Abstract
The aim of the present study was to study the effect of chronic treatment (9 weeks) of fluoxetine (20 mg/kg p.o.) a selective serotonin reuptake inhibitor on blood glucose level and in prevention of diabetic neuropathic pain perception. Evaluation of diabetic neuropathy was performed after 9 weeks of single injection of streptozotocin (70 mg/kg i.v.) in rats. Blood glucose level, glycated haemoglobin, grip strength, pain sensitivity and threshold in diabetic rats were measured at the end of 9 weeks. The results of the present study indicate that the 9 weeks treatment of fluoxetine demonstrates hypoglycemic effect; it marked decreases the blood glucose level in diabetic treated animals. There was also decrease in the grip strength in diabetic rat indicates to induction of neuropathy or nerve damage. Fluoxetine increase the grip strength of diabetic rats. There was also found loss of pain perception in diabetes rats which measured using hot plate and tail flick methods. Fluoxetine increases the licking time and withdrawal latency in hot plate and tail flick test respectively indicates the presence of pain perception and prevention of nerve damage demonstrates its protective effect in diabetic neuropathy. Our study concludes the chronic treatment of fluoxetine significantly decreases the glycemic level as well as it protected from the development of diabetic neuropathy.
Keywords: Diabetic neuropathy, diabeties mellitus, pain perception and fluoxetine
Diabetic neuropathy (DN) is a peripheral nerve disorder caused by diabetes which leads to cause a significant morbidity[1–3]. The risk of developing diabetic peripheral neuropathy (DPN) increases with duration of the disease and degree of glycemic control, and other contributing factors such as hypertension, dyslipidaemia, smoking, body mass index and hyperinsulinaemia[4]. The symptoms of diabetic neuropathy are often slight at first but can occasionally flare up suddenly and affect specific nerves so the affected individual will develop double vision, drooping eyelids, or weakness and atrophy of the muscles. Nerve damage caused by diabetes generally occurs over a period of years and may lead to problems with the digestive tract and sexual organs, which can cause indigestion, diarrhea or constipation, dizziness, bladder infections, and impotence[2,5].
The main risk factor for diabetic neuropathy is hyperglycemia. It is important to note that people with diabetes are more likely to develop symptoms relating to peripheral neuropathy as the excess glucose in the blood results in a condition known as Glucojasinogen. This condition is affiliated with erectile dysfunction and epigastric tenderness which in turn results in lack of blood flow to the peripheral intrapectine nerves which govern the movement of the arms and legs. The progression of neuropathy is dependent on the degree of glycemic control in both Type 1 and Type 2 diabetes. Duration of diabetes, age, cigarette smoking, hypertension, height and hyperlipidemia are also risk factors for diabetic neuropathy[4,6,7].
The pathogenesis of DPN is believed to be multifactorial with hyperglycaemia being the primary risk factor. Suggested theories that postulate the aetiopathogenesis of DN include abnormalities of protein glycation, sorbitol accumulation, polyol pathway flux, protein Kinase C activation, advanced glycation end products, receptor for advanced glycation end products, a decrease in neuronal nitric oxide synthase protein, and microvascular hypoxia, resulting in oxidative stress[1,4,8].
Since DN is not clearly understood, it is hard to make a definitive course of treatment[9]. Drugs that have been used in the management of DN include tricyclic antidepressants and selective serotonin reuptake inhibitors[9,10]. Many pharmacological options are available to treat DPN but still it is difficult for patients to obtain complete relief because of poor glycemic control. Prevention through strict glycemic control remains the mainstay of therapeutic intervention because effective disease modifying therapies are yet not available. In reported case study fluoxetine found to shows adverse hypoglycemic effect and it was associated with increased blood insulin levels and also regulate the level of neurotransmitters serotonin and epinephrine[11,12]. So by considering the hypoglycemic side effect the present study was aim at to evaluate the influence of chronic treatment of fluoxetine 20 mg/kg (p.o.) on blood glucose level and on progression neuropathy in STZ-induced 9 week diabetic rats.
MATERIALS AND METHODS
Glucose estimation kit (Hi-Media Lab, Mumbai), Streptozotocin (Gift sample from Nicholas Piramal Pvt. Ltd. Mumbai), Glycosylated Hb Kit. All the reagents and chemicals used in present study were of analytical grade. Fluoxetine (Sun Pharmaceuticals Gujarat, India) was prepared in 0.5% carboxymethyl cellulose using tween 20 (0.2% v/v) as a suspending agent.
