1 (Figure 5B). However, OGT and/or HCF-1 knockdown can significantly suppress the expression of gluconeogenic genes induced by PGC-1α overexpression (Figure 5B). As PGC-1α expression is induced in primary hepatocytes from overnight-fasted mice, OGT and/or HCF-1 knockdown significantly suppresses G6pc expression, which is abrogated by PGC-1α knockdown (Figure S5B). Compared with fed mice, we observed the increased occupancies of O-GlcNAc and HCF-1 at the FOXO1/HNF4α binding region of the hepatic G6pc promoter in fasted mice (Figure 5C, D). Taken together, these data show that the modulation of gluconeogenic gene expression by OGT and HCF-1 is dependent on PGC-1α.
Figure 5. OGT/HCF-1 regulates gluconeogenesis in a PGC-1α dependent way.
(A-B) Primary hepatocytes from fed C57BL/6 mice (n = 3) were infected with adenoviruses indicated. (A) Protein and (B) mRNA levels were determined by Western blotting and real time PCR, respectively. (C-D) Chromatin immunoprecipitation of (C) O-GlcNAc and (D) HCF-1 on the G6pc promoter from primary hepatocytes of fed or fasted mice. Antibody plus 0.1M free GlcNAc (C) and rabbit IgG (D) were served as negative controls, respectively. The relative signal to the control is shown. (E-H) 12-week-old C57BL/6 male mice (n = 4-6) were injected with shRNA adenoviruses through tail vein. Protein expression (E, day 3), pyruvate tolerance test (F, day 3), glucose tolerance test (G, day 5) and gluconeogenic gene expression (H, day 7) were determined. *, #, P < 0.05; **, P < 0.01; ***, P < 0.001 by ANOVA with a Bonferroni’s post hoc test compared with scrambled shRNA. All values represent mean ± SEM.
To examine the physiological function of OGT/HCF-1, adenoviruses encoding shRNAs were delivered to the liver of C57BL/6 mice by systemic injection to knockdown OGT and/or HCF-1 (Figure 5E). No significant difference in the expression of inflammatory markers TNFα and INFγ in the liver was observed (Figure S5C). Pyruvate tolerance tests were performed to assess gluconeogenesis (Figure 5F). Compared with the scrambled shRNA control, OGT shRNA mice show an initial decrease in blood glucose, but maintain high levels after 60 min (Figure 5F), which may be due to their glucose intolerance (Figure 5G). OGT/HCF-1 double knockdown decreases glucose production from pyruvate to the extent similar to HCF-1 knockdown, suggesting that the effect of OGT on gluconeogenesis is dependent on HCF-1 (Figure 5F). Although OGT shRNA mice are glucose intolerant, and HCF-1 shRNA mice behave similarly to the control, mice with OGT/HCF-1 double knockdown show improved glucose tolerance (Figure 5G). No significant difference in serum insulin was observed between the different groups (Figure S5D). Basal glucose levels are lower in the double knockdown mice, further indicating a gluconeogenic defect (Figure S5E). However, there is no statistically significant difference in insulin sensitivity between those mice (Figure S5F). In further support of our hypothesis, OGT and HCF-1 knockdown decreases PGC-1α protein levels (Figure 5E) and expression of gluconeogenic genes in the liver (Figure 5H). Consistent with the role of PGC-1α as a master regulator of genes involved fatty acid oxidation and mitochondrial biogenesis, we observed decreased expression of genes in fatty acid oxidation and mitochondrial respiration in the livers following OGT/HCF-1 double knockdown (Figure S5G). Taken together, these results strongly argue that OGT/HCF-1 knockdown in C57BL/6 mice can suppress gluconeogenesis through the down-regulation of PGC-1α.
