Abstract
Aim:
The present study was designed to investigate the antidepressant potential of (4-phenylpiperazin-1-yl) (quinoxalin-3-yl) methanone (4a), a novel 5-HT3 receptor antagonist, with an optimal log P (2.84) and pA2 value (7.3) greater than ondansetron (6.9) using rodent behavioural models of depression.
Materials and Methods:
Swiss albino mice were used in actophotometer test, forced swim test (FST) and 5-hydroxytryptophan (5-HTP) induced head twitch response. Reserpine induced hypothermia (RIH) and olfactory bulbectomy were performed in male Wistar rats. Statistical analysis was carried out by using one-way analysis of variance followed by Tukey's test.
Results:
Acute treatment of 4a (1-4 mg/kg, i.p.) in mice produced antidepressant-like effects in FST without affecting the baseline locomotion in actophotometer test. Further, 4a (2-4 mg/kg, i.p.) potentiated the 5-HTP induced head twitches response in mice and also antagonized RIH in rats. Furthermore, sub-chronic (14 days) treatment with 4a (2-4 mg/ kg, p.o.) significantly attenuated the behavioural anomalies induced by bilateral olfactory bulbectomy in rats in modified open field exploration.
Conclusions:
These preliminary investigations confirm that 4a exhibits antidepressant-like activity in behaviour based rodent models of depression.
KEY WORDS: (4-phenylpiperazin-1-yl) (quinoxalin-2-yl) methanone, 4a; 5-HT3 receptor antagonist, depression, forced swim test, olfactory bulbectomy
Introduction
Depression is a severe psychiatric disorder with a lifetime prevalence as high as 21%.[1] According to the World Health Organization (WHO), it will be the second largest global burden of disease by the year 2020, which illustrates the severity and impact of the disorder.[1,2] Depression is not a unitary disorder and most experts agree that it should be considered a syndrome comprised of a spectrum of various symptoms, making animal research into the underlying mechanisms difficult, but feasible.[3] Several promising attempts at explaining the neurobiology of depression have focused on the serotonergic neurotransmitter system as a principal target for several antidepressant agents.[2,4]
Serotonin type-3 (5-HT3) receptor antagonists are currently used in the management of nausea and vomiting associated with cancer chemotherapy.[5] Interestingly, in the last decade, these molecules have been extensively evaluated for their neuro-psychopharmacological potentials in various pre-clinical and few clinical studies.[6] Several pre-clinical (behavioural, neurochemical and genetic) studies have provided evidences linking 5-HT3 receptors and depression. It has been demonstrated that 5-HT3 receptor antagonists reverse escape deficits in rat learned helplessness test, a sensitive antidepressant screening method.[7]
Selective 5-HT3 receptor antagonist, ondansetron (OND) altered local cerebral glucose utilization in the rat median raphe, and also potentiated the anti-immobility effects of Selective serotonin reuptake inhibitors (SSRIs) indicating the role played by 5-HT3 receptors in depression.[8,9] In mouse FST, it has been observed that the antidepressant-like effect of re-uptake inhibitors is associated with potassium ion (K+) channel linked 5-HT3 receptors.[10] A study with 5-HT3 receptor antagonist, ICS 205-930 has been shown to decrease the duration of immobility in forced swim test (FST).[11] The hypothesis of the antidepressant effects of 5-HT3 receptor antagonists and the role of 5-HT3 receptors in the neurobiology of depression is strengthened by recent preclinical reports. MDL72222 (bemesetron), a selective 5-HT3 receptor antagonist, has been shown to reduce the duration of immobility in the mouse tail suspension test (TST) and the antidepressant-like effect was augmented by ketamine.[12] Similarly, female Fischer rats treated with tropisetron spent less time immobile in the FST.[13]
A series of structurally novel quinoxalin -2-carboxamides were designed, synthesized and evaluated for their 5-HT3 antagonistic potential. The compounds were tested for 5-HT3 receptor antagonistic property in isolated guinea pig ileum and the pA2 values were determined against 2-methyl-5-hydroxytryptamine.[14] Animal models of depression have been utilized vigorously to screen novel compounds and were originally designed as screening tests to assess the efficacy of various antidepressants. Hence, we utilized a battery of behavioural tests - FST,[15] 5-hydroxytryptophan (5-HTP) induced head twitch response in mice,[16] reserpine induced hypothermia (RIH)[17] and olfactory bulbectomy[18] in rats to provide significant information about antidepressant-like activity of 4a.
