Abstract
Objectives:
To investigate the effect of Kalanchoe crenata methanolic fraction (MEKC) on proteinuria, glucosuria, and some other biochemical parameters in adriamycin-induced renal impairment in rats.
Materials and Methods:
Ether anesthetized rats received three intravenous injections (days 0, 14, and 28) of 2 mg/kg body weight of adriamycin. Repeated doses of the extract (0, 50, and 68 mg/kg b.w.) and losartan (10 mg/kg b.w.) were administered orally once daily, for 6 weeks, to these rats. Kidney functions were assessed through biochemical parameters.
Results:
MEKC decreased proteinuria and also the urinary excretion of creatinine, glucose, and urea significantly in diseased rats. A decrease in serum levels of creatinine, urea, potassium, alkaline phosphatase, conjugate bilirubin, and alanine transaminase level was also recorded in nephropathic rats, but plasma levels of uric acid and glucose remained unchanged. Moreover, the plant extract markedly (P < 0.05) increased plasma sodium and decreased (P < 0.01) the urinary sodium and potassium levels.
Conclusions:
The results indicated that the treatment with the methanolic fraction of K. crenata may improve proteinuria and all other symptoms due to adriamycin-induced nephropathy and, more than losartan, could ameliorate kidney and liver functions. K. crenata could be a potential source of new oral antinephropathic drug.
KEY WORDS: Adriamycin, antioxidant effect, Kalanchoe crenata methanolic extract, nephropathy, rat
Introduction
Regardless of etiology, glomerulosclerosis and tubule-interstitial fibrosis are the final common pathways of progression seen in most chronic renal diseases.[1] Nephropathy is characterized by specific renal alterations. Features of early renal changes are glomerular hyperfiltration and hypertrophy and increased urinary albumin excretion. Advanced nephropathy is characterized by proteinuria, glucosuria, decline in renal function, increased blood creatinine or decreased creatinine clearance, glomerulosclerosis, and interstitial fibrosis.[1,2] At present, diabetic kidney disease affects about 15–25% of Type 1 diabetic patients,[3] 20–40% of patients with Type 2 diabetes,[4,5] and 2% of patients with drug toxicity.[6] Thus, kidney diseases should be considered as a public health problem. Conventional treatment includes oral enzyme conversion inhibitors such as losartan. However, in places where safe modern drugs and health centers are unavailable, the World Health Organisation has suggested the use of indigenous plants as alternative medicine.[7] About 80% of rural African communities still use phytotherapy to control or treat many diseases. Kalanchoe crenata (Crassulaceae) is a herbaceous plant used in western areas of Cameroon as an antidiabetic and anti-inflammatory drug.[8,9] Adriamycin has been used to induce nephropathic toxicity in rats in several studies.[10,11] The present work was therefore undertaken to assess the effect of the methanolic fraction of K. crenata on adriamycin-induced nephropathy in rats.
Materials and Methods
The whole plant of K. crenata was collected from Batie (West Cameroon) in January and March and was identified by the National Herbarium of Yaounde (Cameroon) where the voucher specimen (50103/YA) was kept. The material was cleaned, shade-dried, and powdered. The powder of K. crenata (2 kg) was macerated in 10 L of methanol for 72 h at room temperature. Removal of the solvent from the extract under reduced pressure yielded 113.6 g (5.68%) of a dark green residue. This residue was dissolved in hexane to remove its hydro-insoluble compounds. The final residue (insoluble in hexane) constituted the methanol fraction of K. crenata (MEKC). The extract yielded 41.8 g (2.09%). Prior to the administration, the extract was dissolved in distilled water.
Preliminary Phytochemical Tests
Phytochemical constituents of the methanolic fraction of K. crenata were determined by standard methods using various reagents.[12] This included Mayer and Dragendoff's reagents for alkaloids, FeCl3 for tannin, frothing test for saponin, magnesium turning and Hcl for flavonoids, NaCl and Fehling's solutions for glycoside, diethyl ether, sulphuric acid and anhydride acetic for steroids, ether-chloroform and NaOH for anthraquinones, and FeCl3 and K3Fe(CN)6 for phenols and polyphenols.
