Abstract
In type 2 Diabetes, oxidative stress plays an important role in development and aggregation of insulin resistance. In the present study, long term administration of the dexamethasone led to the development of insulin resistance in mice. The effect of thiazolidinediones pioglitazone and rosiglitazone, with melatonin on dexamethasone-induced insulin resistance was evaluated in mice. Insulin resistant mice were treated with combination of pioglitazone (10 mg/kg/day, p.o.) or rosiglitazone (5 mg/kg/day, p.o.) with melatonin 10 mg/kg/day p.o. from day 7 to day 22. In the biochemical parameters, the serum glucose, triglyceride levels were significantly lowered (P<0.05) in the combination groups as compared to dexamethasone treated group as well as with individual groups of pioglitazone, rosiglitazone, and melatonin. There was also, significant increased (P<0.05) in the body weight gain in combination treated groups as compared to dexamethasone as well as individual groups. The combination groups proved to be effective in normalizing the levels of superoxide dismutase, catalase, glutathione reductase and lipid peroxidation in liver homogenates may be due to antioxidant effects of melatonin and decreased hyperglycemia induced insulin resistance by thiazolidinediones. The glucose uptake in the isolated hemidiaphragm of mice was significantly increased in combination treated groups (PM and RM) than dexamethasone alone treated mice as well as individual (pioglitazone, rosiglitazone, melatonin) treated groups probably via increased in expression of GLUT-4 by melatonin and thiazolidinediones as well as increased in insulin sensitivity by thiazolidinediones. Hence, it can be concluded that combination of pioglitazone and rosiglitazone, thiazolidinediones, with melatonin may reduces the insulin resistance via decreased in oxidative stress and control on hyperglycemia.
Keywords: Insulin resistance, melatonin, thiazolidinediones, type 2 diabetes
Worldwide prevalence of Type 2 diabetes is near about 246 millions in 2007, and may rise to 380 million in 2025. The socio-economical burden is great consequently; Type 2 diabetes presents a major challenge to healthcare systems around the world[1].
Glucocorticoids, as an endogenous hormones, anti-inflammatory and immunosuppressive drugs, have been reported to induce Cushing's syndrome, which is characterized by central obesity and insulin resistance. The chronic treatment with dexamethasone has been associated with hyperinsulinemia in both animals and humans[2]. The increase level of glucocorticoids via endogenous or exogenous pathways leads to increases glucose production and decrease glucose uptake in peripheral tissues. These effects lead to the insulin resistance due to the decrease in insulin sensitivity[3,4]. Long term glucocorticoid administration lead to insulin resistance by inducing post-receptor defects in the insulin actions[4] as well as inhibition of the insulin secretion from pancreatic β cells[5]. Other mechanisms by which the dexamethasone may induce peripheral insulin resistance, includes inhibition of GLUT4 translocation[6], increased lipoprotein lipase activity in the adipose tissue[7], impaired insulin signalling due to reduction in the phosphatidyl-inositol-3-kinase (PI-3K) activity[8,9].
Long term administration of dexamethasone leads to generation of the free radicals which may contribute to oxidative stress[10]. High glucose concentrations induce mitochondrial ROS, which suppresses the first phase of glucose-induced insulin secretion, at least in part, through the suppression of glyceraldehydes 3-phosphate dehydrogenase activity[11]. In early stages, insulin resistance is compensated by hyperinsulinemia which characterised by the increase in insulin, FFA, and/or glucose levels, may associate with increase in ROS production and oxidative stress. This, in turn, can deteriorate both insulin secretion and action, accelerating the sequence to type 2 diabetes. Many studies support the hypothesis on the basis of in vitro as well as in vivo animal studies that; antioxidants have been shown to improve insulin sensitivity[11].
Oxidative stress induces the expression of NF-KB (Nuclear factor KB) which directly or indirectly contributes to the insulin resistance[12]. Thus, treatment with antioxidants can be used to reduce oxidative stress induced insulin resistance. Thiazolidinediones (pioglitazone and rosiglitazone) are well known agonists of the transcription-factor peroxisome proliferator activated receptor-γ (PPAR-γ) and currently used in the treatment of Type 2 diabetes[13]. Thiazolidinediones increase sensitivity to insulin by restoration of adipokines levels and increased in the expression of GLUT-4[13]. Still, there exists concern about use of these drugs clinically, because of their uncertain cardiovascular safety and other idiosyncratic adverse events[14].