In the present study, compound 4a was selected from a series of compounds[19] for preliminary antidepressant potential based on the optimal log P (2.84) and pA2 value (7.3) greater than 5-HT3 receptor antagonist, OND (6.9) using rodent behavioural models of depression.
Materials and Methods
Animals
Swiss Albino mice (25-30 g) and Wistar rats (225- 300 g) were obtained from Lala Lajpat Rai University of Veterinary and Animal Science, Hisar, Haryana, India (Reg. No. 417/01/a/ CPCSEA). All procedures were in adherence to Institutional Animal Ethics Committee (Protocol No. IAEC/RES/14/4). The animals were housed in laboratory cages and maintained under standard light/ dark cycle (light on 6:00–18:00 hours), temperature (23 ± 2°C) and humidity (50 - 60%) conditions. The animal were given free access to food (standard food pellets) and filtered water. Behavioural studies were carried out during the light phase (9.00 a.m. - 2.00 p.m.).
Chemistry of (4-phenylpiperazin-1-yl) (quinoxalin-2-yl) methanone (4a)
The intermediates 2-(1,2,3,4-tetrahydroxybutyl) quinoxaline (2) and quinoxalin-2-carboxylic acid (3) were synthesized as per scheme 1 (see supplementary data).[19] The oxidative cyclization of o-phenylenediamine with D-fructose in presence of acetic acid afforded the compound 2 in moderate yield, which on oxidation with alkaline hydrogen peroxide afforded the key intermediate quinoxalin-2-carboxylic acid with the yield of 50%. The carboxylic acid group of quinoxalin-2-carboxylic acid (3) was coupled with N-phenylpiperazine in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) and 1-hydroxybenzotriazole (HOBt) under nitrogen atmosphere yield the desired compound 4a in 70% yield. The synthetic protocol is described in scheme 1 (see supplementary data).
Drugs
Fluoxetine was obtained from ipca Laboratories, Mumbai, India as a generous gift samples. Pargyline and 5-Hydroxtryptophan (5-HTP) were procured from Sigma Chemicals, United States of America. Reserpine was purchased from Sisco Research Laboratories, Mumbai, India. Ketamine and xylazine were purchased from Neon Laboratories Ltd. Mumbai, and Indian Immunologicals, Bangalore, India, respectively. Hemostatic sponge was purchased from Sri Gopal Krishan Labs Pvt. Ltd, Mumbai, India. 4a molecule was synthesized in the medicinal chemistry laboratory of department of Pharmacy, Birla Institute of Technology and science, Pilani, Rajasthan, India.
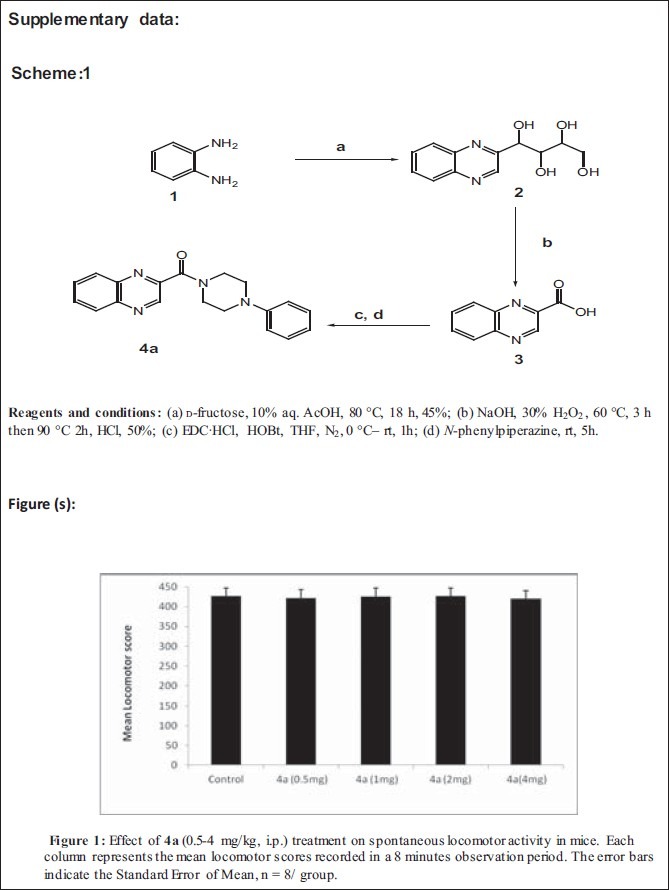
Behavioural Assays
Spontaneous locomotor activity
The spontaneous locomotor activity of mice was assessed using actophotometer (Inco Ambala, India).[20] The mice were individually placed in the centre of the square arena (30 cm ×30 cm) of actophotometer. After an initial familiarization period (2 minutes), the digital locomotor scores were recorded for the next 8 minutes in a dimly lit room. 4a (0.5-4 mg/kg, i.p.) was administered 30 minutes prior to testing.
