Abstract
Gout is a metabolic disorder characterized by elevated uric acid levels in the body, associated with painful arthritis, tophi and nephropathy. The most frequently used pharmacologic urate lowering strategies involve reducing urate production with a xanthine oxidase inhibitor and enhancing urinary excretion of uric acid with a uricosuric agent. Urate lowering agents are limited in number, availability and effectiveness. The emergence of a new medication, febuxostat, to lower serum urate levels is welcome as no new drug have been approved since the introduction of allopurinol, in 1964, and the drugs that are available have limitations owing to inefficacy or toxicity. Febuxostat is a novel, nonpurine selective inhibitor of xanthine oxidase, is a potential alternative to allopurinol for patients with hyperuricemia and gout.
Keywords: Gout, hyperuricemia, xanthine oxidase inhibitor
Gout is the most common form of inflammatory arthritis in men and is caused by the deposition of monosodium urate crystals in tissues[1]. The condition generally occurs after years of sustained high uric acid concentrations and it is estimated to affect approximately 5.1 million people in the United States, according to the most recent National Health and Nutrition Examination Survey (NHANES III)[2]. Prevalence of gout and/or hyperuricemia during the past 10 years has been increasing, possibly because of an increase in the prevalence of two important risk factors for hyperuricemia, namely, obesity and aging[1,3]. Acute and chronic arthritis, tophi and renal diseases are manifestations of gout that reflect the magnitude and duration of hyperuricemia, which is the biological hallmark of gout[4].
Treatment of an acute attack of gout differs from treatment aimed at preventing attacks and other manifestations by reducing the serum urate level. The drugs available for the treatment of hyperuricemia in patients with gout are uricosuric agents (e.g. probenecid, sulfinpyrazone), which increase the excretion of uric acid, and xanthine oxidase inhibitor (e.g. allopurinol and its metabolite oxypurinol), which inhibit the oxidation of xanthine to uric acid. Use of the uricosuric drugs is limited by their inefficacy in patients whose creatinine clearance is less than 50 ml per min per 1.73 m2 of body surface area; this excludes most patients older than 60 years of age. Xanthine oxidase inhibitors are effective in patients with renal insufficiency, but these patients may require a reduction in dose because its clearance is primarily by renal mechanisms. Allopurinol is the most commonly used antihyperuricemic agent because of its efficacy regardless of the cause of hyperuricemia and because of the convenience of once daily dosing[5]. The introduction of febuxostat provided clinicians with an additional hope for the treatment of hyperuricemia and gout in patients who are non responsive to allopurinol. This article reviews pharmacological aspects of febuxostat.
Febuxostat is a novel, orally administered antihyperuricemic drug[6]. Chemically, febuxostat is (2-[3-cyano-4-(2-methylpropoxy)-phenyl]-4-methylthiazole-5-carboxylic acid). It is a non purine analogue inhibitor (fig. 1) of both the oxidized and reduced forms of xanthine oxidase; in contrast, allopurinol an hypoxanthine analog, weakly inhibits the oxidized form[4,6]. It acts as a potent inhibitor of xanthine oxidase and was found to be more than 10- 30 times potent than allopurinol in animal studies[7]. The ki value of febuxostat is 0.7 nM as compared to allopurinol which is 0.7 μM[8]. It has minimal effects on other enzymes involved in purine and pyrimidine metabolism[6].
Fig. 1.
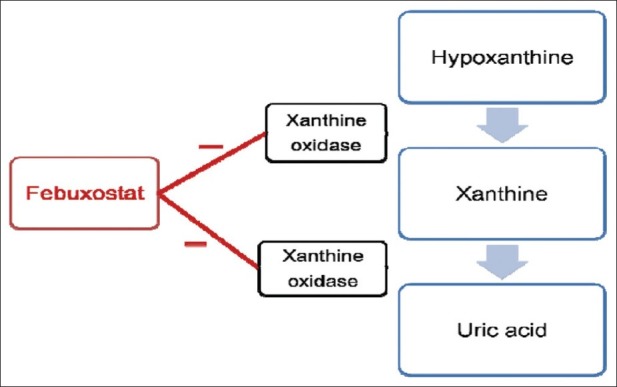
Mechanism of action of febuxostat
Febuxostat is rapidly absorbed after oral administration with a time to reach peak concentration (tmax) of approximately 1 h. The drug is highly bound to albumin in blood (~99%) and appears to have a low to medium apparent volume of distribution of approximately 0.7 l/ kg[9]. The pharmacokinetics of febuxostat appears to be linear in the 10 mg to 120 mg once-daily dose range[9]. It is mainly metabolized to its acylglucuronide metabolite via uridine diphosphate glucuronosyltransferase (UGT) enzymes and to a lesser extent to its active oxidative metabolites 67M-1, 67M-2, and 67M-4 via cytochrome P450 enzymes[10]. Less than 6% of the administered dose is excreted in the urine as unchanged drug[10]. The mean half-life is 4 to 9 h.