Melatonin (N-acetyl-5-methoxytryptamine) is a well known endogenous biological antioxidant synthesized in the pineal gland. It acts as a buffer against oxidative stress, induced by the reactive oxygen and nitrogen species[15]. Melatonin is reported to possess pharmacological actions, other than its antioxidant activity, such as insulin resistance reducing activity[16], enhancing the glucose uptake in skeletal muscle in vitro via IRS-1/PI-3-kinase (Insulin Receptor Substrate-1/ protein kinase-3) pathway stimulates glucose transport[17] and lipid lowering activity[18].
Now in the market different combinations of the oral hypoglycemic agents are used in the treatment of Type 2 diabetes. However, effective treatment for Type 2 diabetes usually requires a combination of two or more oral agents in the longer term, often as an introduction to the insulin therapy. Issues regarding the safety, tolerability, notably weight gain limits the optimal use of the oral hypoglycaemia drugs such as sulphonylureas and thiazolidinediones in combination[19].
On the basis of the reported pharmacological activities of melatonin and thiazolidinediones, in order to have new combination approach in the treatment of Type 2 diabetes. The present study was design to evaluate effect of the combination of thiazolidinediones (pioglitazone and rosiglitazone) with melatonin in the treatment of Type 2 diabetes preclinically.
MATERIALS AND METHODS
Pioglitazone, rosiglitazone, melatonin were obtained from Themis Laboratories Ltd., Mumbai; Glenmark Pharmaceuticals Ltd., Nasik and Arati Bulk Manufacturer Pvt Ltd., Mumbai, India, respectively. Other chemicals and kits for biochemical estimations were obtained from Biolab Diagnostic Pvt. Ltd., India.
Experimental animals:
Forty two Swiss albino mice weighing 25-30 g were used for study and were kept in animal house at 26±2° with relative humidity 44-56% along with light and dark cycles of 12 h respectively. Animals were provided with standard diet and water ad libitum. The food was withdrawn 18–24 h before the start of the experiment. Institution animal ethics committee has approved the experimental protocol (198/99/CPCSEA).
Experimental protocol:
All 42 mice were weighed before treatment, NC (Normal Control) group received equivalent amount of 1% Na-CMC (sodium carboxymethyl cellulose) (1 ml/kg, p.o.), and remaining 36 mice were rendered hyperglycemic by daily administration of a prestandardized dose of dexamethasone (1 mg/kg, i.m.)[20] for consecutive 7 days and then divided into six groups of six each. DC group (dexamethasone control) continued to receive only dexamethasone and 1% CMC (1 ml/kg, p.o.) for next 15 days. PIO group, received pioglitazone (10 mg/kg, p.o.)[21] along with dexamethasone respectively for 15 days. ROSI group, received rosiglitazone (5 mg/kg, p.o.)[21] along with dexamethasone for 15 days. MEL group, received melatonin (10 mg/kg, p.o.)[22] along with dexamethasone for 15 days. PM group received pioglitazone (10 mg/ kg, p.o.) plus melatonin (10 mg/kg, p.o.) along with dexamethasone respectively for 15 days. RM group received rosiglitazone (5 mg/kg, p.o.) plus melatonin (10 mg/kg, p.o.) along with dexamethasone respectively for 15 days.
Biochemical estimations:
Blood samples were collected and used for estimation of glucose and triglyceride on 1, 7 and 22 day. Biochemical estimation of serum glucose and serum triglyceride was done by GOD/POD and GPO/POD method respectively using standard diagnostic kits from Biolabs India ltd., India.
Hepatic antioxidant enzymes assay:
Liver samples were dissected out and washed immediately with ice cold saline to remove as much blood as possible. Liver homogenates (5% w/v) were prepared in cold 50 mM Tris buffer (pH 7.4) using Remi homogenizer. The unbroken cells and cell debris were removed by centrifugation at 5000 rpm for 10 min using a Remi refrigerated centrifuge. The supernatant was used for the estimation of malondialdehyde (MDA)[23], superoxide dismutase (SOD)[24], catalase[25,26] and GSH reductase levels[27].