Forced swim test
The FST was performed with slight modifications[21] from the originally described method.[15] In brief, each mouse was placed individually in a glass cylinder (diameter: 22.5 cm, height: 30 cm) filled with water (depth: 15 cm). The mice were forced to swim for 6 minutes. The total duration of immobility during the last 4 minutes of a single 6 minutes test session was recorded. 4a (0.5-4 mg/kg, i.p.) and fluoxetine (10 mg/kg, i.p.) were administered 30 minutes prior to testing.
5-Hydroxytryptophan induced head twitch response
The method mentioned elsewhere[16] was adopted with slight modifications.[22] Briefly, the mice were treated with a monoamine oxidase inhibitor, pargyline (75 mg/kg, i.p.) 30 min before 5-HTP (5 mg/kg, i.p.) treatment. 4a (2-4 mg/kg, i.p.) and fluoxetine (10 mg/kg, i.p.) were injected 15 minutes prior 5-HTP administration. Fifteen minutes after 5-HTP administration, the number of head twitches exhibited by the mice (vehicle or drug treated) during the next 30 minutes were recorded as head twitch score.
Reserpine induced hypothermia
RIH was performed in rats with slight modifications[22] from the originally described method.[17] Wistar rats were treated with 4a (2-4 mg/kg, i.p.) and reserpine (1 mg/kg, i.p.) 45 and 30 minutes prior to testing respectively. The animals were gently hand-restrained, while inserting the probe rectally. The effect of 4a (2-4 mg/kg, i.p.) and fluoxetine (10 mg/kg, i.p.) treatment on reserpine induced hypothermia (measured with digital thermometer) was recorded by measuring temperature at 0, 30, 60, 90 and 120 minutes following reserpine administration. Hypothermia was measured by calculating temperature difference between 0 minutes and 120th minutes.
Olfactory bulbectomy
Surgery
The rats were bulbectomized at the age of 7-8 weeks. Bilateral olfactory bulbectomy was performed according to the previously described method.[18,23] Briefly, rats were anaesthetized with the cocktail of xylazine and ketamine (5 and 75 mg/kg, i.p., respectively). The animals were fixed in a stereotactic frame (Inco, India), and the skull was exposed by a midline incision. The burr holes (2 mm in diameter) were drilled 8 mm anterior to bregma and 2 mm on either side of the midline at a point corresponding to the posterior margin of the orbit of an eye. The olfactory bulbs were removed by suction, the holes were then filled with haemostatic sponge to control excessive bleeding and the scalp was sutured. Sham-operated rats were subjected to the same surgical procedure, including piercing of the dura mater, but their bulbs were left intact. Following a rehabilitation period of 14 days, olfactory bulbectomized /sham operated rats were treated orally with 4a (2-4 mg/kg), fluoxetine (10 mg/kg) or the vehicle once daily between 09:00-12.00 pm. for 14 days (15th-28th day).
Modified open field behaviour
The olfactory bulbectomized / sham rats were subjected to an open field exploration test on 29th post surgery and 15th day of drug treatment according to the method described earlier.[23–25] The apparatus consisted of a circular (diameter: 90 cm) arena with 75 cm high aluminum walls and floor equally divided into 10 cm squares. A 60 W light bulb was positioned 90 cm above the base of the arena, which was the only source of illumination in the testing room. On the 29th day, each animal was individually placed in the center of the open field apparatus and the ambulation scores (number of squares crossed), rearing and fecal pellets were counted for 5 minutes.
Statistical Analysis
All the data were expressed as mean ± Standard Error of Mean (S.E.M). The data was statistically analyzed using one-way analysis of variance followed by Tukey's test in Graph pad prism 3.
