Abstract
Higher levels of the inflammatory biomarker interleukin-6 (IL-6) correlate with poor clinical outcome in acute ischemic stroke (AIS). Minocycline (MC) is a known anti-inflammatory agent; thus, the effect of MC on IL-6 in the first 24 h of AIS was investigated to determine potential anti-inflammatory activity. The Minocycline to Improve Neurologic Outcome in Stroke (MINOS) study was a non-randomized dose-escalation (3.0–10.0 mg/kg) trial of IV MC for AIS within 6 h of onset. Plasma IL-6 samples were collected prior to MC treatment at 1, 24, and 72 h and compared to those collected in a separate observational study of blood biomarkers in AIS. IL-6 levels were measured by commercially available ELISA kits. The lower limit of detection for IL-6 was 1 pg/ml. Sixty MINOS subjects and 29 non-MINOS subjects were enrolled, and there was no difference in baseline stroke severity. There was no significant difference in IL-6 level pre-MC treatment at 1, 24, or 72 h. However, the odds of a non-detectable IL-6 at 24 h in MINOS were 8.94 (95% CI 2.62–30.46) compared with non-MINOS subjects. It is likely that even low doses of MC have a potent systemic anti-inflammatory effect in AIS. Whether this results in improved outcome will be tested in a randomized clinical trial.
Keywords: Acute cerebral infarction, Emergency treatment of stroke, Inflammation, Interleukin-6, Biomarkers
Introduction
Post-stroke inflammation as characterized by elevated levels of inflammatory mediators, including interleukin-6 (IL-6), is correlated with both stroke severity and poor clinical outcome, prompting an interest in using agents with known anti-inflammatory activity in the acute phase of ischemic stroke (AIS) [1]. Minocycline (MC) is a potent anti-inflammatory agent with multiple mechanisms of action, a well-elucidated pharmacokinetic and safety profile, and compatibility with the only approved pharmacologic treatment for ischemic stroke (t-PA) [2, 3]. In this analysis, the relationship of intravenous MC administered to plasma IL-6 levels in AIS was investigated.
Methods
Subjects
Intravenous MC for AIS within 6 h of symptom onset was investigated in a non-randomized, dose-escalation trial (MINOS) [2]. Between June 2008 and October 2009, 60 subjects were enrolled into four dose tiers using the modified continual reassessment method (mCRM) from 3.0 to 10.0 mg/kg at the Medical College of Georgia (MCG), University of Kentucky and the Oregon Health Sciences University. MC was infused over 1 h every 12 h for 3 days, and blood samples for IL-6 analysis were collected pre-MC, 1 h following the first dose, 24 and 72 h. In a separate observational study of blood biomarkers in AIS, 29 subjects were enrolled between January 2006 and January 2008. Blood samples were collected at 24 h. Data from this population will be referred to as non-MINOS. The local Institutional Review Board approved these studies. Written informed consent was obtained from all participants or their legally authorized representative.
Laboratory Methods
Venous blood samples were collected in chilled, citrate-based, anticoagulated vacutainer tubes. They were centrifuged within 1 h at 2,000×g for 20 min at 4°C, and the plasma was removed, aliquoted, and frozen at −80°C. All measurements were performed in the biomarker laboratory by researchers blinded to the clinical data. IL-6 levels were measured using a commercially available ELISA kit from Thermo Scientific-Pierce (Rockford, IL). The lower limit of detection was 1 pg/ml. Intra- and inter-assay coefficients of variation were <10%. Measured values were graphically displayed alongside the published literature.
Statistical Analysis
All statistical analysis was performed using SAS 9.2. Due to having a large proportion of individuals with IL-6 levels at 24 h below the limit of detection, IL-6 levels were dichotomized into detectable or non-detectable levels. To examine whether an association between detectable IL-6 and MC existed at 24 h, logistic regression was used in controlling for baseline National Institutes of Health Stroke Scale (NIHSS) and demographic variables. MC dose did not affect IL-6 level and was not included in the logistic regression analysis. Odds ratios and 95% confidence intervals were determined for having a non-detectable IL-6 level among those receiving MC compared to those not receiving MC. Statistical significance was assessed using an alpha level of 0.05.
Literature Search
A Medline search was conducted in August 2011 with the search terms interleukin-6 and acute stroke present in the title or abstract. Results were limited to studies on humans and the English language. Studies without plasma IL-6 levels at 24-h post-stroke were excluded.