Effect on glucose uptake in isolated mice hemidiaphragm:
Glucose uptake in mice hemi-diaphragm was estimated by the method described by Ghaisas et al.[20]. Fourteen sets, containing graduated test tubes (n=6) each, were used for study of non-insulin assisted and insulin assisted glucose uptake. The diaphragms were taken out quickly avoiding traumas and divided into two halves. The hemi-diaphragms were rinsed in cold Tyrode solution (without glucose) to remove any blood clots. In non-insulin assisted glucose uptake study, one hemidiaphragm of each animal from all groups was exposed to 2 ml Tyrode solution with glucose (2000 mg/l) in respective graduated test tubes. In insulin assisted glucose uptake study, the remaining hemidiaphragm of each animal from all was exposed to 2 ml Tyrode solution with glucose (2000 mg/l) + Insulin (0.25 IU/ml) in respective graduated test tubes. All the graduated test tubes were incubated for 30 min at 37° in an atmosphere of 95% O2 - 5% CO2 with shaking at 140 cycles per min. Following incubation, the hemidiaphragm were taken out and weighed. The glucose content of the incubated medium was measured by GOD/POD, enzymatic method. Glucose uptake was calculated as the difference between the initial and final glucose content in the incubation medium.
Statistical analysis:
The results were expressed as Mean±SEM and statistically analyzed by ANOVA followed by Dunnett test, with level of significance set at P<0.05.
RESULTS
Effect on the body weight and biochemical parameters:
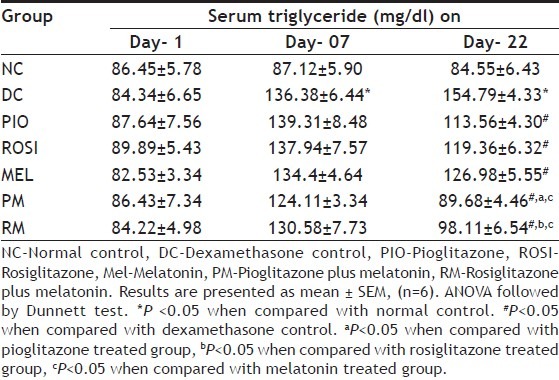
Mice treated with dexamethasone alone showed statistically significant (P<0.05) decrease in the percentage of change in the body weight than normal mice (Table 1), but showed statistically significant (P<0.05) increased in the serum glucose and triglyceride levels on day 7 and day 22 than normal mice (Tables 2 and 3). Pioglitazone plus melatonin and rosiglitazone plus melatonin treated mice showed significant (P<0.05) increase in the percentage of change in the body weight than dexamethasone alone treated mice as well as melatonin treated mice, also showed statistically significant (P<0.05) decrease in the serum glucose and triglyceride levels on day 22 than dexamethasone as well as melatonin alone treated mice.
TABLE 1.
EFFECT OF COMBINATION OF THIAZOLIDINEDIONES WITH MELATONIN ON PERCENTAGE CHANGE IN BODY WEIGHT IN DEXAMETHASONE-INDUCED INSULIN RESISTANCE IN MICE
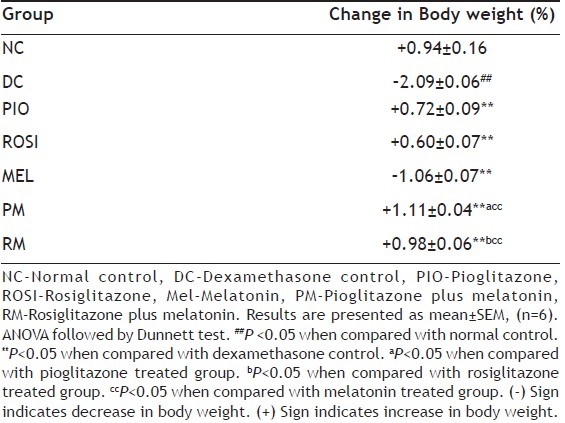
TABLE 2.
EFFECT OF COMBINATION OF THIAZOLIDINEDIONES WITH MELATONIN ON SERUM GLUCOSE IN DEXAMETHASONE-INDUCED INSULIN RESISTANCE IN MICE
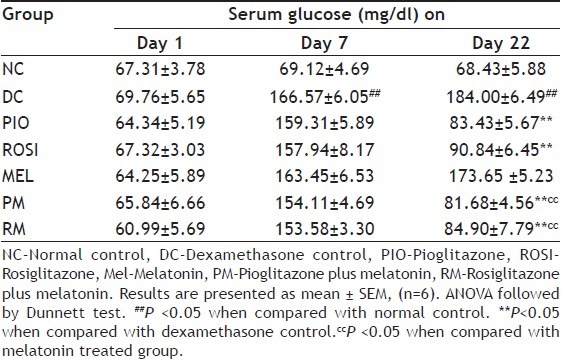
TABLE 3.