Results
Spontaneous locomotor activity
Acute 4a (0.5-4 mg/kg, i.p.) treatment had no significant effect on the baseline locomotor activity of mice when compared to the control group (see supplementary data).
Forced swim test (FST)
One- way analysis of variance showed that acute treatment with 4a (1-4 mg/kg, i.p.) produced a significant [F (5, 42) = 24.59, P < 0.05] reduction of immobility time as compared to the control group in mouse FST [Figure 1]. The positive control fluoxetine (10 mg/kg, i.p.) also significantly reduced the immobility duration [P < 0.05] as compared to the control group. 4a at 0.5 mg/kg dose failed to reach the level of significance in comparison to control group in FST.
Figure 1.
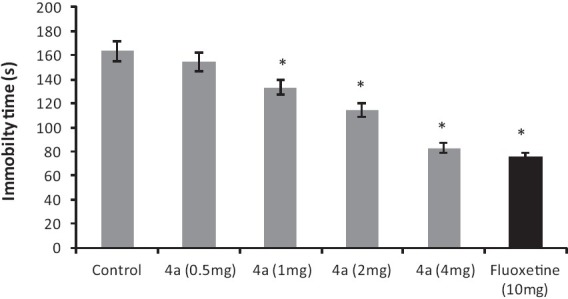
Effect of 4a (0.5-4 mg/kg, i.p.) and fluoxetine (FLX) (10 mg/ kg, i.p.) treatment on the duration of immobility in mouse Forced swim test. Each column represents the mean duration of immobility in sec. (s). The error bars indicate the Standard Error of Mean, *P < 0.05 when compared with the vehicle treated group; n = 8 / group
5-Hydroxytryptophan induced head twitches response
The co-administration of pargyline (75 mg/kg, i.p.) and 5-HTP (5 mg/kg, i.p.) induced the characteristic head twitch response. 4a (2-4 mg/kg, i.p.) and fluoxetine (10 mg/kg, i.p.) treatment significantly [F (3, 20) = 31.55, P < 0.05] potentiated the head twitch response as compared to combination of pargyline and 5-HTP treatment alone [Figure 2].
Figure 2.
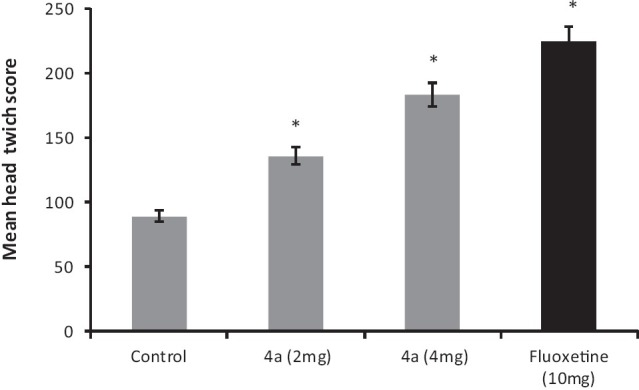
The effect of 4a (2-4 mg/kg, i.p.) and fluoxetine (FLX) (10 mg/kg, i.p.) on the 5-HTP and Pargyline induced head twitch response in mice. Each column represents the mean number of head twitches during a 30 minutes. Control group animals were administered 5-hydroxytryptophan (5 mg/kg, i.p.) and pargyline (75 mg/kg, i.p.). Error bars represent the Standard Error of Mean, *P < 0.05 when compared to the control group; n = 6 / group
Reserpine induced hypothermia (RIH)
Administration of reserpine (1 mg/kg, i.p.) elicited a pronounced decrease [P < 0.05] in core body temperature of control group's rats. This effect was significantly antagonized by 4a (2-4 mg/kg, i.p.) treatment [F (3, 20) = 11.17, P < 0.05]. Similarly, fluoxetine (10 mg/kg, i.p.) also attenuated the hypothermic response induced by reserpine treatment [Figure 3].
Figure 3.