O-GlcNAcylation recruits BAP1 to deubiquitinate PGC-1α
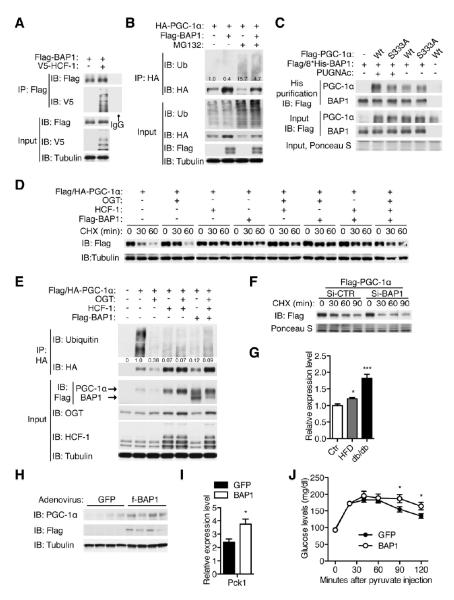
BAP1, a deubiquitinating enzyme, has been shown to interact with HCF-1 (Figure 6A), yet its targets are largely unknown (Machida et al., 2009; Misaghi et al., 2009). We tested the hypothesis that BAP1 is responsible for PGC-1α deubiquitination. Overexpression of BAP1 does not increase global ubiquitination, but substantially increases the level of PGC-1α and decreases PGC-1α ubiquitination as normalized to the protein level (Figure 6B). This suggests that regulation of PGC-1α by BAP1 is target-specific. PUGNAc treatment promotes the interaction between PGC-1α and BAP1, while it has no effect on the S333A mutant (Figure 6C), suggesting that O-GlcNAcylation of PGC-1α facilitates BAP1 binding. Overexpression of OGT, HCF-1 and BAP1 individually or in combination enhances PGC-1α stability (Figure 6D). OGT, HCF-1, and BAP1 stabilize PGC-1α by blocking its ubiquitination (Figure 6E). Conversely, knockdown of BAP1 in cells (Figure S6A) decreases the stability of PGC-1α (Figure 6F). In agreement with others’ observation (Daou et al., 2011), we noticed that HCF-1 can stabilize OGT (Figure 6E, Figure S6B). BAP1 also increases OGT protein levels, suggesting that BAP1 is a potential deubiquitinase for OGT as well (Figure S6C). These data indicate that HCF-1 recruits OGT to O-GlcNAcylate PGC-1α, which facilitates the recruitment of BAP1 to deubiquitinate PGC-1α and OGT, thereby sustaining levels of PGC-1α in the cell.
Figure 6. O-GlcNAcylation and HCF-1 recruits BAP1 to deubiquitinate PGC-1α.
(A-F) HEK 293T cells were transfected and treated as indicated. (A) BAP1 interacts with HCF-1. (B) Ubiquitination of PGC-1α was determined with/without BAP1 overexpression. Relative ubiquitination normalized to the protein level is shown. (C) Interaction between PGC-1α and BAP1 was examined by checking recovery of PGC-1α from metal affinity purification of His-tagged BAP1. (D) Stability and (E) ubiquitination of PGC-1α are shown when co-transfected with OGT, HCF-1 and BAP1. Relative ubiquitination normalized to protein level is shown. (F) Stability of PGC-1α in response to BAP1 knockdown. Knockdown efficiency is shown in Figure S6A. (G) BAP1 gene expression in HFD and db/db mice compared to age-matched wildtype mice revealed by real time PCR (n = 4-5). (H-J) 12-week-old C57BL/6 male mice (n = 4) were injected with GFP or f-BAP1 adenoviruses through tail vein. Protein expression (H, day 5), Pck1 gene expression (I, day 5) and pyruvate tolerance test (J, day 3) were determined. (G, I) *, P < 0.05; ***, P < 0.001 by two-tailed t-test. (J) *, P < 0.05 by ANOVA with a Bonferroni’s post hoc test compared with the control. All values represent mean ± SEM.
Interestingly, Bap1 gene expression is significantly increased in high fat diet (HFD) and db/db mice (Figure 6G). To further determine the physiological role of BAP1 in gluconeogenesis, Flag-tagged BAP1 was overexpressed in mouse liver by adenoviral infection. In line with our observation that BAP1 is a deubiquitinase for PGC-1α in vitro, hepatic BAP1 overexpression increases the protein level of PGC-1α (Figure 6H). Furthermore, BAP1-overexpressing mice show increased expression of Pck1 transcripts (Figure 6I) and increased gluconeogenesis as seen in the pyruvate tolerance test (Figure 6J). These data demonstrate that BAP1 deubiquitinase stabilizes PGC-1α and promotes gluconeogenesis in vivo.