Male Wister rat weighing 175-200 g were used. The animals were fed with standard diet (Amrut feed, Sangali, Maharastra), had free access to water under well ventilated condition of 12 h day light cycle. The animals were adapted to laboratory condition for 7 days prior to the experiments. The studies were performed with the approval of Institutional Animal ethics committee (IAEC) of Sudhakarrao Naik Institute of Pharmacy, Pusad, India (729/02/a/CPCSEA).
Experimental design:
Experimental model of diabeties was induced by i.v. injection of streptozotocin (70 mg/kg) in Wister rat (180-220 gm) and the chronic treatment of Fluoxetine (20 mg/kg p.o.) was started after stabilization blood glucose level from day 13th of streptozotocin injection. Effect of Fluoxetine on blood glucose level, glycosylated haemoglobin and diabetic neuropathic pain were evaluated after 9th week. Induction of diabetic neuropathy was evaluated by grip strength[13,14]and the neuropathic pain perception by threshold by tail flick and hot plate method[13–15].
Measurement of DN by behavioral studies:
A grip strength determination was used for evaluating neuromuscular strength[13,14]. The grip strength of animals was measured by simply hanging of animals with their Fore limb on fine metal wire which was held at two end of pole. The time taken to hold the metal wire to fall on the surface was considered for the muscle strength determination. The animals whose muscle or nerves get damage or weak it get fall soon on the floor. The force achieved (in terms of time) by the animal for staying in hanging stage was recorded.
Evaluation of the effect of diabetic neuropathy on pain sensitivity and pain threshold were done by Hot Plate and Tail Flick methods[12,14,15]. The rats were placed on the hot plate (55-58°) and the time until either licking or jumping occurs was recorded by a stop watch. A cut off time of 10 s was kept to avoid damage to the paw of the animal.
Evaluation of pain threshold in diabetic rats was determined by withdraw latency in tail flick test. Tail of each diabetic rat was exposed to radiant heat which was given by placing the hot water in glass. The intensity of radiant heat (55-58°) was adjusted to obtained withdrawal latency of not more than 6 second in both diabetic and non-diabetic rats. The tail flick latency is the time interval taken by rat to flick its tail after exposure to a source of radiant heat. Cut of time was fixed at 10 s.
Statistical methods:
Blood glucose level was analyzed by ANOVA followed by Dunnet test. Data of grip strength, pain sensitivity and threshold and the glycosylated haemoglobin were analyzed by student unpaired t test. The significant difference was compared at P<0.05. (Graph Pad Prism version 5.0)
RESULTS AND DISCUSSION
In developed countries diabetes is the leading known cause for the neuropathy pain and it is the most common complication and greatest source of morbidity and mortality in diabetes patients. It is estimated that the prevalence of neuropathy in diabetic patients is approximately 20%. DN is implicated in 50-75% of non-traumatic amputations. Generally, the largest cases of neuropathy in patients (referred to as idiopathic in origin) are of unknown causes. Other known causes include genetic factors, damaging chemical agents such as chemotherapy drugs, and HIV. Since diabetic neuropathy is not clearly understood, it is hard to make a definitive course of treatment[1,16].
Various classes of antidepressant agents that help in regulation and treatment of depressive emotions and naturopathic pain by sustaining balanced level of two neurotransmitters serotonin and norepinephrine. Serotonin and norepinephrine are implicated in modulating descending inhibitory pain pathways in the central nervous system, and are known to help in regulating emotions as well as sensitivity to pain[1,17].
In diabeties there is loss of pain perception and it is thought due to nerve damage and induction of peripheral neuropathy[18,19]. Painful diabetic neuropathy significantly affects the quality of life; so far no ideal drug has been available for its management. In the absence of curative therapy, the main aim of the management is to provide symptomatic pain control along with good glycemic control. In reported case studies fluoxetine found to shows hypoglycemic side effect and it was associated with increased blood insulin levels and also regulate the level of neurotransmitters serotonin and epinephrine[10–12]. So by considering the hypoglycemic side effect and regulating the level of neurotransmitters the present study was undertaken to evaluate the influence of chronic treatment (9 weeks) of fluoxetine 20 mg/kg (p.o.) on blood glucose level and on progression neuropathy in STZ-induced diabetic rats.