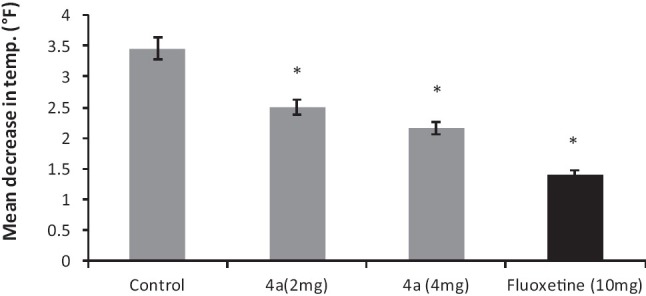
The effect of 4a (2-4 mg/kg, i.p.) and fluoxetine (FLX) (10 mg/ kg, i.p.) treatment on reserpine induced hypothermia in rats. Each column represents the mean decrease in rectal temperature (°F). Error bars represent the Standard Error of Mean, *P < 0.05 when compared to the 2nd hour value of vehicle treated group, n = 6 / group
Olfactory bulbectomy
Modified open field behaviour
The effects of 4a treatment (14 days) on the behaviour of olfactory bulbectomized (OBX) /sham rats were analyzed in the modified open field paradigm [Figures 4–6]. Removal of the olfactory bulbs produced a characteristic hyperactivity in the OBX rats when compared to sham rats in the modified open field test. Sub-chronic (14 days) treatment with 4a (2-4 mg/ kg, p.o.) significantly [F (7, 40) = 30.6, P < 0.05] reduced the ambulation [Figure 4], rearing [Figure 5] (F (7, 40) = 10.22, P < 0.05] and fecal pellets [Figure 6] in olfactory bulbectomized rats [F (7, 40) = 5.66, P < 0.05] as compared to the vehicle treated olfactory bulbectomized rats. Fluoxetine treatment (10 mg/ kg) also significantly (P < 0.05) attenuated all of the effects of bilateral olfactory bulbectomy.
Figure 4.
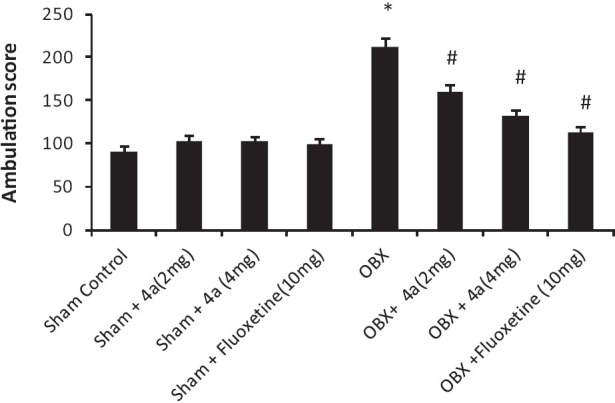
Effect of 4a (2-4 mg /kg, p.o.) and fl uoxetine (10 mg/kg, p.o.) on the ambulation scores of sham/olfactory bulbectomized (OBX) rats in the modifi ed open field test. Each column represents the mean ambulation score. Error bars represent the Standard Error of Mean. *P < 0.05 when compared to the sham control, #P < 0.05 when compared to the vehicle treated OBX group. n = 6/ group
Figure 6.
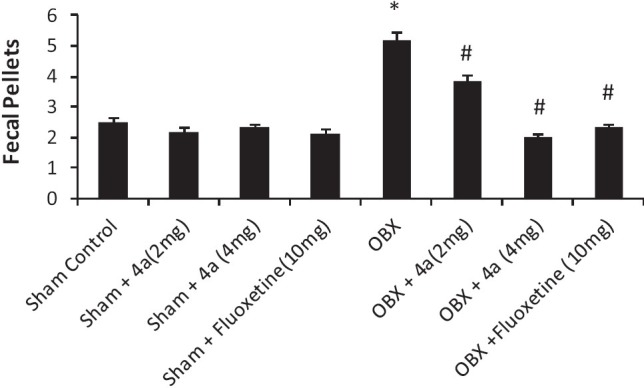
Effect of 4a (2-4 mg /kg, p.o.) and fl uoxetine (10 mg/kg, p.o.) on the fecal pellets of sham/olfactory bulbectomized (OBX) rats in the modifi ed open fi eld test. Each column represents the mean fecal pellets. Error bars represent the Standard Error of Mean. *P < 0.05 when compared to the sham control, #P < 0.05 when compared to the vehicle treated OBX group. n = 6/ group
Figure 5.