EFFECT OF COMBINATION OF THIAZOLIDINEDIONES WITH ANTIOXIDANT ON SERUM TRIGLYCERIDE LEVEL IN DEXAMETHASONE-INDUCED INSULIN RESISTANCE IN MICE
Pioglitazone and rosiglitazone alone treated mice showed significant (P<0.05) decrease in the serum glucose level on day 22 than dexamethasone alone treated mice. Pioglitazone, rosiglitazone and melatonin alone treated mice showed significant (P<0.05) increase in the percentage of change in the body weight than dexamethasone alone treated mice, also showed significant (P<0.05) decrease in the serum glucose level on day 22 than dexamethasone alone treated mice.
The combination of pioglitazone plus melatonin and rosiglitazone plus melatonin also showed statistically significant (P<0.05) increased in the body weight than individual pioglitazone and rosiglitazone treated groups (Table 1).
Effect on the glucose uptake in isolated hemidiaphragm of mice:
The glucose uptake in the isolated hemidiaphragm of mice in the insulin stimulated as well as non insulin stimulated glucose uptake was statistically significant (P<0.05) decrease in the dexamethasone alone treated group mice than normal group mice (fig. 1). The Pioglitazone plus melatonin and rosiglitazone plus melatonin treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in both insulin as well as non insulin assisted than dexamethasone alone treated mice and melatonin treated mice. Pioglitazone plus melatonin and rosiglitazone plus melatonin treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in insulin assisted than pioglitazone, rosiglitazone treated mice respectively.
Fig. 1.
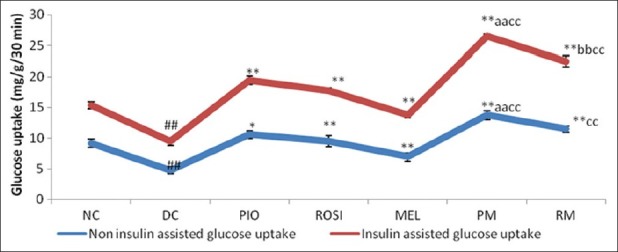
Effect of combination of thiazolidinediones with melatonin on Non-insulin assisted and insulin assisted glucose uptake using isolated hemidiaphragm of mice in dexamethasone-induced insulin resistance.
NC–Normal control, DC –Dexamethasone control, PIO-Pioglitazone, ROSI-Rosiglitazone, MEL-Melatonin, PM-Pioglitazone plus melatonin, RM-Rosiglitazone plus melatonin. Results are expressed as Mean±SEM, (n=6). ANOVA followed by Dunnet test. ##P <0.05 when compared with normal control. *P <0.05, **P <0.05 when compared with dexamethasone control. aaP<0.05 when compared with pioglitazone treated group. bbP<0.05 when compared with rosiglitazone treated group. ccP<0.05 when compared with melatonin treated group.
Rosiglitazone plus melatonin treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in insulin assisted than melatonin treated mice also. Pioglitazone and rosiglitazone alone treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in insulin assisted and non-insulin assisted than dexamethasone alone treated mice. Melatonin alone treated mice did not showed any change in the glucose uptake in the isolated hemidiaphragm in insulin assisted and non-insulin assisted than dexamethasone alone treated mice.
Effect on the oxidative stress parameters in the liver:
Mice treated with dexamethasone alone showed statistically significant decrease (P<0.05) in the hepatic levels of GSH, SOD, Catalase while statistically significant increase in MDA (P<0.05) level than normal mice (Table 4). Pioglitazone plus melatonin treated mice showed statistically significant increase (P<0.05) in hepatic levels of GSH, SOD, catalase, while statistically significant decrease (P<0.05) in LPO level when compared with dexamethasone alone treated mice, pioglitazone treated and melatonin treated mice. Rosiglitazone plus melatonin treated mice showed statistically significant increase (P<0.05) in hepatic levels of GSH, SOD and Catalase while statistically significant decrease (P<0.05) in LPO level than dexamethasone alone mice, but when compared with rosiglitazone treated mice, rosiglitazone plus melatonin treated mice showed statistically significant increase (P<0.05) in GSH, SOD only and statistically significant decrease in LPO levels. Also rosiglitazone plus melatonin treated mice when compared with melatonin treated group showed statistically significant increase (P<0.05) in the SOD level.
TABLE 4.