Knockdown of OGT and HCF-1 improves glucose homeostasis in diabetic mice
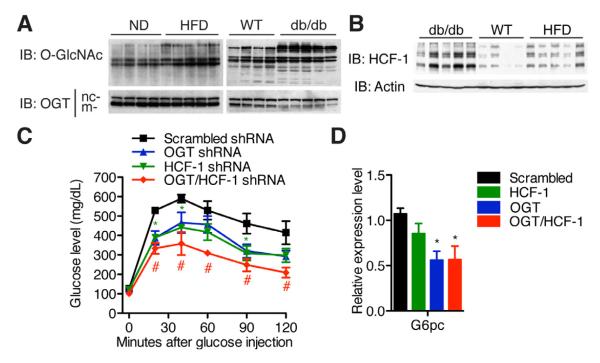
Insulin resistance and diabetes are associated with increased O-GlcNAc modification (Cooksey and McClain, 2002; Hart et al., 2011). We observed an increase in OGT expression and global O-GlcNAcylation in the liver of HFD mice (Figure 7A, and Figure S7A, C). Although hepatic OGT expression is slightly decreased in genetically diabetic db/db mice, global O-GlcNAcylation is increased, suggesting a possible increase in OGT activity (Figure 7A, and Figure S7B, D). Interestingly, HCF-1 protein levels are also significantly elevated in both HFD and db/db mice (Figure 7B, Figure S7E). To evaluate whether OGT/HCF-1 knockdown ameliorates diabetes, shRNA adenoviruses were administered to db/db mice. No significant differences in expression of inflammatory genes were observed between the different groups (Figure S7F). Individual knockdown of OGT or HCF-1 improves glucose homeostasis, and double knockdown further normalizes blood glucose, as shown by the glucose tolerance test (Figure 7C). Insulin sensitivity is relatively unchanged (Figure S7G). The salutary effects of OGT/HCF-1 knockdown in db/db mice can be attributed, at least in part, to the suppression of gluconeogenesis through the downregulation of G6pc expression (Figure 7D). These results suggest that the OGT/HCF-1 complex may contribute to the pathogenesis of diabetes by upregulating gluconeogenesis in vivo.
Figure 7. Hepatic knockdown of OGT and HCF-1 improves glucose homeostasis in diabetic mice.
(A) Global O-GlcNAcylation level and OGT expression and (B) HCF-1 protein expression in livers of age-matched C57Bl/6 mice on normal diet (WT and ND), on high fat diet (HFD) and db/db mice (n = 4-5). nc- and m-refer to nucleocytoplasmic and mitochondrial forms of OGT, respectively. Densitometric analysis is shown in Figure S7 C-E. (C, D) 12-week-old db/db male mice (n = 4-6) were infected with shRNA adenoviruses. (C) Glucose tolerance test (day 3). (D) G6pc mRNA expression in liver (day 7). *, #, P < 0.05; **, P < 0.01 by ANOVA with a Bonferroni’s post hoc test compared with scrambled shRNA. All values represent mean ± SEM.
DISCUSSION
PGC-1α is a critical metabolic node subjected to both transcriptional and post-translational regulation (Fernandez-Marcos and Auwerx, 2011). The expression of PGC-1α can be stimulated by exercise, cold and fasting through Ca2+, p38MAPK, AMPK, and PKA pathways (Fernandez-Marcos and Auwerx, 2011). The activity of PGC-1α is fine-tuned by phosphorylation, acetylation, and methylation. PGC-1α has also been shown to be O-GlcNAcylated (Housley et al., 2009), and PGC-1α acts to target OGT to FOXO1 and FOXO3, resulting in their increased O-GlcNAcylation and transcriptional activity. Nevertheless, the role of O-GlcNAcylation on PGC-1α remains elusive. In this study, we show that O-GlcNAcylation of PGC-1α can stabilize PGC-1α by inhibiting its ubiquitination. PGC-1α can be regulated by ubiquitin-dependent proteasomal degradation (Olson et al., 2008; Trausch-Azar et al., 2010). Skp1/Cullin/F-box-cell division control 4 (SCFCdc4) was identified as the E3 ubiquitin ligase for PGC-1α. Here, we identify BAP1, a member of ubiquitin C-terminal hydrolases, as the PGC-1α deubiquitinase. This suggests that a dynamic balance of ubiquitination and deubiquitination is a key mechanism controlling cellular PGC-1α levels. Along with the findings by Housley et al., it is conceivable that O-GlcNAcylation of PGC-1α and FOXO transcription factors may have sequential and concerted action in promoting gluconeogenesis.
Interplay between O-GlcNAcylation and phosphorylation has been well documented (Zeidan and Hart, 2010). These two modifications can either competitively occupy single or proximal sites, or coexist at different sites to cooperatively regulate protein functions. The co-purification of protein kinase(s) and phosphatase(s) with OGT and OGA also suggests an intrinsic relationship between O-GlcNAcylation and phosphorylation (Slawson et al., 2008; Wells et al., 2004). However, a central question yet to be answered is whether and how O-GlcNAcylation interplays with other post-translational modifications. An important study by the Lefebvre group reveals that O-GlcNAcylation can positively regulate global protein ubiquitination (Guinez et al., 2008). Here we report that O-GlcNAcylation of PGC-1α does not affect phosphorylation, but antagonizes ubiquitination of PGC-1α by recruiting the deubiquitinase BAP1 (Figure 6). This is the first evidence for a direct crosstalk between O-GlcNAcylation and ubiquitination on a single protein. Previous studies show that O-GlcNAcylation can modulate proteasome function (Guinez et al., 2008; Zhang et al., 2003). However, this is unlikely to account for the stabilization of PGC-1α by OGT because 1) the S333A mutation, which bypasses the effects of O-GlcNAcylation on the proteasome, increases PGC-1α ubiquitination and decreases its stability, 2) HCF-1 and BAP1 have no effect on proteasome function or global ubiquitination (Figure 6B and data not shown), but can decrease PGC-1α ubiquitination and increase its stability.