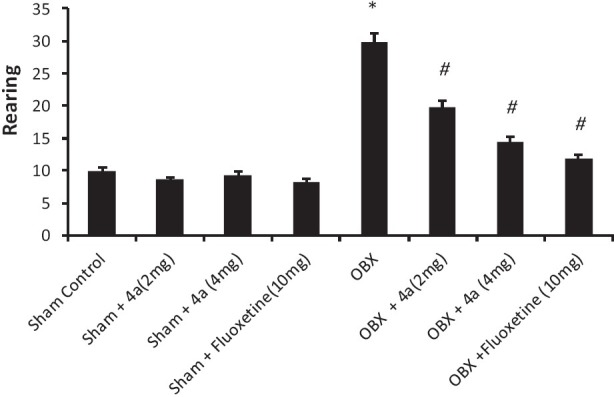
Effect of 4a (2-4 mg /kg, p.o.) and fl uoxetine (10 mg/kg, p.o.) on the rearing behaviour of sham/olfactory bulbectomized (OBX) rats in the modifi ed open fi eld test. Each column represents the mean rearing score. Error bars represent the standard error of mean. *P < 0.05 when compared to the sham control, #P < 0.05 when compared to the vehicle treated OBX group. n = 6/ group
Discussion
The results of this behavioural investigation divulge the antidepressant- like effects of ‘4a’. The preliminary attributes of 4a namely (i) Log P value of 2.84, which is optimum for blood brain barrier permeability[26] (ii) pA2 value (7.3) greater than selective 5- HT3 receptor antagonist ondansetron (6.9) (iii) antidepressant- like effects observed in mouse FST, incentivized the present study which comprised a series of standardized antidepressant assays. In spite of differences between laboratories, predictive assays of antidepressant activity such as FST and TST in mice are responsive to major classes of antidepressants including tricyclics, SSRIs and Serotonin-norepinephrine reuptake inhibitors (SNRIs).[27] The central nervous system (CNS) stimulatory or sedative property of a test compound mimics antidepressant-like or depressogenic-like behavioural outcome of rodents respectively, in FST.[25] 4a treatment (1-4 mg/kg) exhibited significant antidepressant-like effects in FST and the tested dose range did not alter the basal spontaneous locomotor activity (supplementary data). Hence, the behaviour of mice in the above mentioned assays does not reflect a sheer CNS stimulatory effect of 4a. The present study showed that 4a treatment significantly decreased the duration of immobility in FST in mice, an antidepressant-like profile also shown by the standard antidepressant drugs in clinical use and various 5-HT3 receptor antagonists.[11–13,25] Furthermore, Rajkumar et al.[25] have demonstrated that OND produces antidepressant-like effects only at low doses (0.01 and 0.1 mg/kg), and high dose of OND (1 mg/kg) did not reduce the immobility time in FST, probably due to the presynaptic receptor inhibition. However, 4a didn’t show such biphasic response in FST at the tested doses in comparison to OND as reported in the earlier study.
The enhancement of synaptic concentrations of monoamine, particularly serotonin is considered as the well-known mechanism of several antidepressants. The serotonergic property of 4a was confirmed by an in vivo study in mice. The potentiation of 5-HTP induced behaviour has previously been related to serotonergic mechanisms.[26] In the present study, the pargyline (MAO inhibitor) and 5-HTP induced head twitches response was significantly potentiated by 4a. The depletion of brain biogenic amines induced by biogenic amine depletors such as reserpine is reported to induce hypothermia and ptosis response in rodents.[28] The decrease in body temperature induced by reserpine treatment has been reported to be antagonized by antidepressants.[29] Both 4a and fluoxetine significantly antagonized the hypothermic response induced by reserpine which indicates that the antidepressant effect in this sensitive model is possibly due to the increased serotonin levels through 5-HT3 receptor blockade.
The rat olfactory bulbectomy model of chronic depression (with adequate face and predictive validity) is known to espial novel agents that are unmarked by more conventional models of depression.[30] Olfactory bulbectomy was proposed as a model for agitated hyposerotonergic model of depression and is used to explore the antidepressant potential of novel agents.[23] The OBX rats exhibited a specific abnormal behavioural pattern in the brightly lit, circular, open field arena, characterized by increased ambulation, rearing and fecal pellets.[30] Such changes are attenuated by antidepressants of many pharmacological classes.[23] In the present investigation, 4a significantly attenuated the behavioural abnormalities exhibited by OBX rats which strongly support the clinical potential of 4a as an antidepressant agent.