EFFECT OF COMBINATION OF THIAZOLIDINEDIONES WITH MELATONIN ON HEPATIC LEVELS OF GSH, SOD, CATALASE, AND LPO IN DEXAMETHASONE-INDUCED INSULIN RESISTANCE IN MICE
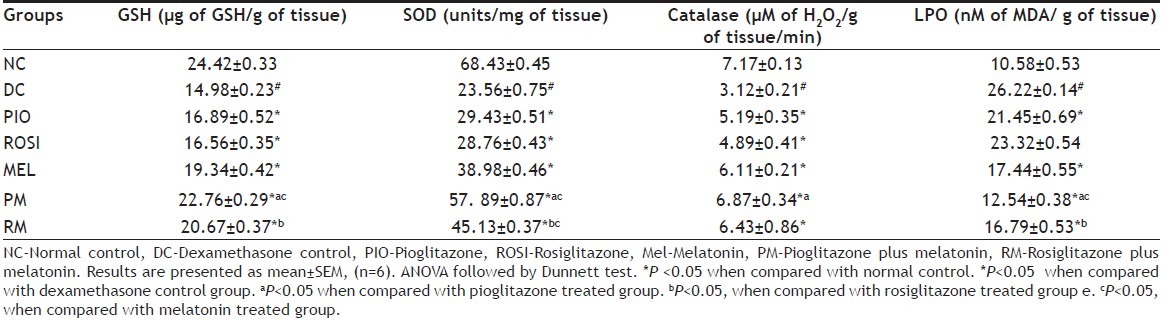
Pioglitazone, rosiglitazone and melatonin alone treated mice showed statistically significant increase (P<0.05) in hepatic levels of GSH, SOD, catalase, while statistically significant decrease (P<0.05) in LPO level when compared with dexamethasone alone treated mice.
DISCUSSION
The present investigation focussed mainly on the adverse effects induced by the long-term administration of dexamethasone such as muscle catabolism, hyperphagia, increased adiposity and induction of insulin resistance[28]. A dexamethasone-induced decrease in IRS-1 expression has been suggested to be also mechanism for insulin resistance[29–31]. It has been also proved that, dexamethasone treatment for longer period may leads to a decrease in insulin binding and that this was attributed to a reduction in the number of available cell-surface insulin receptors[32]. This could contribute to insensitivity to insulin but probably not to the reduction in maximal insulin response in glucose uptake, since fat cells have a large proportion of spare receptors[33].
Dexamethasone is a synthetic glucocorticoid which has a 50-fold greater affinity for the glucocorticoid receptor relative to cortisol. When administered in excess, dexamethasone induces adverse effects such as muscle catabolism, hyperphagia, increased adiposity, and increased insulin resistance[28]. Insulin antagonistic hormone, glucocorticoids, is under control of hypothalamic-pituitary–adrenal axis[34,35]. The insulin resistance by the glucocorticoid is via suppresses hepatic glucose production, stimulate peripheral glucose utilization and a direct inhibitory effect on glucose-induced insulin release in the β-cells[36].
In the present study, dexamethasone administration resulted in significant increase in the serum glucose and triglyceride levels. The combinations pioglitazone plus melatonin and rosiglitazone plus melatonin, decrease the elevated blood glucose level may be via increase in the glucose uptake though translocation of GLUT-4 by melatonin[17] and thiazolidinediones along with the increased insulin sensitivity[28,37]. The decrease in the serum triglyceride level in combination groups, pioglitazone plus melatonin and rosiglitazone plus melatonin might be due to increase in the lipoprotein lipase activity which causes uptake of triglyceride in fat cells[13] by thiazolidinediones and melatonin may decreases Δ-5 desaturase activity in liver microsomes which may help in decrease in hyperlipidemia in the combination treated group[18].
As the insulin resistance is characterized by decrease in the glucose uptake in muscles, hence, the glucose uptake in the isolated hemidiaphragm was evaluated. The glucose uptake decreased in isolated hemidiaphragm in dexamethasone alone treated mice, which indicates the development of insulin resistance. The insulin stimulated glucose uptake in skeletal muscle and fat cells is by the facilitative glucose transporter isoform GLUT4[38–40]. Most of GLUT4 (90%) is in the basal state sequestered in an intracellular pool, and insulin stimulates glucose uptake mainly by recruiting this intracellular GLUT4 pool to the plasma membrane[40–42]. Dexamethasone may interact with the intrinsic activity of GLUT4 with support to this it has been reported that, the translocation process of GLUT4 from intracellular compartments to the plasma membrane in response to insulin is reduced by dexamethasone[43,44]. The probable mechanisms behind the increase in glucose uptake in combination treated mice may be due to enhanced insulin sensitivity and GLUT- 4 expression in the muscle by thiazolidinediones[28] and melatonin[16,17]. The results of pioglitazone plus melatonin and rosiglitazone plus melatonin groups were more statistically significant than individual pioglitazone, rosiglitazone and melatonin treated groups which indicated that the combination treated groups showed better improvement in reducing insulin resistance than alone treated group of thiazolidinediones and melatonin.
