Abstract
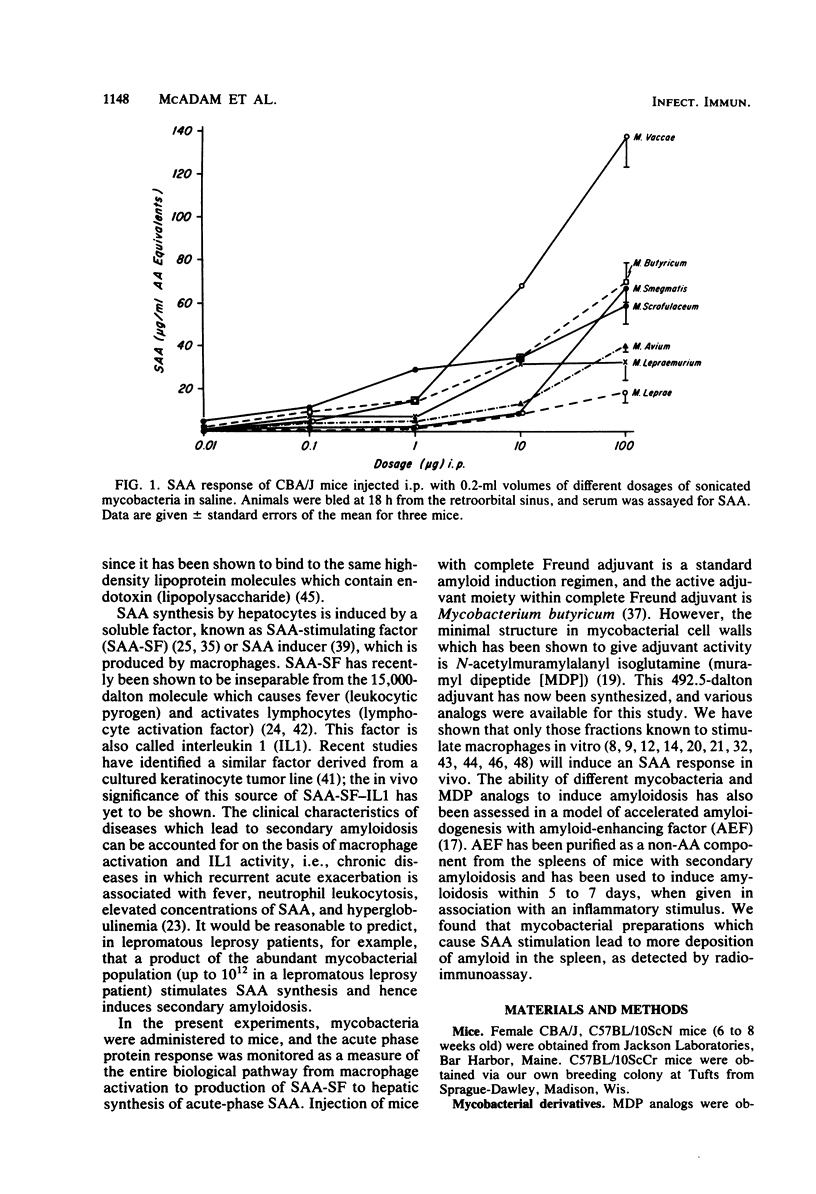
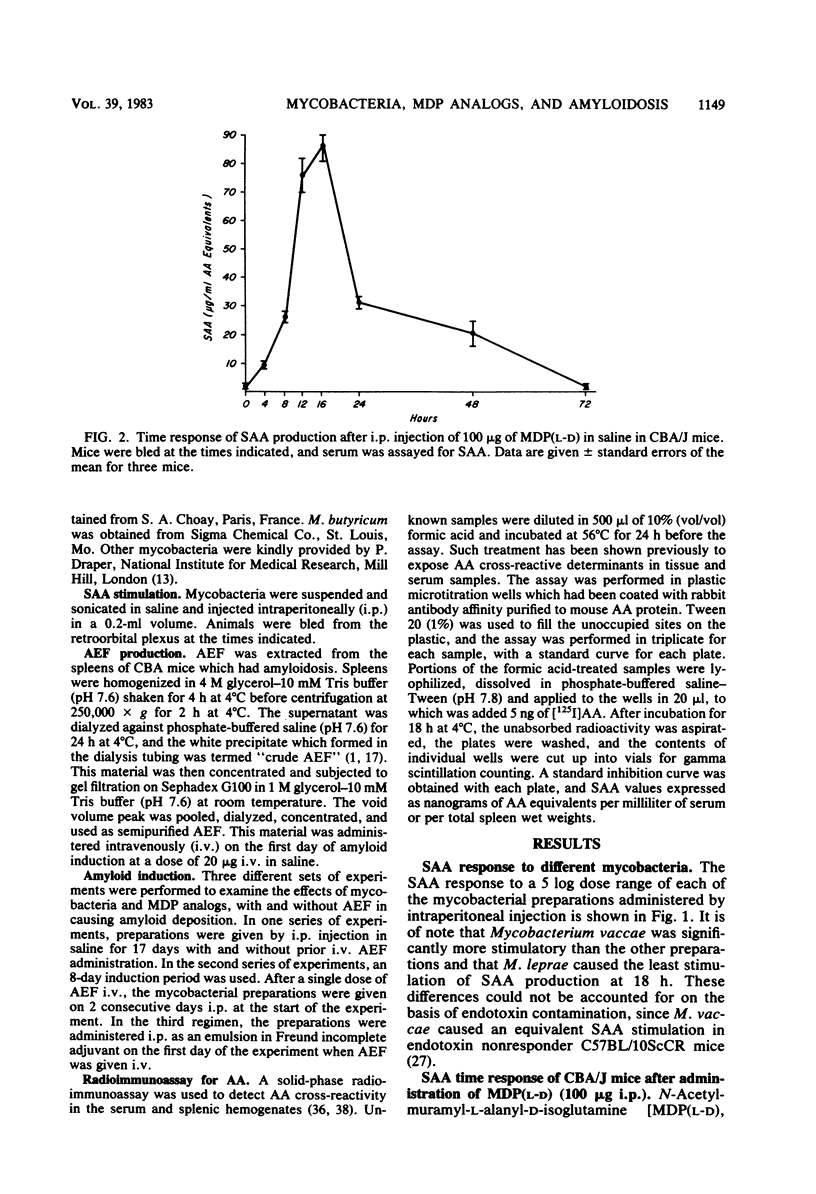
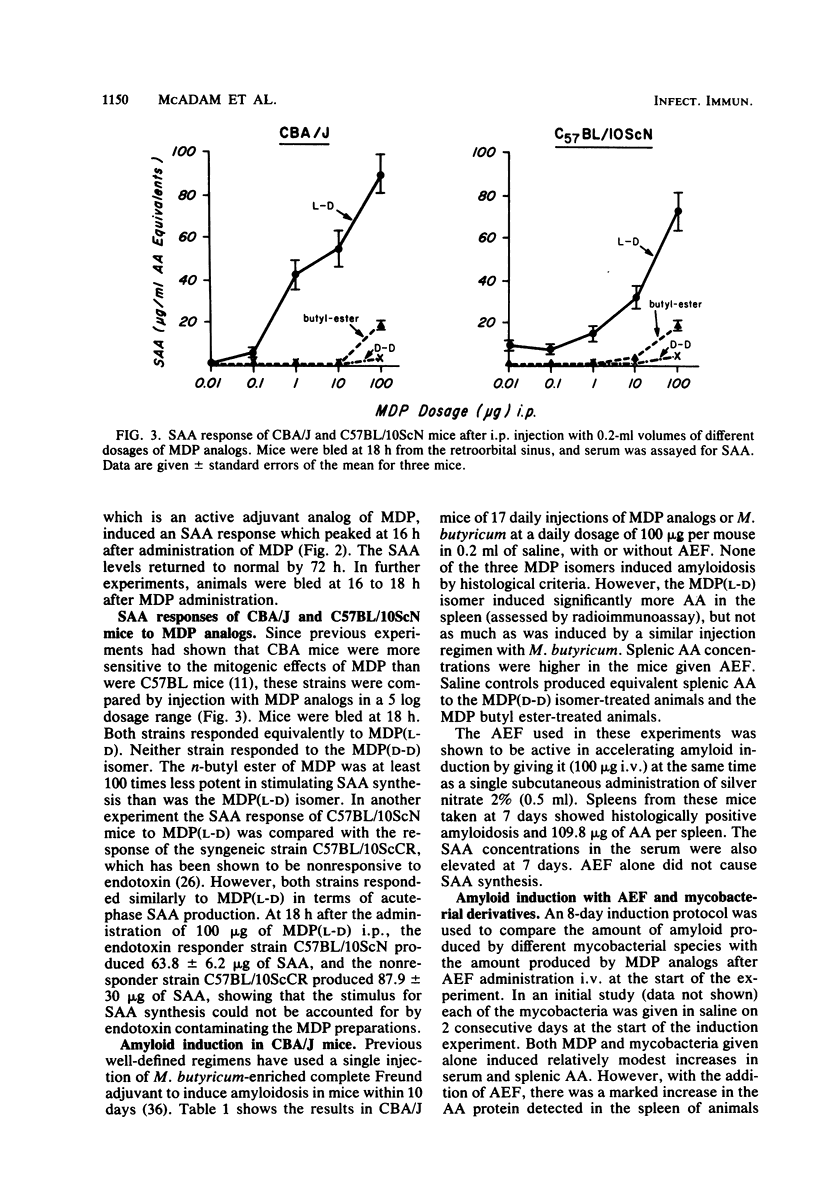
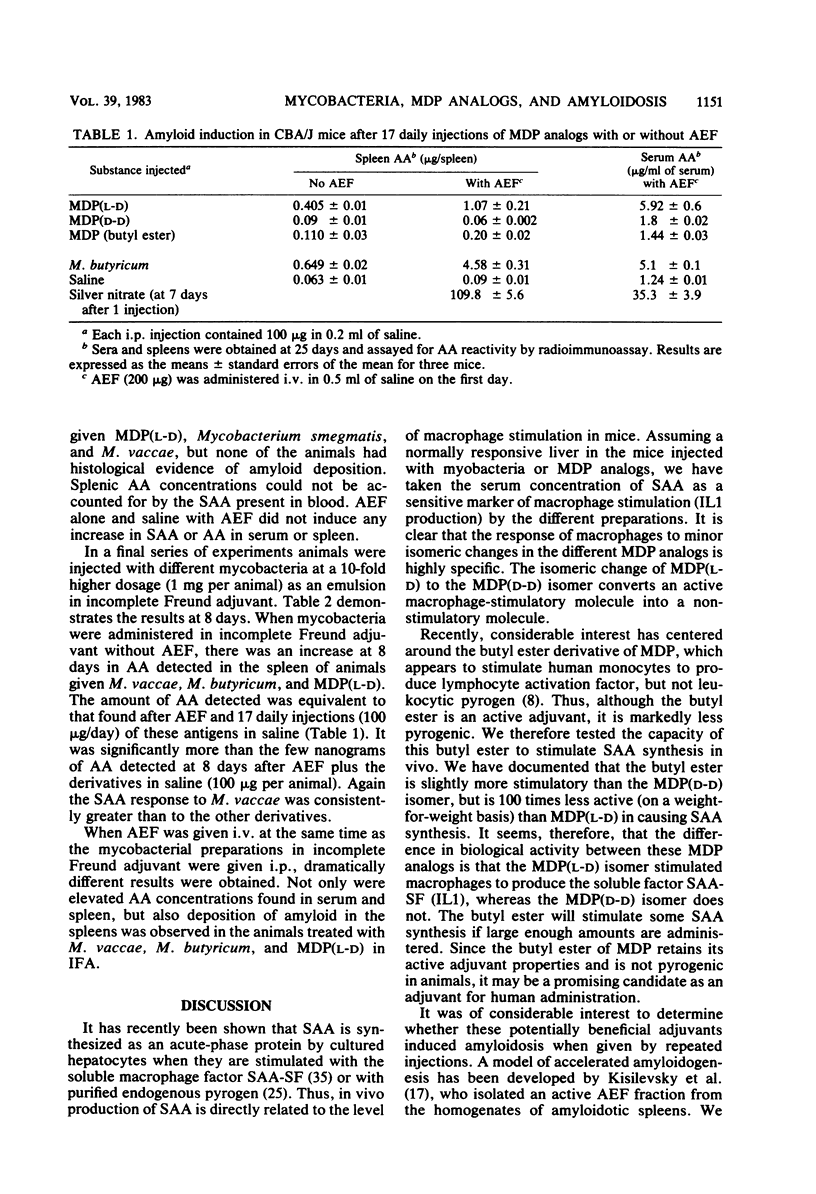
Serum amyloid A protein (SAA) elevation accompanies induction of secondary amyloidosis in mice given Mycobacterium butyricum in Freund adjuvant. The synthesis of SAA by cultured hepatocytes is induced by a macrophage-derived mediator, which has been identified as interleukin 1. In these studies, SAA synthesis has been used as an index of macrophage activation to examine the in vivo response of mice to challenge with seven different mycobacteria and with synthetic analogs of the immunoadjuvant N-acetylmuramyl-L-alanyl-D-isoglutamine [MDP(L-D)]. SAA synthesis was stimulated by administration (by the intraperitoneal route) of the mycobacteria dissolved in saline, with Mycobacterium vaccae being the most active and Mycobacterium leprae being the least stimulatory. MDP(L-D), which is the minimal structure (molecular weight, 492) able to substitute for mycobacteria in Freund adjuvant, stimulated SAA synthesis, whereas the MDP(D-D) isomer was inactive. The butyl ester of MDP, which induces no detectable pyrogenicity but retains adjuvanticity, required a 100-fold greater dosage than MDP(L-D) in stimulating SAA synthesis. Amyloidosis was detected histologically only when active SAA inducers MDP(L-D), M. vaccae, and M. butyricum, were administered in incomplete Freund adjuvant, with amyloid-enhancing factor. These studies demonstrated that SAA elevation was a sensitive in vivo marker of the capacity of antigens to stimulate macrophages to produce interleukin 1. A point of considerable relevance to the human use of MDP was the observation that repeated injections of the adjuvant MDP in saline did not induce secondary amyloidosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr G. M., Modabber F. Z., Rook G. A., Mehrotra M. L., Stanford J. L., Chedid L. Absence of antibodies to muramyl dipeptide in patients with tuberculosis or leprosy. Clin Exp Immunol. 1982 Jan;47(1):53–58. [PMC free article] [PubMed] [Google Scholar]