HCF-1 has been identified in complex with OGT in different contexts (Fujiki et al., 2009; Mazars et al., 2010; Wysocka et al., 2003). Recent studies show that O-GlcNAcylation of HCF-1 is required for its proteolytic maturation, and OGT itself is suggested as the protease that catalyzes HCF-1 cleavage (Capotosti et al., 2011; Daou et al., 2011). Approximately 50% of nuclear OGT is associated with HCF-1, yet the targets and functions of this abundant nuclear complex are poorly understood (Daou et al., 2011). HCF-1 is known as a scaffold protein for multiple histone-modifying enzymes (Kristie et al., 2010). We also identified that a variety of enzymes that catalyze diverse post-translational modifications are associated with OGT and/or HCF-1 (Supplementary Table 1 and data not shown). Drosophila OGT is one of the polycomb group genes involved in epigenetic silencing (Gambetta et al., 2009) and, remarkably, histones are modified by O-GlcNAc (Sakabe et al., 2010). Therefore, we propose that HCF-1, as an adaptor protein, facilitates the recognition of OGT substrates involved in epigenetic regulation.
Metabolism is tightly controlled by hormonal and nutritional signals. Glucose effectiveness refers to the ability of high glucose per se to suppress endogenous glucose production, and it has an important role in glucose homeostasis (Tonelli et al., 2005). Here we describe a mechanism whereby glucose determines its own production, which is through regulation of OGT/HCF-1 complex formation and subsequent O-GlcNAcylation and stabilization of PGC-1α. Compared to no glucose, low levels of glucose stimulate gluconeogenesis; on the other hand, hyperglycemia inhibits gluconeogenesis (Rigoulet et al., 1987; Seglen, 1974). Consistent with this phenomenon, we found that OGT/HCF-1 interaction, O-GlcNAcylation and expression of PGC-1α, enrichment of O-GlcNAc and HCF-1 on the G6pc promoter, and expression of gluconeogenic genes all peak under euglycemic conditions. This indicates a vital role for the OGT/HCF-1/PGC-1α pathway in maintaining normal glucose levels and mediating glucose effectiveness. It has been known that loss of glucose effectiveness contributes to type 2 diabetes (Mevorach et al., 1998). Our finding that expression of HCF-1 and BAP1 is upregulated in diabetic mice strongly suggests that the hyperactivation of the OGT/HCF-1/PGC-1α pathway diminishes glucose effectiveness. Hence, pharmacological inhibition of this pathway represents a potential strategy for preventing hyperglycemia and treating type 2 diabetes.
EXPERIMENTAL PROCEDURES
Cell culture
HEK 293T cells were cultured in DMEM with 10% fetal bovine serum (FBS). HepG2 and FAO hepatoma cells were cultured in RPMI with 10% FBS. Primary hepatocytes were isolated by Yale Liver Center Core Facility and plated in DMEM with 10% FBS, 2 mM Sodium Pyruvate, 1 M Dexamethasome, 0.1 M Insulin on Collagen I coated plates. HEK 293T and HepG2 cells were transfected with FuGENE HD (Roche). FAO and primary hepatocytes were infected with adenovirus in medium containing 0.5% BSA. Treatment with different glucose was performed with no glucose DMEM medium plus 10% dialyzed FBS and various concentrations of glucose for 6 h. PUGNAc (10 M, 16 h), MG132 (20 M, 4 h), Cycloheximide (100 g/ml) were treated as indicated.
Plasmids and adenovirus preparation
pAdTrack, 3*IRS-luciferase, flag-PGC-1α and Gal4-PGC-1α plasmids were from Addgene. pAdTrack-Flag/HA-OGT/OGA and pAdTrack-Flag-HCF-1-Myc plasmids were constructed by subcloning of PCR products. Point mutants of PGC-1α were generated with the QuikChange XL II sitedirected mutagenesis kit (Stratagene). OGT shRNA plasmids were purchased from Sigma. Overexpression or shRNA adenovirus constructs were established using AdEasy system (Luo et al., 2007). Adenoviruses were amplified in HEK 293 cells and purified using the kit from Virapur.