Dexamethasone administration in mice showed reduction in the body weight when compared with normal control mice, which may be due to complex metabolic changes such as hyperleptinemia, decreased food consumption, weight loss etc. induced by increased ob gene expression[21,45]. The combination groups, pioglitazone plus melatonin and rosiglitazone plus melatonin tends to restore the body weight loss in mice, the mechanism behind restoration of body weight in the combination groups may be due to decreased leptin expression and protein catabolism by the thiazolidinediones and melatonin[13,46].
In hyperglycemic conditions, there is reduction in the endogenous antioxidant levels and increase in the free radical generation[47] which may directly or indirectly contributes to the insulin resistance. Insulin resistance induces release of cytokines like TNF-α (Tumor necrosis factor alpha), IL-8 (Interleukine-8) which leads to further development of the oxidative stress in liver by reducing endogenous mitochondrial levels of Cu/Zn SOD, glutathione and production H2O2 radicals[48].
Mice treated with dexamethasone alone showed decrease in the levels of antioxidant enzymes SOD, catalase, GSH and increased lipid peroxidation (MDA) in the liver homogenate. The pioglitazone plus melatonin and rosiglitazone plus melatonin showed increase levels of antioxidant enzymes such as GSH, SOD catalase in the liver and decrease in lipid peroxidation in liver homogenates. The antioxidant potential exhibited by combination of pioglitazone plus melatonin and rosiglitazone plus melatonin, might be due to potent antioxidant effect of melatonin which scavenges a variety of reactive oxygen and nitrogen species including hydroxyl radical, hydrogen peroxide, singlet oxygen, nitric oxide and peroxynitirite anion and also ability to reduce lipid peroxidation[49] as well as oxidative stress reducing effect of thiazolidinediones by decreased hyperglycemia[50,51].
In conclusion, the combination of thiazolidinediones with melatonin showed greater improvement in reducing the insulin resistance via antioxidant as well as hypoglycemia effects of melatonin and thiazolidinediones than individual agent's monotherapy and warrants further investigation.
Footnotes
Ghaisas, et al.: Thiazolidinediones in Combination with Melatonin for Insulin Resistance
REFERENCES
- 1.Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: Targets and new therapies. Pharmacol Ther. 2010;125:328–61. doi: 10.1016/j.pharmthera.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab. 2007;292:654–67. doi: 10.1152/ajpendo.00453.2006. [DOI] [PubMed] [Google Scholar]
- 3.Gholap S, Kar A. Gymnemic acids from Gymnema sylvestre. Potentially regulates dexamethasone-induced hyperglycemia in mice. Pharm Biol. 2005;43:192–5. [Google Scholar]
- 4.Thomas DM, Udagawa N, Hards DK, Gidsii J, Resret K, Injytt A, et al. Insulin receptor expression in primary and cultured oseoclast like cells. Bone. 1998;23:181–6. doi: 10.1016/s8756-3282(98)00095-7. [DOI] [PubMed] [Google Scholar]
- 5.Lambillotte C, Gilon P, Henquin J. Direct Glucocorticoid Inhibition of Insulin Secretion. J Clin Invest. 1999;99:414–23. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, Krause U, et al. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J. 2007;321:707–12. doi: 10.1042/bj3210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong JM, Simsolo RB, Saffari B, Kern PA. The regulation of lipoprotein lipase gene expression by dexamethasone in isolated rat adipocytes. Endocrinol. 1992;130:2310–6. doi: 10.1210/endo.130.4.1547742. [DOI] [PubMed] [Google Scholar]
- 8.Rask E, Olsson T, Soderberg S, Andrew RW, Livingstone DE, Johnson O. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–21. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 9.Buren J, Lai YC, Lundgren M, Eriksson J W, Jensen J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem Biophys. 2008;474:91–101. doi: 10.1016/j.abb.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Bjelakovic G, Beninati S, Pavlovic D, Kocic G, Jevtovic T, Kamenov B, et al. Dexamethasone and oxidative stress. J Basic Clin Physiol Pharmacol. 2007;18:115–27. doi: 10.1515/jbcpp.2007.18.2.115. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Motz E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 12.Ogihara T, Asano T, Katagiri H, Sakoda H, Anai M, Shojima N, et al. Oxidative stress induces insulin resistance by activating the nuclear factor-κB pathway and disrupting normal subcellular distribution of phosphatidylinositol-3-kinase. Diabetologia. 2004;47:794–805. doi: 10.1007/s00125-004-1391-x. [DOI] [PubMed] [Google Scholar]