Acute Toxicity Evaluation
The MEKC was tested for its acute toxicity in mice. Five groups of six mice each were administered orally one of the different doses of the extract: 2, 4, 6, 8, and 10 g/kg body weight. Control group received only vehicle (water). Animals were observed continuously for initial 2 h, intermittently for the next 6 h, and then at 24 h and 48 h following drug administration for death and overt behavior: lethargy, jerkiness, sensitivity to noise and touch, and respiratory rate. The lethal dose 50 (LD50) was determined with the following formulae.[13]
LD50 = Xs – d (Sp - ½)
Xs = Lethal dose 100; d = Interval between the doses
p = Death proportion per group; Sp = sum of death proportions
Induction of Renal Impairment
Male Wistar albino rats weighting 200–250 g, raised in the Faculty of Science, University of Yaoundé I, were used. They were maintained under natural laboratory conditions (temperature and dark/light cycle) and allowed access to food and water ad libitum. Animal housing and experiments in vivo were done according to the Guidelines of the European Union directive on Ethical Evaluation of Animal Experiments (CEE Council 86/609)[14] and approved by the Institutional Committee of the Ministry of the Scientific Research and Innovation of Cameroon.
To induce the kidney disease, the rats anesthetized with ether received three intravenous (penile vein) injections (days 0, 14, and 28) of 2 mg/kg b.w. of adriamycin (2 mg/mL doxorubicin hydrochloride: Pharmacia Italia, S.P.A., Italy) in 9% NaCl.[10,11] The control group received normal saline. Rats with proteinuria levels >3 g/L, serum creatinine >57 mmol/L, and creatinuria <10 mmol/L were used in the experiments as nephropathic rats.
Animal Treatment
The nephropathic rats were randomly divided into four groups of five animals each: one group of nephropathic control rats (NeC) received, as (five) normal control rats (NC), distilled water (5 mL/kg); two groups received 50 mg/kg b.w. (NeK50) and 68 mg/kg b.w. (NeK68) K. crenata extract; the last group (standard reference: NeL10) received 10 mg/kg b.w. losartan (losartan + hydrochlorothiazide; HYZAAR, Merck Sharp and Dohmet-Chibret, MSD, Paris). The extract doses were obtained from the tradipractitioner method. The drugs were administered orally daily for 6 weeks.
Serum and Urine Samples
Urine and blood samples were obtained from each rat at day 0 and at 2 weeks interval thereafter until week 12. After 6 weeks of treatment, the rats were housed individually in the metabolic cages for 24 h for urine collection. Xylol was put in the collection container to prevent the evaporation. The rats were fasted for 24 h; spot urine samples were collected for protein, creatinine, urea, uric acid, sodium, and potassium level estimation. The biochemical assays were performed within 24 h of collection. The rats then were ether anesthetized, killed, and blood samples collected into normal tubes and were allowed to clot at room temperature. Serum was separated by centrifugation (3000 tr/min at 30°C, 10 min), aliquoted, and kept frozen at -20°C for the estimation of protein, creatinine, sodium, potassium, alkaline phosphatase, transaminase, urea, and uric acid levels within 3 months.
Serum and Urine Analysis
Glucose, total protein, creatinine, alkaline phosphatase, alanine aminotransferase (ALAT), urea, and uric acid levels were analyzed in serum using commercial diagnostic kits (Bio Direct Laboratories, La villeneuve-france and Elitect Laboratories, SEPPIMS A. France). Sodium and potassium were analyzed in blood and urine by a selective electrode ion autoanalyser (ILLYTE). Urinary creatinine, urea, uric acid, and glucose were estimated spectrophotometrically with commercially available kits (Biodirect and Elitech). The osmolality of plasma or urine was determined using the following formula:[15]
Osmolality = [(Na+ + K+) × 2] (mEq/L) + [urea × 16] (g/L) + [glucose × 5.5] (g/L)
Statistical Analysis
The results were expressed as the mean ± SEM. These were analyzed statistically using one-way analysis of variance followed by Dunnett's test using Graphpad Insad version 3.6 software. P < 0.05 was considered as statistically significant.
Results
The preliminary phytochemical analysis revealed the presence of different classes of compounds such as tannins, phenols, sterol, anthraquinones, triterpens, phobotanins, and polyphenol in the MEKC.
In acute toxicity tests, MEKC (4, 6, 8, and 10 g/kg) reduced the sensitivity to noise and touch, jerkiness, lethargy, and caused soft feces and 66% mice died within 30 min of administration. The dose 10 g/kg caused 100% mortality and the LD50 was found to be 4.4 g/kg. There were no gross behavioral changes. Macroscopically, the organs (liver, kidney, heart) did not show any discolouration.