Tandem purification and MudPIT
FH-OGT and FH-PGC-1α protein complexes were isolated from cell extracts by immunoprecipitation with α-Flag antibody followed by α-HA antibody according to established procedures with certain modifications (Nakatani and Ogryzko, 2003). For the MudPIT analysis, the precipitated protein was resuspended and subjected to digestion with trypsin. The resulted protein digest was pressure-loaded onto a Kasil-fritted fused silica capillary column (250-μm i.d.) packed with 3 cm 5-μm Partisphere strong cation exchange resins (Whatman, Clifton, NJ) and 3 cm 5-μm Aqua C18 resins (Phenomenex, Ventura, CA). After desalting, this sample-loaded back-end column was then connected to a 100-μm i.d. capillary column with a 5-μm pulled tip packed with 12 cm 3-μm Aqua C18 material through a zero-dead-volume union (UpChurch Scientific, Oak Harbor, WA), and the entire three phase column was placed inline with an Agilent 1200 quaternary HPLC (Agilent, Palo Alto, CA) and analyzed using a modified 11-step separation described previously. As peptides were eluted from the microcapillary column, they were electrosprayed directly into a hybrid LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific, San Jose, CA) with the application of a distal 2.5 kV spray voltage. A cycle of one FT full-scan mass spectrum (400-1400 m/z, 60000 resolution) followed by 8 data-dependent LTQ MS/MS spectra at a 35% normalized collision energy was repeated continuously throughout each step of the multidimensional separation. Application of mass spectrometer scan functions and HPLC solvent gradients was controlled by the Xcalibur data system (Thermo Fisher Scientific, San Jose, CA). MS/MS spectra were processed and searched with the ProLucid algorithm (Xu et al., 2006) against the EBI human IPI database (ftp://ftp.ebi.ac.uk/pub/databases/IPI/, version 3.30), that was concatenated to a decoy database in which the sequence for each entry in the original database was reversed. ProLuCID results were assembled and filtered using the DTASelect (version 2.0) program (Cociorva et al., 2007; Tabb et al., 2002). The MudPIT data from FH-OGT, FH-PGC-1α, and EGFP control was compared. Proteins were designated as putative interacting proteins if they were identified only in FH-OGT or FH-PGC-1α samples but not in the EGFP control, or the spectral count of each protein co-purified with FH-OGT or FH-PGC-1α was at least four times larger than that in the EGFP control.
Antibodies, immunoprecipitation and western blotting
Anti-OGT, anti-Flag, anti-HA antibodies were from Sigma. Anti-O-GlcNAc (RL2), anti-V5 antibodies were from Abcam. Anti-HCF-1 antibody was from Bethyl Laboratories. Anti-Ubiquitin antibody was from Santa Cruz Biotechnology. Anti-PGC-1α (4C1.3) antibody was from EMD chemicals. Tissue and cell proteins were lysed in 1% Nonidet P-40, 50 mM Tris·HCl, 0.1 mM EDTA, 150 mM NaCl, proteinase inhibitors and protein phosphatase inhibitors. For immunoprecipitation, whole-cell lysates were mixed with various antibodies as specified in text and precipitated by Protein A/G agarose beads (Santa Cruz). Equal amounts of whole lysates or immunoprecipitation samples were electrophoresed on 8% SDS-PAGE gels and transferred to PVDF membrane. Primary antibodies were incubated at 4 oC for overnight. Western blotting was visualized by peroxidase conjugated secondary antibodies and ECL chemiluminescent substrate.
Transcriptional activity assay
HepG2 and FAO cells were transfected with expression plasmids, luciferase reporters and β-galactosidase. Cells were lysed, luciferase and β-galactosidase enzyme activities were measured using kits from Promega. Relative luciferase activity was determined by normalizing to β-galactosidase activity.
Chromatin immunoprecipitation
Primary hepatocytes were fixed with formaldehyde, lysed in buffer containing 50 mM Tris·HCl PH8.0, 10 mM EDTA and 1% SDS, and then sonicated. Soluble chromatin was diluted, and then immunoprecipitated with anti-O-GlcNAc (plus 1M free GlcNAc as a negative control), anti-HCF-1 (H12) antiserum or IgG. DNA was extracted using Chelex-100 and subjected to real time PCR using primers flanking the FOXO1/HNF4α binding region of G6pc promoter. The 3′UTR of G6pc was used as the negative control (Nelson et al., 2006).