- 13.Spiegelman BM. PPAR-γ: Adipogenic regulator and thiazolidinediones receptor. Diabetes. 1998;47:507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 14.Ruano G, Bernene J, Windemuth A, Bower B, Wencker D, Richard L, et al. Physiogenomic Comparison of Edema and BMI in Patients Receiving Rosiglitazone or Pioglitazone. Clinica Chimica Acta. 2008;400:48–55. doi: 10.1016/j.cca.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Reiter RJ, Acuna-Castroviejo D, Tan DX, Burkhardt S. Free radical mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann NY Acad Sci. 2001;939:200–15. [PubMed] [Google Scholar]
- 16.Nishida S, Segawa T, Murai I, Nakagawa S. Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of δ-5 desaturase activity. J Pineal Res. 2002;32:26–33. doi: 10.1034/j.1600-079x.2002.10797.x. [DOI] [PubMed] [Google Scholar]
- 17.Ha E, Yim S, Chung J, Yoon K, Kang I, Cho Y, et al. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J Pineal Res. 2006;41:67–72. doi: 10.1111/j.1600-079X.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 18.Kadhim HM, Ismail SH, Hussein KI, Bakir IH, Sahib AS, Khalaf BH, et al. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with Metformin. J Pineal Res. 2006;41:189–93. doi: 10.1111/j.1600-079X.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 19.Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 20.Ghaisas M, Navghare V, Takawale A, Zope V, Tanwar M, Deshpande A. Effect of Tectona grandis Linn. on dexamethasone-induced insulin resistance in mice. J Ethnopharmacol. 2009;122:304–7. doi: 10.1016/j.jep.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Gaikwad AB, Viswanad B, Ramarao P. PPAR-γ agonists partially restores hyperglycemia induced aggravation of vascular dysfunction to angiotensin II in thoracic aorta isolated from rats with insulin resistance. Pharmacol Res. 2007;55:400–7. doi: 10.1016/j.phrs.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Paskaloglu K, Xener G, Ayanoglu-Dulger G. Melatonin treatment protects against diabetes induced functional and biochemical changes in rat aorta and corpus cavernosum. Eur J Pharmacol. 2004;499:345–54. doi: 10.1016/j.ejphar.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Slater TF, Sawyer BC. The stimulatory effect of carbon tetrachloride and other halogenoalkanes or peroxidative reactions in rat liver fraction in vitro. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra HP, Fridovich I. Role of superoxide anion in auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 25.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. pp. 673–85. [Google Scholar]
- 26.Colowick SP, Kaplan NO, Packer L. Methods in Enzymology. London: Academic Press; 1984. pp. 21–125. [Google Scholar]
- 27.Ellaman GL. Tissue sulfhydryl group. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Prelovsek O, Mars T, Jevsek M, Podbregar M, Grubic Z. High dexamethasone concentration prevents stimulatory effects of TNF- alpha and LPS on IL-6 secretion from the precursors of human muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1651–6. doi: 10.1152/ajpregu.00020.2006. [DOI] [PubMed] [Google Scholar]
- 29.Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, et al. Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: possible protection of beta cells from oxidative stress. Metabol. 2004;53:488–94. doi: 10.1016/j.metabol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Saad MJ, Folli F, Araki E, Hashimoto N, Csermely P, Kahn CR. Regulation of insulin receptor, insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-F442A adipocytes. Effects of differentiation, insulin, and dexamethasone. Mol Endocrinol. 1994;8:545–57. doi: 10.1210/mend.8.5.7520127. [DOI] [PubMed] [Google Scholar]