Febuxostat was found to be well tolerated. The most common adverse events include abnormal liver function test results, abdominal pain, diarrhea, headache, joint related signs and symptoms, and musculoskeletal and connective tissue signs and symptoms[11]. All adverse events were of mild intensity. The overall incidence of adverse events during dosing was higher for subjects in the moderate hepatic impairment (75%) and mild hepatic impairment (63%) groups as compared to subjects in the normal hepatic function group (25%)[10]. There were no serious adverse events or clinically significant changes from baseline in laboratory values, physical examination, vital signs, or ECG readings during the study period.
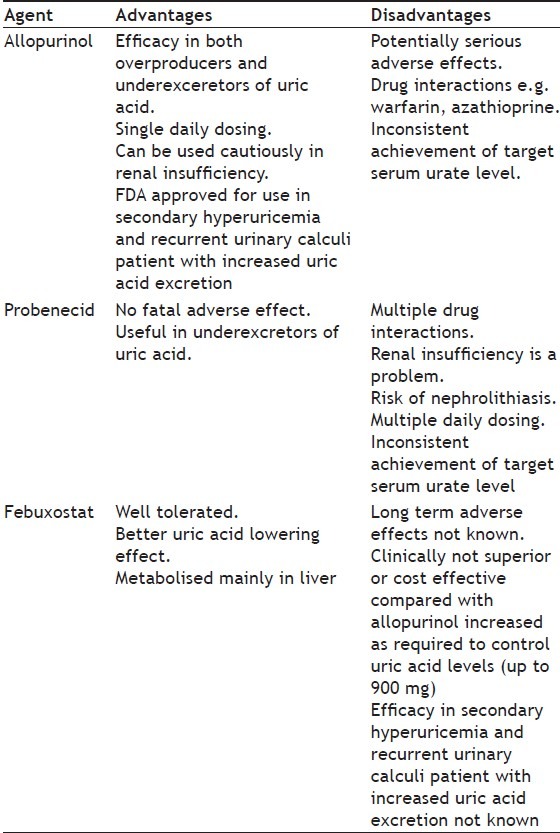
Febuxostat is indicated for the long-term management of hyperuricemia in patients with gout. It was found to be more effective in the doses of 40-120 mg per day in lowering serum urate levels than the fixed daily dose of 300 mg of allopurinol. The results of various clinical trials conducted for assessing the uric acid lowering effect of febuxostat is summarized in Table 1. The reduction in the frequency of gout flares and decrease in the size or number of tophi was similar to allopurinol. It is a potential alternative to allopurinol for patients with hyperuricemia and gout. The relative costs of allopurinol and febuxostat may influence the decision of use of these agents. The ability of febuxostat was not altered in patients with mild to moderate renal insufficiency[12]. Comparative features of the common antihyperuricemic agents are given in Table 2. However, further studies are needed to define long term safety profile of febuxostat, especially when it is administered in patients with secondary hyperuricemia, renal insufficiency, or in those with other coexisting conditions or receiving medications that may cause hepatotoxicity[13].
TABLE 1.