Real time PCR
Total RNA was extracted from cells or mouse liver using TRIzol reagent (Invitrogen). Complementary DNA was synthesized from total RNA with Superscript III enzyme and random hexamer primers (Invitrogen). cDNAs were amplified with SYBR Green I Master and the LightCycler 480 Real time PCR system (Roche). All data were normalized to the expression of 18S or 36B4. Primer sequences are available from the authors on request.
Glucose output assay
Medium of primary hepaocytes or Fao cells was replaced with 1ml of glucose-free DMEM, supplemented with 20mM sodium lactate and 2mM sodium pyruvate and various glucose levels when indicated. After a 6-h incubation, medium was collected and the glucose concentration was measured using glucose oxidation kit (Sigma). The readings were then normalized to the total protein content. To measure gluconeogenesis, primary hepatocytes were treated with gluconeogenic medium containing 13C pyruvate, lactate and different levels of glucose. Newly synthesized glucose was calculated based on the distribution of glucose M+1 isotopomers.
Animal studies
C57Bl/6 mice were purchased from NCI/NIH, and db/db mice (Stock No. 000697) were purchased from Jackson Laboratory. Recombinant adenovirus (6 × 108 plaque forming units (pfu) to C57Bl/6 mice, and 2 × 109 pfu to db/db mice) was delivered by systemic tail-vein injection to mice. 3-7 days after viral infection, mice were subjected to pyruvate, glucose and insulin tolerance tests. 16 h fasted mice were injected intraperitoneally with sodium pyruvate (2 g/kg body weight) or glucose (1.5 g/kg body weight); 6 h fasted mice were injected with insulin (0.75 U/kg body weight to C57Bl/6 mice, 2.5 U/kg body weight to db/db mice). Blood glucose was measured from tail-vein blood collected at the designated times using Nova Max Glucometer.
Statistical analyses
Results are shown as mean ± SEM. The comparisons between two groups were carried out using two-tailed unpaired Student’s t-test. For multiple-group comparisons, one-way ANOVAs were used to test for no differences among the group means. Post hoc comparisons were adjusted using Bonferroni corrections. Mixed-model ANOVA’s were used to analyze data from glucose and pyruvate tolerance tests. Covariance structure was optimized for each dataset using the Corrected Akaike Information Criterion and the Schwarz Bayesian Information Criterion. Bonferroni corrections were employed for post hoc comparisons (contrasts at each time period) when the global test was significant (p < 0.05). All statistical calculations were performed in SAS 9.3 (MIXED/GLIMMIX/MULTTEST procedures).
Supplementary Material
Highlights.
The OGT/HCF-1 complex targets, O-GlcNAcylates, and co-activates PGC-1α.
O-GlcNAcylation of PGC-1α facilitates the recruitment of deubiquitinase BAP1 to stabilize PGC-1α.
OGT/HCF-1 interaction and O-GlcNAcylation of PGC-1α are sensitive to food and glucose availability.
Hepatic knockdown of OGT and HCF-1 improves glucose homeostasis.
ACKNOWLEDGEMENT
We thank Marc Montminy for providing G6pc-luc plasmids, Winship Herr for HA tagged FL-, N-, and C-HCF-1 plasmids and HCF-1 (H12) antibody, Thomas Kristie for HCF-1/V5 and pU6-Si-HCF-1 plasmids, Yuichi Machida for 3*Flag/His8-BAP1 plasmid, Fredric Wondisford for pFA-CREB, pFA-CRTC2 plasmids, Terry Unterman for pEGFP-FOXO1 and pEGFP-FOXO1 (3A) plasmids, Pere Puigserver for Flag/HA-PGC-1α and PGC-1α shRNA adenovirus, and Sohail Malik for A*2.luc plasmid. We thank Peter Van Ness and Mark Trentalange for assistance in statistic analysis, and Colleen Feriod for critical reading of the manuscript. This work was supported by NIH R01-DK089098, P30-DK34989, P30-DK045735, P30-AG021342, American Diabetes Association Junior Faculty Award, and Ellison Medical Foundation New Scholar Award to X.Y., NIH P01-DK057751 to A.M.B. and X.Y., the Brown-Coxe fellowship to H.B.R., the China Scholarship Council-Yale World Scholars fellowship to M.L., and NIH P41-RR011823 to J.R.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Andrali SS, Qian Q, Ozcan S. Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J Biol Chem. 2007;282:15589–15596. doi: 10.1074/jbc.M701762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkinaree C, Park K, Hart GW. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta. 2010;1800:96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosti F, Guernier S, Lammers F, Waridel P, Cai Y, Jin J, Conaway JW, Conaway RC, Herr W. O-GlcNAc Transferase Catalyzes Site-Specific Proteolysis of HCF-1. Cell. 2011;144:376–388. doi: 10.1016/j.cell.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283:13009–13020. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikanishi T, Fujiki R, Hashiba W, Sekine H, Yokoyama A, Kato S. Glucose-induced expression of MIP-1 genes requires O-GlcNAc transferase in monocytes. Biochem Biophys Res Commun. 2010;394:865–870. doi: 10.1016/j.bbrc.2010.02.167. [DOI] [PubMed] [Google Scholar]