The present neuro-behavioural study shows the acute and chronic effects of 4a, a novel 5-HT3 receptor antagonist in animal models of depression. Based on the results obtained, i.e., potentiation of head twitches response (due to increased serotonin level) and reversal of reserpine induced hypothermia, it is suggested that 4a produced antidepressant-like effect probably by increasing the concentration of neurotransmitter.[31] An increase in the serotonin concentration due to postsynaptic 5-HT3 receptor antagonism could be responsible for the antidepressant-like effect of 4a.[32]
Conclusions
Based on the results obtained from these preliminary investigations, it is suggested that 4a, a novel 5-HT3 receptor antagonist, could be a potential candidate for the management of depression. Hopefully, further studies will provide a better understanding of the promiscuous nature of the novel 5-HT3 antagonist that could also be useful for the management of depression. Though no abnormal behaviour was observed at the tested dose levels, assessment of the safety profile of the molecule is essential.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Yi LT, Li JM, Li YC, Pan Y, Xu Q, Kong LD. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008;82:741–51. doi: 10.1016/j.lfs.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 3.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci. 2006;7:137–51. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 4.Adell A, Castro A, Celada P, Bortolozzi A, Pazos A, Artigas F. Strategies for producing faster acting antidepressants. Drug Discov Today. 2005;10:578–85. doi: 10.1016/S1359-6446(05)03398-2. [DOI] [PubMed] [Google Scholar]
- 5.Mahesh R, Perumal RV, Pandi PV. Cancer chemotherapy induced nausea and vomiting: Role of mediators, development of drugs and treatment methods. Pharmazie. 2005;60:83–96. [PubMed] [Google Scholar]
- 6.Wolf H. Preclinical and clinical pharmacology of the 5-HT3 receptor Antagonists. Scand J Rheumatol. 2000;29:37–45. doi: 10.1080/030097400446625. [DOI] [PubMed] [Google Scholar]
- 7.Martin KF, Hannon S, Phillips I, Heal DJ. Opposing roles for 5-HT1B and 5-HT3 receptors in the control of 5-HT release in rat hippocampus in vivo. Br J Pharmacol. 1992;106:139–42. doi: 10.1111/j.1476-5381.1992.tb14306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell EA, Pratt JA. Neuroanatomical structures involved in the action of the 5-HT3 antagonist ondansetron: A 2-deoxyglucose autoradiographic study in the rat. Brain Res. 1991;538:289–94. doi: 10.1016/0006-8993(91)90442-x. [DOI] [PubMed] [Google Scholar]
- 9.Redrobe JP, Bourin M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol. 1997;325:129–35. doi: 10.1016/s0014-2999(97)00115-5. [DOI] [PubMed] [Google Scholar]
- 10.Redrobe JP, Pinot P, Bourin M. The effect of the potassium channel activator, cromakalim, on antidepressant drugs in the forced swimming test in mice. Fundam Clin Pharmacol. 1996;10:524–8. doi: 10.1111/j.1472-8206.1996.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa Y, Ishima T, Takashima T. The 5-HT3 receptor agonist attenuates the action of antidepressants in the forced swim test in rats. Brain Res. 1998;786:189–93. doi: 10.1016/s0006-8993(97)01459-5. [DOI] [PubMed] [Google Scholar]
- 12.Kos T, Popik P, Pietraszek M, Schafer D, Danysz W, Dravolina O, et al. Effect of 5-HT3 receptor antagonist MDL 72222 on behaviours induced by ketamine in rats and mice. Eur Neuropsychopharmacol. 2006;6:297–10. doi: 10.1016/j.euroneuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Bravo G, Maswood S. Acute treatment with 5-HT3 receptor antagonist, tropisetron, reduces immobility in intact female rats exposed to the forced swim test. Pharmacol Biochem Behav. 2006;85:362–8. doi: 10.1016/j.pbb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Mahesh R, Perumal RV, Pandi PV. Microwave assisted synthesis of 2-(4-substituted piperazin-1-yl)-1, 8-naphthyridine-3-carbonitrile as a new class of serotonin 5-HT3 receptor antagonists. Bioorg Med Chem Lett. 2004;14:5179–81. doi: 10.1016/j.bmcl.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 15.Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 16.Martin P, Massol J, Soubrie P, Puech AJ. Effects of triiodothyronine (T3) on the potentiation by antidepressants of L-5-hydroxytryptophan-induced head-twitches in mice. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:735–48. doi: 10.1016/0278-5846(89)90061-4. [DOI] [PubMed] [Google Scholar]