- Bausserman L. L., Herbert P. N., McAdam K. P. Heterogeneity of human serum amyloid A proteins. J Exp Med. 1980 Sep 1;152(3):641–656. doi: 10.1084/jem.152.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Aldo-Benson M. Effect of purified protein SAA on immune response in vitro: mechanisms of suppression. J Immunol. 1979 May;122(5):2077–2082. [PubMed] [Google Scholar]
- Chedid L. A., Parant M. A., Audibert F. M., Riveau G. J., Parant F. J., Lederer E., Choay J. P., Lefrancier P. L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982 Feb;35(2):417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rodríguez Ochoa G., Ulrich M., Avila J. L., Goihman M. Elimination of Mycobacterium leprae subsequent to local in vivo activation of macrophages in lepromatous leprosy by other mycobacteria. Clin Exp Immunol. 1974 Jun;17(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L., Lefrancier P., Choay J. In vitro spleen cell responsiveness to various analogs of MDP (N-acetylmuramyl-L-alanyl-D-isoglutamine), a synthetic immunoadjuvant, in MDP high-responder mice. Cell Immunol. 1978 Jan;35(1):173–179. doi: 10.1016/0008-8749(78)90137-5. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Elin R. J., Chedid L., Wolff S. M. The pyrogenicity of the synthetic adjuvant muramyl dipeptide and two structural analogues. J Infect Dis. 1978 Dec;138(6):760–767. doi: 10.1093/infdis/138.6.760. [DOI] [PubMed] [Google Scholar]
- Draper P. Cell walls of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):95–98. [PubMed] [Google Scholar]
- Fidler I. J., Sone S., Fogler W. E., Barnes Z. L. Eradication of spontaneous metastases and activation of alveolar macrophages by intravenous injection of liposomes containing muramyl dipeptide. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1680–1684. doi: 10.1073/pnas.78.