- Cociorva D, D LT, Yates JR. Validation of tandem mass spectrometry database search results using DTASelect. Curr Protoc Bioinformatics. 2007;16:1–14. doi: 10.1002/0471250953.bi1304s16. [DOI] [PubMed] [Google Scholar]
- Cooksey RC, McClain DA. Transgenic mice overexpressing the rate-limiting enzyme for hexosamine synthesis in skeletal muscle or adipose tissue exhibit total body insulin resistance. Ann N Y Acad Sci. 2002;967:102–111. doi: 10.1111/j.1749-6632.2002.tb04268.x. [DOI] [PubMed] [Google Scholar]
- Daou S, Mashtalir N, Hammond-Martel I, Pak H, Yu H, Sui G, Vogel JL, Kristie TM, Affar EB. Crosstalk between O-GlcNAcylation and proteolytic cleavage regulates the host cell factor-1 maturation pathway. Proc Natl Acad Sci U S A. 2011;108:2747–2752. doi: 10.1073/pnas.1013822108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejosez M, Levine SS, Frampton GM, Whyte WA, Stratton SA, Barton MC, Gunaratne PH, Young RA, Zwaka TP. Ronin/Hcf-1 binds to a hyperconserved enhancer element and regulates genes involved in the growth of embryonic stem cells. Genes Dev. 2010;24:1479–1484. doi: 10.1101/gad.1935210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319:1402–1405. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1{alpha}, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki R, Chikanishi T, Hashiba W, Ito H, Takada I, Roeder RG, Kitagawa H, Kato S. GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature. 2009;459:455–459. doi: 10.1038/nature07954. [DOI] [PubMed] [Google Scholar]
- Gambetta MC, Oktaba K, Müller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009;325:93–96. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys. 2003;415:155–163. doi: 10.1016/s0003-9861(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Guinez C, Mir AM, Dehennaut V, Cacan R, Harduin-Lepers A, Michalski JC, Lefebvre T. Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J. 2008;22:2901–2911. doi: 10.1096/fj.07-102509. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross Talk Between O-GlcNAcylation and Phosphorylation: Roles in Signaling, Transcription, and Chronic Disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LF, Jr., Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW. A PGC-1{alpha}-O-GlcNAc Transferase Complex Regulates FoxO Transcription Factor Activity in Response to Glucose. J Biol Chem. 2009;284:5148–5157. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien E, Herr W. Proteolytic processing is necessary to separate and ensure proper cell growth and cytokinesis functions of HCF-1. EMBO J. 2003;22:2360–2369. doi: 10.1093/emboj/cdg242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie TM, Liang Y, Vogel JL. Control of alpha-herpesvirus IE gene expression by HCF-1 coupled chromatin modification activities. Biochim Biophys Acta. 2010;1799:257–265. doi: 10.1016/j.bbagrm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-GlcNAc modification of FoxO1 increases its transcriptional activity: a role in the glucotoxicity phenomenon? Biochimie. 2008;90:679–685. doi: 10.1016/j.biochi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Lazarus BD, Love DC, Hanover JA. O-GlcNAc cycling: implications for neurodegenerative disorders. Int J Biochem Cell Biol. 2009;41:2134–2146. doi: 10.1016/j.biocel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta ), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem. 2009;284:34179–34188. doi: 10.1074/jbc.M109.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazars R, Gonzalez-de-Peredo A, Cayrol C, Lavigne A-C, Vogel JL, Ortega N, Lacroix C, Gautier V, Huet G, Ray A, et al. The THAP-zinc finger protein THAP1 associates with coactivator HCF-1 and O-GlcNAc transferase: a link between DYT6 and DYT3 dystonias. J Biol Chem. 2010;285:13364–13371. doi: 10.1074/jbc.M109.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci U S A. 2002;99:10695–10699. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach M, Giacca A, Aharon Y, Hawkins M, Shamoon H, Rossetti L. Regulation of endogenous glucose production by glucose per se is impaired in type 2 diabetes mellitus. J Clin Invest. 1998;102:744–753. doi: 10.1172/JCI2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaghi S, Ottosen S, Izrael-Tomasevic A, Arnott D, Lamkanfi M, Lee J, Liu J, O’Rourke K, Dixit VM, Wilson AC. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol. 2009;29:2181–2192. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y, Ogryzko V. Immunoaffinity purification of mammalian protein complexes. Methods Enzymol. 2003;370:430–444. doi: 10.1016/S0076-6879(03)70037-8. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc. 2006;1:179–185. doi: 10.1038/nprot.2006.27. [DOI] [PubMed] [Google Scholar]
- Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson BL, Hock MB, Ekholm-Reed S, Wohlschlegel JA, Dev KK, Kralli A, Reed SI. SCFCdc4 acts antagonistically to the PGC-1alpha transcriptional coactivator by targeting it for ubiquitin-mediated proteolysis. Genes Dev. 2008;22:252–264. doi: 10.1101/gad.1624208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Nogueira ML, Vogel JL, Kristie TM. Transcriptional coactivator HCF-1 couples the histone chaperone Asf1b to HSV-1 DNA replication components. Proc Natl Acad Sci U S A. 2010;107:2461–2466. doi: 10.1073/pnas.0911128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Rigoulet M, Leverve XM, Plomp PJ, Meijer AJ. Stimulation by glucose of gluconeogenesis in hepatocytes isolated from starved rats. Biochem J. 1987;245:661–668. doi: 10.1042/bj2450661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RJ, Le AM, Zhang L, Kahn M, Samuel VT, Shulman GI, Bennett AM. MAPK phosphatase-1 facilitates the loss of oxidative myofibers associated with obesity in mice. J Clin Invest. 2009;119:3817–3829. doi: 10.1172/JCI39054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca L, Hendler R, Sherwin RS. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978;47:1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Sakabe K, Wang Z, Hart GW. {beta}-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci USA. 2010;107:19915–19920. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. Autoregulation of glycolysis, respiration, gluconeogenesis and glycogen synthesis in isolated parenchymal rat liver cells under aerobic and anaerobic conditions. Biochim Biophys Acta. 1974;338:317–336. [Google Scholar]
- Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell. 2008;19:4130–4140. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb DL, McDonald WH, Yates JR., 3rd DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res. 2002;1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli J, Kishore P, Lee D-E, Hawkins M. The regulation of glucose effectiveness: how glucose modulates its own production. Curr Opin Clin Nutr Metab Care. 2005;8:450–456. doi: 10.1097/01.mco.0000172588.47811.63. [DOI] [PubMed] [Google Scholar]
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- Trausch-Azar J, Leone TC, Kelly DP, Schwartz AL. Ubiquitin proteasome-dependent degradation of the transcriptional coactivator PGC-1{alpha} via the N-terminal pathway. J Biol Chem. 2010;285:40192–40200. doi: 10.1074/jbc.M110.131615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerababu G, Tang J, Hoffman RT, Daniels MC, Hebert LF, Jr., Crook ED, Cooksey RC, McClain DA. Overexpression of glutamine: fructose-6-phosphate amidotransferase in the liver of transgenic mice results in enhanced glycogen storage, hyperlipidemia, obesity, and impaired glucose tolerance. Diabetes. 2000;49:2070–2078. doi: 10.2337/diabetes.49.12.2070. [DOI] [PubMed] [Google Scholar]
- Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem. 2004;279:38466–38470. doi: 10.1074/jbc.M406481200. [DOI] [PubMed] [Google Scholar]
- Whelan SA, Dias WB, Thiruneelakantapillai L, Lane MD, Hart GW. Regulation of Insulin Receptor Substrate 1 (IRS-1)/AKT Kinase-mediated Insulin Signaling by O-Linked {beta}-N-Acetylglucosamine in 3T3-L1 Adipocytes. J Biol Chem. 2010;285:5204–5211. doi: 10.1074/jbc.M109.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AC, LaMarco K, Peterson MG, Herr W. The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell. 1993;74:115–125. doi: 10.1016/0092-8674(93)90299-6. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Venable JD, Park SK, Cociorva D, Lu B, Liao L, Wohlschlegel J, Hewel J, Yates JR. ProLuCID, a fast and sensitive tandem mass spectra-based protein identification program. Molecular & Cellular Proteomics. 2006;5:S174–S174. [Google Scholar]
- Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. O-GlcNAc modification: a nutritional sensor that modulates proteasome function. Trends Cell Biol. 2004;14:218–221. doi: 10.1016/j.tcb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci. 2010;123:13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–725. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.