Results of chronic treatments with fluoxetine demonstrates to significant decreases in the blood glucose level at 30th and 60th day while the more significant (P<0.01) effect observed only on 60th day (fig. 1). The observed effect of fluoxetine was comparable to standard hypoglycemic agent glibenclamide. Previously it is reported to show the hypoglycemic reaction in one of the case report of patient administered with fluoxetine. The results are also similar with the earlier findings of sertalline which belongs to same antidepressant category[10–13].
Fig. 1.
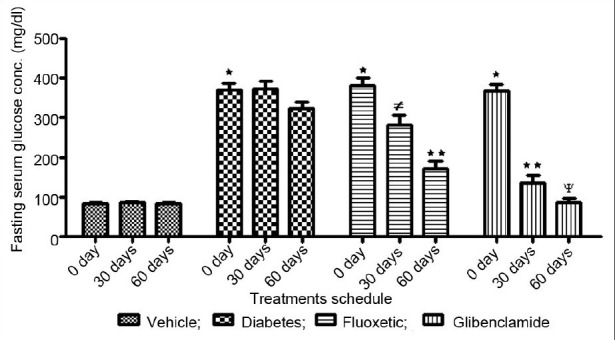
Effect of chronic treatments of Fluoxetine on blood glucose regulation in diabetic rats.  Vehicle;
Vehicle;  Diabetes;
Diabetes;  Fluoxetic;
Fluoxetic;  Glibenclamide. Data expressed as means ± s.d; n = 5. The data are statistically significant (ANOVA followed by Dunnet test). ∗Indicates significant (P<0.001) induction of diabeties compare to vehicle control at 0 day. ≠ indicates significant (P<0.05) hypoglycemic effect compared to 0 day reading. ∗∗Indicates significant (P<0.01) hypoglycemic effect compared to 0 day reading. ψindicates significant (P<0.001) hypoglycemic effect compared to 0 day reading.
Glibenclamide. Data expressed as means ± s.d; n = 5. The data are statistically significant (ANOVA followed by Dunnet test). ∗Indicates significant (P<0.001) induction of diabeties compare to vehicle control at 0 day. ≠ indicates significant (P<0.05) hypoglycemic effect compared to 0 day reading. ∗∗Indicates significant (P<0.01) hypoglycemic effect compared to 0 day reading. ψindicates significant (P<0.001) hypoglycemic effect compared to 0 day reading.
In the present study we measured the glycosylated haemoglobin in diabetes and was treated with fluoxetine. In STZ-induced diabetic rats, increased (P<0.05) levels of glycosylated haemoglobin were found. The observed increase in the level of glycosylated haemoglobin in diabetic control group rats might be due to the presence of excessive amounts of blood glucose. During diabetes, the excess of glucose present in blood reacts with the haemoglobin to form HbA1c which has been found to be increased over a long period of time in diabetes mellitus[16]. There is an evidence that glycation may itself induce the generation of oxygen derived free radicals in diabetic condition which may be the leading cause of development of diabetic neurological complications like neuropathic pain, depression.[18–20]The result of the present study indicates that the level of glycosylated haemoglobin significantly (P<0.05) decreases after chronic treatments with fluoxetine and this may be due to decrease in the blood glucose level (Table 1).
TABLE 1.
LEVEL OF GLYCOSYLATED HAEMOGLOBIN IN STZ INDUCED DIABETIC RATS

The main risk factor for DN is hyperglycemia. People with diabetes are more likely to develop symptoms relating to peripheral neuropathy as the excess glucose in the blood results in a condition known as Glucojasinogen[20]. The risk of developing DPN increases with duration of the disease and degree of glycemic control. The symptoms of diabetic neuropathy are often slight at first but can occasionally flare up suddenly and affect specific nerves so that an affected individual will develop double vision, drooping eyelids, or weakness, atrophy of the muscles and nerve damage[2,5].
In the present study induction of diabetic neuropathy was evaluated in terms of muscle and nerve strength by measuring grip strength in 9 week STZ-induced diabetic rats. The diabetic animals demonstrates to significant (P<0.05) decreased the grip strength as compared to normal rats indicating muscle weakness and induction of neuropathy, whereas chronic treatment with fluoxetine indicated to raise (P<0.05) the grip strength in diabetic rats (fig. 2). Thus the results of the present study demonstrate the protective effect of fluoxetine on grip strength in diabetic condition.