Results
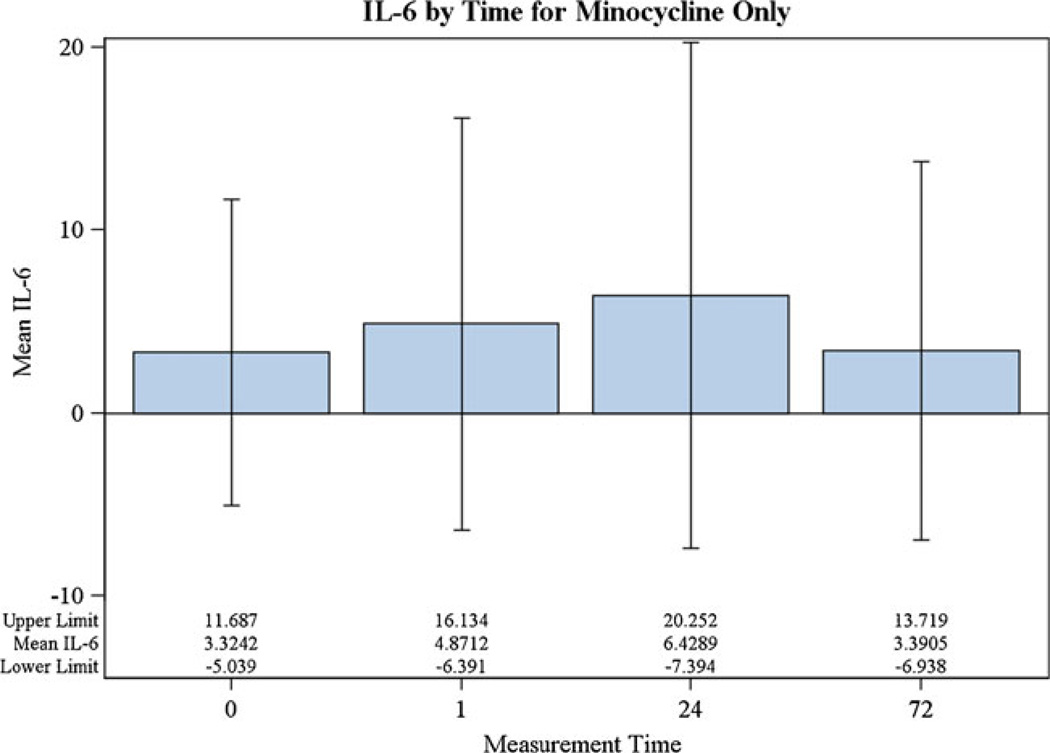
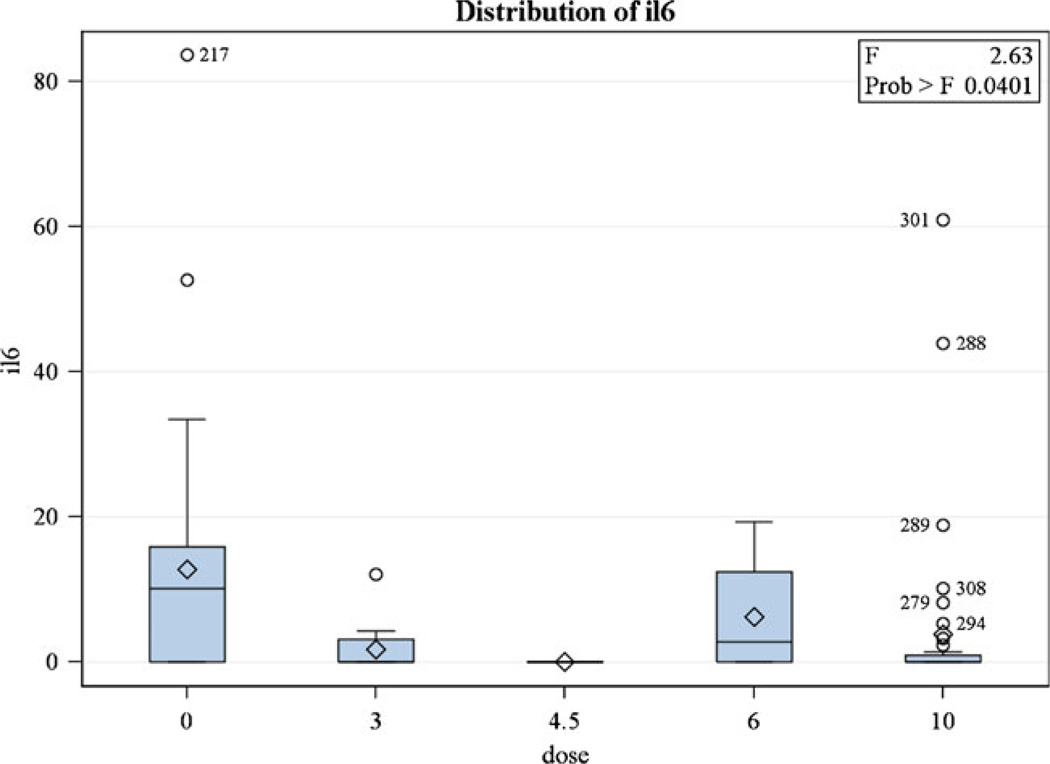
There were 60 individuals in MINOS and 29 individuals in the comparison group. Using the mCRM, 11 MINOS subjects received 3.0 mg/kg, 4 received 4.5 mg/kg, 4 received 6.0 mg/kg, and 41 received 10.0 mg/kg [2]. MINOS and non-MINOS demographics were similar for age, sex, hypertension, stroke subtype (lacunar vs. non-lacunar), and baseline stroke severity (NIHSS). The non-MINOS group had more African-Americans and diabetics, while MINOS had more subjects with atrial fibrillation (Table 1). There was no significant difference in IL-6 level pre-MC treatment at 1, 24, or 72 h (Fig. 1). However, the mean IL-6 at 24 h was 3.34 (SD = 10.18, median = 0) in MINOS and 12.82 (SD = 17.87, median = 10.10) in the non-MINOS group. A statistically significant association between MC and non-detectable IL-6 was found, with those given MC 8.94 times (95% CI 2.62–30.46) more likely to have a non-detectable IL-6 level at 24 h than those in the non-MINOS comparison group (p = 0.0005). IL-6 values with different doses were not statistically different from each other in post hoc tests (Fig. 2). At 90 days, the mean NIHSS in MINOS was 2.16 (SD = 3.27, median = 1.0) and 4.50 (SD = 4.99, median = 3.0) in the comparison group (p = 0.1064).