- 17.Costa E, Garatini S, Valzelli L. Interaction between reserpine, imipramine and chlorpromazine. Experientia. 1960;16:461–3. doi: 10.1007/BF02171155. [DOI] [PubMed] [Google Scholar]
- 18.Song C, Leonard BE. The olfactory bulbectomized rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–47. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Mahesh R, Devadoss T, Pandey DK, Yadav SK. Quinoxalin-2-carboxamides: Synthesis and pharmacological evaluation as serotonin type-3 (5-HT3) receptor antagonists. J Enzyme Inhib Med Chem. 2011;26:610–5. doi: 10.3109/14756366.2010.543419. [DOI] [PubMed] [Google Scholar]
- 20.Boissier JR, Simon P. Action of caffeine on the spontaneous motility of the mouse. Arch Int Pharmacodyn Ther. 1965;158:212–21. [PubMed] [Google Scholar]
- 21.Mahesh R, Rajkumar R, Minasri B, Perumal V. Potential antidepressants: Pharmacology of 2-(4-methyl piperazin-1-yl)-1, 8-naphthyridine-3-carbonitrile in rodent behavioural models. Pharmazie. 2007;62:919–24. [PubMed] [Google Scholar]
- 22.Devadoss T, Pandey DK, Mahesh R, Yadav SK. Effect of acute and chronic treatment with QCF-3 (4-benzylpiperazin-1-yl) (quinoxalin-2-yl) methanone, a novel 5-HT3 receptor antagonist, in animal models of depression. Pharmacol Rep. 2010;62:245–57. doi: 10.1016/s1734-1140(10)70263-2. [DOI] [PubMed] [Google Scholar]
- 23.Van Reizen H, Leonard BE. Effects of psychotropic drugs on the behaviour and neurochemistry of olfactory Bulbectomised rats. Pharmacol Ther. 1990;47:21–34. doi: 10.1016/0163-7258(90)90043-2. [DOI] [PubMed] [Google Scholar]
- 24.Kelly JP, Wyrnn AS, Leonard BE. Olfactory bulbectomized rat as a model of depression: An update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 25.Rajkumar R, Mahesh R, Borah M. Antidepressant- like effects of serotonin type- 3 antagonist, ondansetron: An investigation in behaviour-based rodent models. Behav Pharmacol. 2008;19:29–40. doi: 10.1097/FBP.0b013e3282f3cfd4. [DOI] [PubMed] [Google Scholar]
- 26.Ter Laak AM, Tsai RS, Donne-Op den Kelder GM, Carrupt PA, Testa B, Timmerman H. Lipophilicity and hydrogen-bonding capacity of H1 -antihistaminic agents in relation to their central sedative side-effects. Eur J Pharm Sci. 1994;2:373–84. [Google Scholar]
- 27.Millan MJ, Dekeyne A, Papp M, La Rochelle CD, MacSweeny C, Peglion JL, et al. S33005, a novel ligand at both serotonin and norepinephrine transporters: II. Behavioural profile in comparison with venlafaxine, reboxetine, citalopram and clomipramine. J Pharmacol Exp Ther. 2001;298:581–91. [PubMed] [Google Scholar]
- 28.Englert LF, Ho BT, Taylor D. The effects of (-) Δ9 -9-tetrahydrocannabinol on reserpine-induced hypothermia in rats. Br J Pharmacol. 1973;49:243–52. doi: 10.1111/j.1476-5381.1973.tb08369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourin M, Fiocco AJ, Clenet F. How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol. 2001;16:9–21. doi: 10.1002/hup.178. [DOI] [PubMed] [Google Scholar]
- 30.Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B. Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res. 1992;587:181–5. doi: 10.1016/0006-8993(92)90995-l. [DOI] [PubMed] [Google Scholar]
- 31.Blandina P, Goldfarb J, Walcott J, Green JP. Serotonergic modulation of the release of endogenous norepinephrine from rat hypothalamic slices. J Pharmacol Exp Ther. 1991;256:341–7. [PubMed] [Google Scholar]
- 32.Schafer WR. How do antidepressants work.? Prospect for genetic analysis of drug mechanism. Cell. 1999;98:551–4. doi: 10.1016/s0092-8674(00)80042-2. [DOI] [PubMed] [Google Scholar]