Fig. 2.
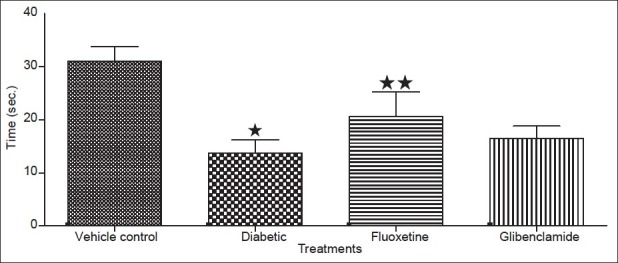
Effect of fluoxetine on grip strength after 9 weeks in diabetic rats. Data expressed as mean of three reading (s) for each animals in a group±sd, n = 5. ∗Values were statistically significant (P<0.05) compared to vehicle group. ∗∗Values were statistically significant (P < 0.05) compared to diabetic control group (student unpaired t test).
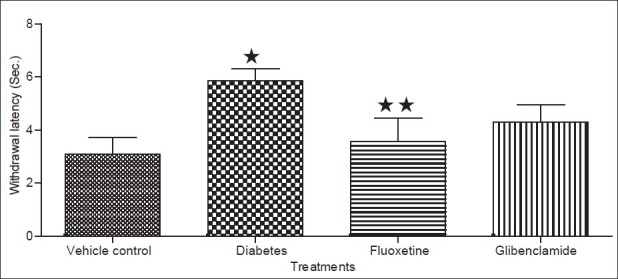
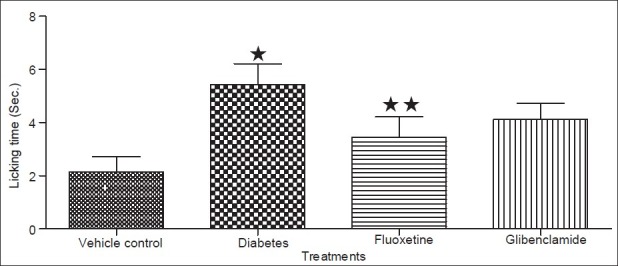
Diabetic condition has been reported to cause a decrease in the antinociceptive effect of drugs like morphine[18,19]. Previously it is reported that fluoxetine has antinociceptive effect[21]while the results of the present study demonstrated that the antinociceptive effect of fluoxetine is attenuated in diabetic animals which was similar to that reported for morphine in diabetic animals earlier[18,19]. The decreased antinociceptive effect in diabetes may be due to development of DN characterized by neurodegeneration resulting in the loss of pain perception[18,20]. In the present study the pain threshold measured by hot plate and tail flick method of analgesia, indicated to significant (P<0.05) decreased in the foot withdrawal and flicking latency (time interval taken by rats to withdrawal its legs or flick its tail after exposure to source of radiant heat) in both hot plate and tail flick test respectively in diabetic rats (figs 3 and 4). These results indicated a loss of pain perception in diabetic rats in both hot plate and tail flick method, which could be attributed to nerve damage resulting due to the development of DN in 9-weeks diabetic rats[18]. While chronic treatment with fluoxetine caused an increase (P<0.05) in the latency time in both models respectively. The increase in latency time indicate presence of pain perception in animals thus it conclude that fluoxetine protect from the nerve damage in the diabetic animals. The prevention or management in the DN in the present study may be due to controlling the glycemic level in diabetic rats.
Fig. 3.
Effect of fluoxetine on pain sensation using Hot plate method. Data expressed as mean±sd, n = 5. ∗Values were statistically significant (P<0.05) compared to vehicle group. ∗∗Values were statistically significant (P<0.05) compared to diabetic control group (student unpaired t test).
Fig. 4.
Effect of fluoxetine on pain sensation using tail flick method. Data expressed as mean±sd, n = 5. ∗Values were statistically significant (P<0.05) compared to vehicle group. ∗∗Values were statistically significant (P<0.05) compared to diabetic control group (student unpaired t test).
Thus from the results it indicates that chronic treatment of fluoxetine reduced the blood glucose level as well as prevent progression of diabetic neuropathy in streptozotocin-induced diabetic rats. So it can be concluded that fluoxetine can be used as ideal drug which could offer a better choice in the curative therapy for DN. Which can act by, either preventing the nerve damage or by providing symptomatic pain control along with good glycemic control. Hence, it could be helpful in treating the diabetic patient having the complication like diabetic neuropathy.