Table 1.
Descriptive statistics by MINOS and non-MINOS groups
| Variable | Level | MINOS N=60 | Non-MINOS N=29 | p value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Race | Caucasian | 50 | 83.3 | 16 | 55.2 | 0.0054 |
| African-American | 9 | 15.0 | 13 | 44.8 | ||
| Asian | 1 | 1.7 | 0 | 0.0 | ||
| Gender | Male | 32 | 53.3 | 15 | 51.7 | 1.0000 |
| Female | 28 | 46.7 | 14 | 48.3 | ||
| tPA | No tPA | 36 | 40.0 | 9 | 31.0 | 0.4867 |
| tPA | 24 | 60.0 | 20 | 69.0 | ||
| Lacunar | Non-lacunar | 52 | 86.7 | 22 | 75.9 | 0.2341 |
| Lacunar | 8 | 13.3 | 7 | 24.1 | ||
| Hypertension | No | 12 | 20.0 | 4 | 13.8 | 0.8415 |
| Yes | 48 | 80.0 | 25 | 86.2 | ||
| Diabetes | No | 44 | 73.3 | 13 | 44.8 | 0.0107 |
| Yes | 16 | 26.7 | 16 | 55.7 | ||
| Atrial fibrillation | No | 46 | 76.7 | 28 | 95.6 | 0.0311 |
| Yes | 14 | 23.3 | 1 | 3.5 | ||
| Non-detectable IL-6 at 24 h | No | 16 | 26.7 | 21 | 72.4 | <0.0001 |
| Yes | 44 | 73.3 | 8 | 27.6 | ||
| Mean | SD | Mean | SD | |||
| Age | 65.04 | 13.72 | 64.45 | 13.93 | 0.8502 | |
| NIHSS at baseline | 8.67 (median = 7.0) | 5.83 | 9.52 (median = 8.0) | 6.22 | 0.5564 | |
Fig. 1.
IL-6 by measurement time in MINOS (F(3,44.2) = 2.75, p = 0.0541). No post hoc pairwise Bonferroni alpha-adjusted multiple comparison tests showed statistically significant differences
Fig. 2.