SUMMARY OF CLINICAL TRIALS CONDUCTED TO ASSESS EFFICACY OF FEBUXOSTAT
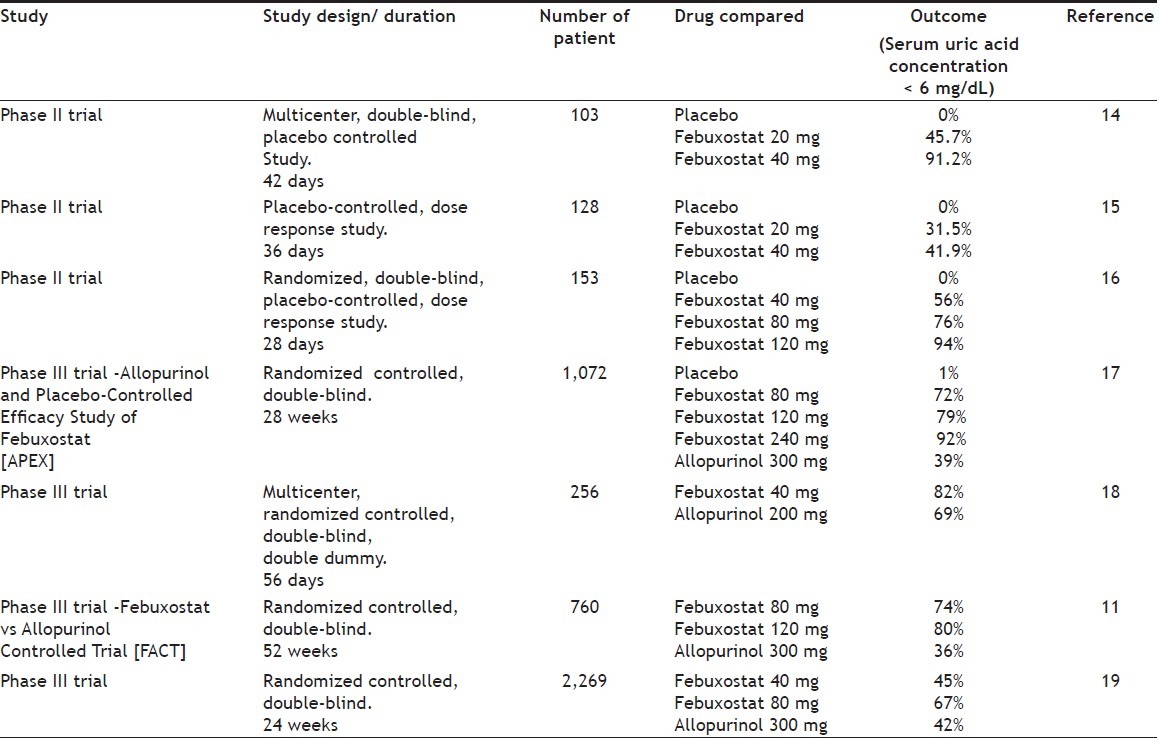
TABLE 2.
COMPARISION OF COMMONLY USED ANTIHYPERURICEMIC AGENTS WITH FEBUXOSTAT
Febuxostat is contraindicated in patients being treated with the xanthine oxidase substrates azathioprine, mercaptopurine, and theophylline. Like allopurinol, febuxostat is not effective for the treatment of acute gouty attacks and may even precipitate them during the first 6 months of therapy. Prophylactic therapy with nonsteroidal anti-inflammatory drug or colchicine is advised to prevent the same. However, discontinuation of febuxostat is not necessary if a gout flare occurs[10]. Periodic liver function tests are advised for patients receiving febuxostat as transaminase elevations greater than 3 times the upper limit of normal were observed in patients treated with febuxostat in clinical trials[10]. A higher rate of cardiovascular thromboembolic events was observed in patients treated with febuxostat. Although a causal relationship has not been established, it is recommended that patients be monitored for signs and symptoms of myocardial infarction and stroke[10]. Caution is advised with the use of febuxostat in patients with severe renal or hepatic impairment because of a lack of data in this population[10].
Coadministration of febuxostat with xanthine oxidase substrate drugs (azathioprine, mercaptopurine or theophylline) could increase plasma concentrations of these drugs, since these drugs are metabolized by xanthine oxidase, resulting in severe toxicity; hence their concomitant use is contraindicated. Since febuxostat does not inhibit or induce cytochrome P450 enzymes it lacks significant drug interactions with other drugs metabolized of these enzymes.
Febuxostat is an orally active drug found to be effective in the dosage of 40-120 mg/day. The pharmacokinetics of the drug allows it to be suitable for once a day dosing. The recommended starting dosage of febuxostat is 40 mg once daily, if the patients do not achieve the target serum uric acid concentration (<6 mg/dl) after 2 weeks then the dose is increased to 80 mg once daily. Although febuxostat is metabolized mainly in liver, but no dosage adjustment is necessary for patients with mild to moderate hepatic impairment, however caution is required in severe hepatic impairment as there is lack of data in this population[10]. No dose adjustment required for mild to moderate renal failure but caution is required for severe impairment[10]. Febuxostat is pregnancy category C drug, and therefore its use in pregnancy is recommended only if the potential benefit outweighs the potential risk to the fetus. Human breast milk excretion data is not known hence caution is advised if febuxostat is administered to women who are breast-feeding. Safety and effectiveness have not been established in children therefore it should not be used in them[10].