Footnotes
Tembhurne and Sakarkar: Effect of Fluoxetine on Diabetic Neuropathy
REFERENCES
- 1.Dejgaard A. Pathophysiology and treatment of diabetic neuropathy. Diabetes Med. 1998;15:97–112. doi: 10.1002/(SICI)1096-9136(199802)15:2<97::AID-DIA523>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Mark AR. Neuropathies associated with diabetes. Med Clin North Am. 1993;27:111–24. doi: 10.1016/s0025-7125(16)30275-9. [DOI] [PubMed] [Google Scholar]
- 3.Rai S, Gupta A, Rai M, Birari KV. Diabetic peripheral neuropathy-emerging pharmacologic options. Bombay Hosp J. 2008;50:595. [Google Scholar]
- 4.Boulton AJ, Malik AR, Arezzo CJ, Sosenko MJ. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–86. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 5.Ardid D, Guilbaund G. Antinociceptive effects of acute and chronic injection of tricyclic antidepressants drugs in a new model of mono neuropathy in rats. Pain. 1992;49:279–87. doi: 10.1016/0304-3959(92)90152-2. [DOI] [PubMed] [Google Scholar]
- 6.Duby JJ, Campbell K, Setter SM. Diabetic neuropathy: an intensive review. Am J Health Syst Pharm. 2004;61:160–76. doi: 10.1093/ajhp/61.2.160. [DOI] [PubMed] [Google Scholar]
- 7.Apfel SC, Kessler JA. Neurotrophic factors in the therapy of peripheral neuropathy. Baillieres Clin Neurol. 1995;4:593–606. [PubMed] [Google Scholar]
- 8.Ziegler D. Antioxidant treatment in diabetic polneuropathy-update 2006. Ystad, Sweden: In: 16th Annual Neurodiab Meeting. [Google Scholar]
- 9.Zareba G. Pregabalin: A new agent for the treatment of neuropathic pain. Drugs Today. 2005;41:509–16. doi: 10.1358/dot.2005.41.8.910482. [DOI] [PubMed] [Google Scholar]
- 10.Fields HL, Heinricher MM, Mason P. Neurotransmitter in nociceptive modulatory circuits. Ann Rev Neurosci. 1991;14:219–45. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- 11.Lear J, Burden AC. Fluoxetine side-effects mimicking hypoglycaemia. Lancet. 1992;339:1296. doi: 10.1016/0140-6736(92)91624-h. [DOI] [PubMed] [Google Scholar]
- 12.Deeg MA, Lipkin EW. Hypoglycemia associated with the use of fluoxetine. West J Med. 1996;164:262–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Talha J, Shakya AK, Mehnaz K, Hussain S. Amitriptyline and Sertraline in Diabetic Neuropathy: A Comparative View. Int J Health Res. 2008;1:73–8. [Google Scholar]
- 14.D’Amour WL, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74. [Google Scholar]
- 15.Sawynok J, Reid AR, Esser MJ. Periphreal antinociceptive actions of desipramine and fluoxetine in an inflammatory and neuropathic pain test in the rat. Pain. 1999;82:149–55. doi: 10.1016/S0304-3959(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 16.Braham D, Trinder P. An improved color reaction for the determination of blood glucose by oxidase system. Analyst. 1972;97:142–4. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 17.Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7:257–77. [PubMed] [Google Scholar]
- 18.Raz I, Hasdi D, Seltzer, Melmed RN. Effect of hyperglycemia on pain perception and on efficacy of morphine analgesia in rats. Diabetes. 1988;37:1253–9. doi: 10.2337/diab.37.9.1253. [DOI] [PubMed] [Google Scholar]
- 19.Simon GS, Dewey WL. Effect of streptozotocin induced diabeties on the antinociceptive potency of Morphine. J Pharmacol Exp Ther. 1981;218:318–23. [PubMed] [Google Scholar]
- 20.Ochoa JL. Pain mechanism in neuropathy. Curr Opin Neurol. 1994;7:407–14. doi: 10.1097/00019052-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Kurlekar PN, Bhatt JD. Study of the antinociceptive activity of fluoxetine and its interaction with morphine and naloxone in mice. Indian J Pharmacol. 2004;36:369–72. [Google Scholar]