IL-6 by dose of minocycline at 24 h. The 0 dose is the non-MINOS comparison group
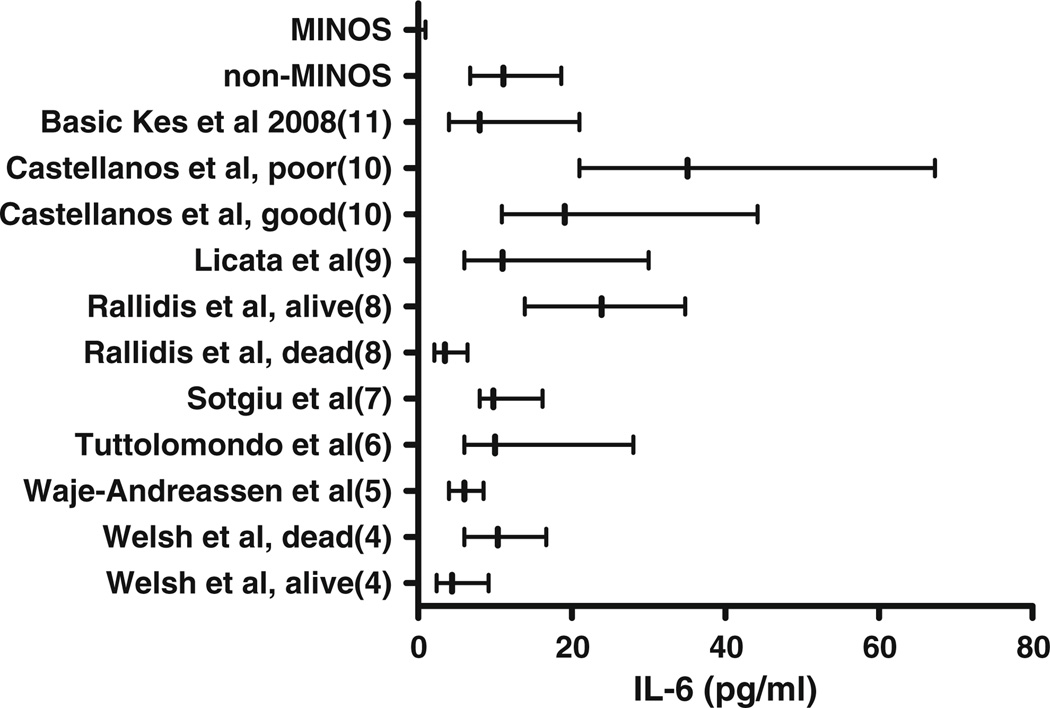
Twenty-one studies fit our systematic review criteria. Subsequently, only studies that reported data as median and IQR and that either did not dichotomize their results or only dichotomized IL-6 levels based on pre-defined good and poor outcomes were included, leaving eight studies for review [4–11]. When plotted against previous reports of IL-6 at 24 h after ischemic stroke, MINOS patients appeared markedly lower (Fig. 3).
Fig. 3.
Levels of IL-6 within 24 h of onset of symptoms in acute ischemic stroke. Data displayed as median and IQR. Non-MINOS group was similar to other AIS populations, while the MINOS group visually was much lower than that of the published literature
Discussion
Following an acute stroke, serum markers of inflammation, including IL-6, are elevated [12]. Increased IL-6 is correlated with larger infarct volume, greater stroke severity, and worse clinical outcome [1]. In acute stroke, levels of IL-6 increase between admission and 24 h and remain elevated out to 1 week [14]. MC has potent anti-inflammatory properties and inhibits neutrophil, macrophage, and microglial cell activation [13]. No pharmacologic intervention has previously been shown to prevent this increase in IL-6 post-stroke. In MINOS, IL-6 levels were not statistically different between pre-treatment and 1, 24, and 72 h. However, compared with a separate cohort of ischemic stroke patients, MINOS patients had lower IL-6 at 24 h. Our data suggest that MC may blunt the normal rise in IL-6 after stroke, possibly reflecting anti-inflammatory activity.
Several limitations preclude more definitive conclusions about the impact of MC on IL-6. The study was not a randomized controlled trial; thus, despite subjects in the two groups being of similar age, sex, stroke severity, and stroke subtype, there were more African-Americans and diabetics in the non-MINOS group. However, there was no interaction between race or diabetic status and MC on IL-6, and we controlled for race and diabetes in our analysis. Bias resulting from an unrecognized cofounder is also possible. Further, the non-MINOS group had a disproportionate population of diabetic and African-American patients compared to the MINOS group; however, this did not appear to affect IL-6. Also, IL-6 may be raised by concomitant infections or other inflammatory complications of stroke such as urinary tract infections, pneumonia, or deep vein thrombosis, but it is unlikely that these events occurred prior to 24 h [15]. Finally, although IL-6 has been correlated to the outcome, the clinical significance of lowering IL-6 levels remains to be fully elucidated.
In summary, IL-6 levels were lower at 24 h in patients from the MINOS study group compared to the non-MINOS patient group who did not receive MC. These findings support the anti-inflammatory activity of MC and potential in AIS. Due to its well-elucidated safety profile, compatibility with t-PA and anti-inflammatory activity, MC is an exciting prospect for use in AIS and supports a randomized controlled trial to investigate its effect on clinical outcome.