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S. Presence of auto-anti-idiotypic antibody during the normal human immune response to tetanus toxoid antigen. J Immunol. 1982 Jul;129(1):139–144. [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Keizman I., Rimon A., Sohar E., Gafni J. Amyloid accelerating factor. Purification of a substance from human amyloidotic spleen that accelerated the formation of casein-induced murine amyloid. Acta Pathol Microbiol Scand Suppl. 1972;233:172–177. [PubMed] [Google Scholar]
- Kisilevsky R., Axelrad M., Corbett W., Brunet S., Scott F. The role of inflammatory cells in the pathogenesis of amyloidosis. Lab Invest. 1977 Dec;37(6):544–553. [PubMed] [Google Scholar]
- Kronvall G., Husby G., Samuel D., Bjune G., Wheate H. Amyloid-related serum component (protein ASC) IN LEPROSY PATIENTS. Infect Immun. 1975 May;11(5):969–972. doi: 10.1128/iai.11.5.969-972.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie G., Zucker-Franklin D., Franklin E. C. Elastase-type proteases on the surface of human blood monocytes: possible role in amyloid formation. J Immunol. 1980 Jul;125(1):175–180. [PubMed] [Google Scholar]
- Lederer E., Adam A., Ciorbaru R., Petit J. F., Wietzerbin J. Cell walls of Mycobacteria and related organisms; chemistry and immunostimulant properties. Mol Cell Biochem. 1975 May 30;7(2):87–104. doi: 10.1007/BF01792076. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Li J., Knowles J., Foss N. T., Dinarello C. A., Rosenwasser L. J., Selinger M. J., Kaplan M. M., Goodman R., Herbert P. N. The biology of SAA: identification of the inducer, in vitro synthesis, and heterogeneity demonstrated with monoclonal antibodies. Ann N Y Acad Sci. 1982;389:126–136. doi: 10.1111/j.1749-6632.1982.tb22131.x. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Ryan J. L. C57BL/10/CR mice: nonresponders to activation by the lipid a moiety of bacterial lipopolysaccharide. J Immunol. 1978 Jan;120(1):249–253. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Rothman W., Reinherz E., Schlossman S. F., Bloom B. R. Delineation of a human T cell subset responsible for lepromin-induced suppression in leprosy patients. J Immunol. 1980 Sep;125(3):1183–1188. [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Nath I., Van Rood J. J., Mehra N. K., Vaidya M. C. Natural suppressor cells in human leprosy: the role of HLA-D-identical peripheral lymphocytes and macrophages in the in vitro modulation of lymphoproliferative responses. Clin Exp Immunol. 1980 Nov;42(2):203–210. [PMC free article] [PubMed] [Google Scholar]
- Nelson D. S., Penrose J. M., Waters M. F., Pearson J. M., Nelson M. Depressive effect of serum from patients with leprosy on mixed lymphocyte reactions. Influence of anti-leprosy treatment. Clin Exp Immunol. 1975 Dec;22(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Sullivan L. Serum amyloid A: evidence for its origin in polymorphonuclear leukocytes. J Clin Invest. 1978 Dec;62(6):1181–1186. doi: 10.1172/JCI109237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg M. A., Masuda A., Benson M. D., Mendes N. F. Serum amyloid protein SAA, C-reactive protein and lysozyme in leprosy. Int J Lepr Other Mycobact Dis. 1979 Jun;47(2):133–137. [PubMed] [Google Scholar]
- Selinger M. J., McAdam K. P., Kaplan M. M., Sipe J. D., Vogel S. N., Rosenstreich D. L. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980 Jun 12;285(5765):498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Ignaczak T. F., Pollock P. S., Glenner G. G. Amyloid fibril protein AA: purification and properties of the antigenically related serum component as determined by solid phase radioimmunoassay. J Immunol. 1976 Apr;116(4):1151–1156. [PubMed] [Google Scholar]
- Sipe J. D., McAdam K. P., Torain B. F., Pollock P. S. Isolation and structural properties of murine SAA--the acute phase serum precursor of amyloid AA. Immunol Commun. 1977;6(1):1–12. doi: 10.3109/08820137709055799. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., McAdam K. P., Uchino F. Biochemical evidence for the biphasic development of experimental amyloidosis. Lab Invest. 