Febuxostat, is the latest drug for the treatment of hyperuricemia and gout developed after a gap of nearly 40 years. It has been approved by European medicines agency and US. Availability of febuxostat provides an alternative to the patients not tolerating or having inadequate reduction in serum uric acid level with allopurinol,
Footnotes
Bisht and Bist: Febuxostat: Novel Drug for Gout
REFERENCES
- 1.Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA. 1991;266:3004–7. [PubMed] [Google Scholar]
- 2.Kramer HM, Curhan G. The association between gout and nephrolithiasis: The National Health and Nutrition Examination Survey III, 1988-1994. Am J Kidney Dis. 2002;40:37–42. doi: 10.1053/ajkd.2002.33911. [DOI] [PubMed] [Google Scholar]
- 3.Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–7. [PubMed] [Google Scholar]
- 4.Becker MA, Jolly M. Metabolic bone and joint disease. In: Koopman WJ, Moreland LW, editors. Arthritis and allied conditions: A textbook of rheumatology. 15th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 2303–39. [Google Scholar]
- 5.Terkeltaub RA. Gout. N Engl J Med. 2003;349:1647–55. doi: 10.1056/NEJMcp030733. [DOI] [PubMed] [Google Scholar]
- 6.Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, et al. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–47. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi H, Ota M, Kobayashi M. A comparative study on the hypouricemic activity and potency in renal xanthine calculus formation of two xanthine oxidase/xanthine dehydrogenase inhibitors: TEI-6720 and allopurinol in rats. Res Commun Mol Pathol Pharmacol. 1999;104:307–19. [PubMed] [Google Scholar]
- 8.Takano Y, Hase-Aoki K, Horiuchi H, Kasahara Y, Kondo S, Becker MA, et al. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;76:1835–47. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Becker MA, Kisicki J, Khosravan R, Wu J, Mulford D, Hunt B, et al. Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids. 2004;23:1111–6. doi: 10.1081/NCN-200027372. [DOI] [PubMed] [Google Scholar]
- 10.Cada DJ, Levien TL, Bake DE. Febuxostat. Hosp Pharm. 2009;44:688–99. [Google Scholar]
- 11.Becker MA, Schumacher HR, Wortmann RL, MacDonald PA, Eustace D, Pala WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 12.Mayer MD, Khosravan R, Vernillet L, Wu JT, Joseph-Ridge N, Mulford DJ. Pharmacokinetics and dynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase, in subjects with renal impairment. Am J Ther. 2005;12:22–34. doi: 10.1097/00045391-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Moreland LW. Febuxostat- Treatment for hyperuricemia and Gout? N Engl J Med. 2005;353:2505–7. doi: 10.1056/NEJMe058247. [DOI] [PubMed] [Google Scholar]
- 14.Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. Febuxostat, a novel non-purine selective inhibitor of xanthine oxidase, in a phase III placebo-controlled double-blind clinical trial in Japanese subjects with gout or hyperuricemia [abstract]. American College of Rheumatology 2004 Annual Scientific Meeting. Arthritis Rheum. 2004;50:S337. [Google Scholar]
- 15.Kamatani N, Fujimori S, Hada T, Hosoya T, Kato R, Matsuzawa T, et al. Phase II dose-response clinical trial using febuxostat (TMX-67), a novel-type xanthine oxidase/xanthine dehydrogenase inhibitor, for gout and hyperuricemia[abstract]. American College of Rheumatology, 2003 Annual Scientific Meeting. Arthritis Rheum. 2003;48:1349. [Google Scholar]
- 16.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Pala WA, Eustace D, et al. Febuxostat, a novelnonpurine selective inhibitor of xanthineoxidase: A twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy inpatients with gout. Arthritis Rheum. 2005;52:916–23. doi: 10.1002/art.20935. [DOI] [PubMed] [Google Scholar]
- 17.Schumacher HR, Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540–8. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 18.Kamatani N, Fujimori S, Hada T, Wclls A, MacDonald PA, Lloyd E, et al. Febuxostat, a novel non-purine selective inhibitor of xanthine oxidase, in an allopurinol-controlled phase III clinical trial in Japanese subjects with gout or hyperuricemia [abstract].American College of Rheumatology 2004 Annual Scientific Meeting. Arthritis Rheum. 2004;50:S336–7. [Google Scholar]
- 19.Becker M, Schumacher HR, Jr, Espinoza L, Wclls A, MacDonald PA, Lloyd E, et al. A phase 3 randomized, controlled, multicenter, double-blind trial (RCT) comparing efficacy and safety of daily febuxostat (FEB) and allopurinol (ALLO) in subjects with gout [abstract] Arthritis Rheum. 2008;58:4029. [Google Scholar]