Acknowledgments
This study was supported by the NIH/NINDS grants (DCH, R01 NS055728; SCF, RO1 NS063965; and AE, R21 NS070239), AHA EIA (AE, 0740002N), and the MCG Intramural Grants Program Scientist Training Program to JAS.
Contributor Information
Jeffrey A. Switzer, Email: jswitzer@me.com, Department of Neurology, Georgia Health Sciences University, 1120 15th St, Augusta, GA 30912, USA.
Andrea Sikora, Program in Clinical and Experimental Therapeutics, College of Pharmacy, University of Georgia, Augusta, GA 30912, USA.
Adviye Ergul, Department of Physiology, Georgia Health Sciences University, Augusta, GA 30912, USA; Department of Biostatistics, Georgia Health Sciences University, Augusta, GA 30912, USA; Program in Clinical and Experimental Therapeutics, College of Pharmacy, University of Georgia, Augusta, GA 30912, USA; Charlie Norwood VA Medical Center, Augusta, GA 30912, USA.
Jennifer L. Waller, Department of Biostatistics, Georgia Health Sciences University, Augusta, GA 30912, USA
David C. Hess, Department of Neurology, Georgia Health Sciences University, 1120 15th St, Augusta, GA 30912, USA Program in Clinical and Experimental Therapeutics, College of Pharmacy, University of Georgia, Augusta, GA 30912, USA.
Susan C. Fagan, Department of Neurology, Georgia Health Sciences University, 1120 15th St, Augusta, GA 30912, USA Program in Clinical and Experimental Therapeutics, College of Pharmacy, University of Georgia, Augusta, GA 30912, USA; Charlie Norwood VA Medical Center, Augusta, GA 30912, USA.
References
- 1.Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, Wardlaw J, Dennis M, Sudlow C. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000145. e1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. Minocycline to improve neurologic outcome in stroke (minos): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Switzer JA, Hess DC, Ergul A, Waller JL, Machado LS, Portik-Dobos V, Pettigrew LC, Clark WM, Fagan SC. Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke. 2011;42:2633–2635. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. 2009;27:247–253. doi: 10.1159/000196823. [DOI] [PubMed] [Google Scholar]
- 5.Waje-Andreassen U, Krakenes J, Ulvestad E, Thomassen L, Myhr KM, Aarseth J, Vedeler CA. Il-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 6.Tuttolomondo A, Pecoraro R, Pinto A, Licata G. A precise stroke classification for evaluation of ischemic stroke subtypes and their relation with diabetes: is toast the best? Stroke. 2011;42:e10. doi: 10.1161/STROKEAHA.110.602508. [DOI] [PubMed] [Google Scholar]
- 7.Sotgiu S, Barone R, Zanda B, Arru G, Fois ML, Arru A, Rosati G, Marchetti B, Musumeci S. Chitotriosidase in patients with acute ischemic stroke. Eur Neurol. 2005;54:149–153. doi: 10.1159/000089935. [DOI] [PubMed] [Google Scholar]
- 8.Rallidis LS, Vikelis M, Panagiotakos DB, Rizos I, Zolindaki MG, Kaliva K, Kremastinos DT. Inflammatory markers and in-hospital mortality in acute ischaemic stroke. Atherosclerosis. 2006;189:193–197. doi: 10.1016/j.atherosclerosis.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Licata G, Tuttolomondo A, Di Raimondo D, Corrao S, Di Sciacca R, Pinto A. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost. 2009;101:929–937. [PubMed] [Google Scholar]
- 10.Castellanos M, Sobrino T, Pedraza S, Moldes O, Pumar JM, Silva Y, Serena J, Garcia-Gil M, Castillo J, Davalos A. High plasma glutamate concentrations are associated with infarct growth in acute ischemic stroke. Neurology. 2008;71:1862–1868. doi: 10.1212/01.wnl.0000326064.42186.7e. [DOI] [PubMed] [Google Scholar]
- 11.Basic Kes V, Simundic AM, Nikolac N, Topic E, Demarin V. Proinflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin Biochem. 2008;41:1330–1334. doi: 10.1016/j.clinbiochem.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 12.Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994;122:135–139. doi: 10.1016/0022-510x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 13.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, Hallenbeck JM, del Zoppo GJ, Rothwell NJ, Tyrrell PJ, Hopkins SJ. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. doi: 10.1016/s0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 15.Salobir B, Sabovic M. Possible vascular-bed-specific role of interleukin-6 in young women with a history of myocardial infarction, lacunar cerebral infarction and deep vein thrombosis. Cytokine. 2004;25:265–272. doi: 10.1016/j.cyto.2003.11.011. [DOI] [PubMed] [Google Scholar]