- 31.Rondinone CM, Wang LM, Lonnroth P, Wesslau C, Pierce JH, Smith U. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus PNAS. 1997;94:4171–5. doi: 10.1073/pnas.94.8.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho E, Jansson PA, Axelsen M, Eriksson JW, Huang X, Groop L, et al. Low cellular IRS 1 gene and protein expression predict insulin resistance and NIDDM. FASEB J. 1999;13:2173–8. doi: 10.1096/fasebj.13.15.2173. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson J, Lonnroth P, Smith U. Insulin can rapidly increase cell surface insulin binding capacity in rat adipocytes. A novel mechanism related to insulin sensitivity. Diabetes. 1992;41:707–14. doi: 10.2337/diab.41.6.707. [DOI] [PubMed] [Google Scholar]
- 34.Kono T, Barham FW. The relationship between the insulin binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971;246:6210–6. [PubMed] [Google Scholar]
- 35.Pagano G, Cavallo-Perin P, Cassader M, Bruno A, Ozzello A, Masciola P, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest. 1983;72:1814–20. doi: 10.1172/JCI111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amatruda JM, Livingston JN, Lockwood DH. Cellular mechanisms in selected states of insulin resistance: human obesity, glucocorticoid excess, and chronic renal failure. Diabetes Metab Rev. 1985;1:293–317. doi: 10.1002/dmr.5610010304. [DOI] [PubMed] [Google Scholar]
- 37.Young PW, Cawthorne MA, Coyle PJ, Holder JC, Holman GD, Kozka IJ, et al. Repeat treatment of obese mice with BRL 49653, a new potent insulin sensitizer, enhances insulin action in white adipocytes: Association with increased insulin binding and cell-surface GLUT4 as measured by photoaffinity labeling. Diabetes. 1995;44:1087–92. doi: 10.2337/diab.44.9.1087. [DOI] [PubMed] [Google Scholar]
- 38.Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, et al. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100:2094–8. doi: 10.1172/JCI119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–8. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 40.Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin stimulated GLUT4 vesicle trafficking. J Biol Chem. 1999;274:2593–6. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 41.Charron MJ, Katz EB, Olson AL. GLUT4 gene regulation and manipulation. J Biol Chem. 1999;274:3253–6. doi: 10.1074/jbc.274.6.3253. [DOI] [PubMed] [Google Scholar]
- 42.Kandror KV, Pilch PF. Compartmentalization of protein traffic in insulin-sensitive cells. Am J Physiol. 1996;271:E1–14. doi: 10.1152/ajpendo.1996.271.1.E1. [DOI] [PubMed] [Google Scholar]
- 43.Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–77. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- 44.Garvey WT, Huecksteadt TP, Monzon R, Marshall S. Dexamethasone regulates the glucose transport system in primary cultured adipocytes: Different mechanisms of insulin resistance after acute and chronic exposure. Endocrinology. 1989;124:2063–73. doi: 10.1210/endo-124-5-2063. [DOI] [PubMed] [Google Scholar]
- 45.Kim DS, Kim TW, Park IK, Kang JS, Om AS. Effect of chromium picolinate supplementation on Insulin sensitivity, serum lipid, and body weight in dexamethasone-treated rats. Metabol. 2002;51:589–94. doi: 10.1053/meta.2002.31985. [DOI] [PubMed] [Google Scholar]
- 46.Shalam M, Harish MS, Farhana SA. Prevention of dexamethasone and fructose-induced insulin resistance in rats by SH-01D, a herbal preparation. Indian J Pharmacol. 2006;38:419–22. [Google Scholar]
- 47.Willi SM, Kennedy A, Brant BP, Wallace P, Rogers NL, Garvey WT. Effective use of thiazolidinediones for the treatment of glucocorticoid-induced diabetes. Diabetes Res Clin Pract. 2002;58:87–96. doi: 10.1016/s0168-8227(02)00127-4. [DOI] [PubMed] [Google Scholar]
- 48.Baydas G, Nedzvetsky VS, Nerush PA, Kirichenko SV, Demchenko HM, Reiter RJ. A novel role for melatonin: regulation of the expression of cell adhesion molecules in the rat hippocampus and cortex. Neurosci Lett. 2002;326:109–12. doi: 10.1016/s0304-3940(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 49.Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest. 2001;108:635–6. doi: 10.1172/JCI13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cam M, Yavuz O, Guven A, Ercan F, Bukan N, Ustundag S. Protective effects of chronic melatonin treatment against renal injury in streptozotocin-induced diabetic rats. J Pineal Res. 2003;35:212–20. doi: 10.1034/j.1600-079x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 51.Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension. 2004;43:48–56. doi: 10.1161/01.HYP.0000103629.01745.59. [DOI] [PubMed] [Google Scholar]