1978 Jan;38(1):110–114. [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Ryan J. L., McAdam K. P., Rosenstreich D. L. Detection of a mediator derived from endotoxin-stimulated macrohpages that induces the acute phase serum amyloid A response in mice. J Exp Med. 1979 Sep 19;150(3):597–606. doi: 10.1084/jem.150.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Souvannavong V., Adam A. Opposite effects of the synthetic adjuvant N-acetyl-muramyl-L-alanyl-D-isoglutamine on the immune response in mice depending on experimental conditions. Eur J Immunol. 1980 Aug;10(8):654–656. doi: 10.1002/eji.1830100814. [DOI] [PubMed] [Google Scholar]
- Sztein M. B., Vogel S. N., Sipe J. D., Murphy P. A., Mizel S. B., Oppenheim J. J., Rosenstreich D. L. The role of macrophages in the acute-phase response: SAA inducer is closely related to lymphocyte activating factor and endogenous pyrogen. Cell Immunol. 1981 Sep 1;63(1):164–176. doi: 10.1016/0008-8749(81)90037-x. [DOI] [PubMed] [Google Scholar]
- Tenu J. P., Lederer E., Petit J. F. Stimulation of thymocyte mitogenic protein secretion and of cytostatic activity of mouse peritoneal macrophages by trehalose dimycolate and muramyldipeptide. Eur J Immunol. 1980 Aug;10(8):647–653. doi: 10.1002/eji.1830100813. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., McAdam K. P., Ulevitch R. J. Interactions of bacterial lipopolysaccharide with acute-phase rabbit serum and isolation of two forms of rabbit serum amyloid A. J Immunol. 1982 Mar;128(3):1420–1427. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Ward P. A., Goralnick S., Bullock W. E. Defective leukotaxis in patients with lepromatous leprosy. J Lab Clin Med. 1976 Jun;87(6):1025–1032. [PubMed] [Google Scholar]
- Watson J., Whitlock C. Effect of a synthetic adjuvant on the induction of primary immune responses in T cell-depleted spleen cultures. J Immunol. 1978 Jul;121(1):383–389. [PubMed] [Google Scholar]