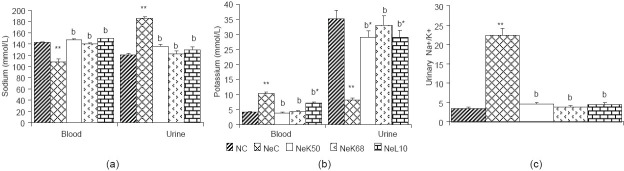
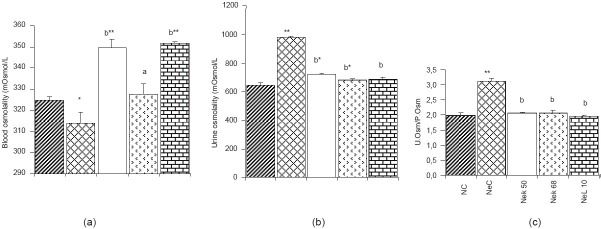
Animals with adriamycin-induced nephropathy showed a significant increase (P < 0.01) in the levels of creatinine, urea, and uric acid [Table 1a]. They also had increased urinary volume, protein, urea, uric acid, and glucose [Table 1b], while proteinemia and creatinuria decreased significantly (P < 0.01). The blood glucose level was not reduced significantly. The serum level of sodium and the urinary level of potassium also decreased significantly (P < 0.01), while blood potassium, urinary sodium and urinary Na+/K+ ratio [Figure 1] increased significantly (P < 0.01). The blood osmolality was significantly decreased (P < 0.05) as compared to normal rats, while urine osmolality and urinary osmolality/blood osmolality ratio were significantly (P < 0.01) increased [Figure 2]. The nephropathy also caused a significant increase (P < 0.01) of ALAT, alkaline phosphatase (ALP), and conjugate bilirubin levels in blood [Figure 3].
Table 1.
Effect of methanolic extract of K. crenata on blood (a) and urinary (b) chemistry in adriamycin-induced nephropathic rats
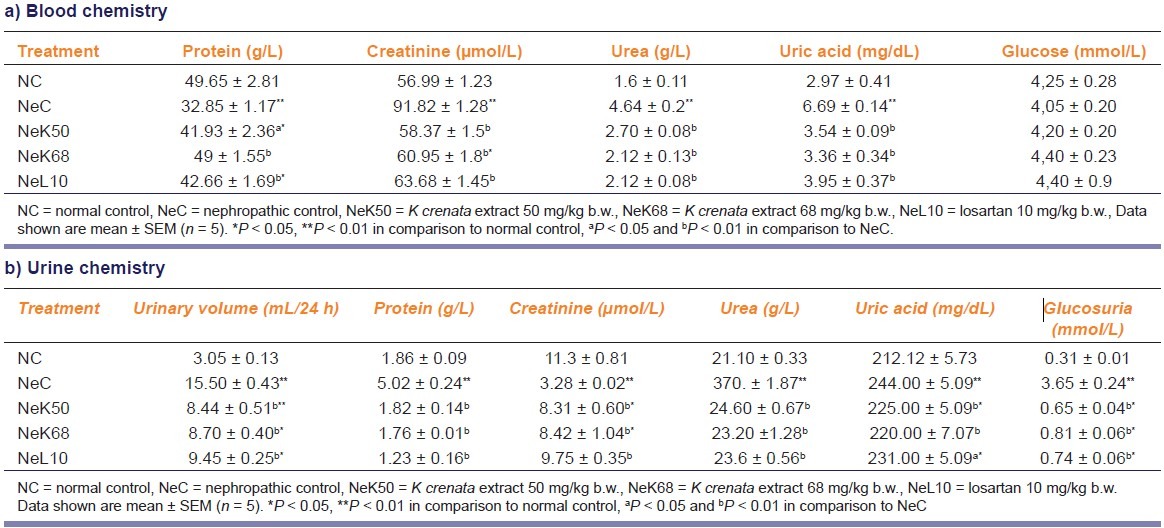
Figure 1.
Effect of methanolic extract of K. crenata on sodium (a), potassium (b), and urinary Na+/K+ ratio (c) in adriamycin-induced nephropathy rats. NC = normal control, NeC = nephropathic control, NeK50 = K crenata extract 50 mg/kg b.w., NeK68 = K. crenata extract 68 mg/kg b.w., NeL10 = losartan 10 mg/kg b.w. Data shown are mean ± SEM (n = 5). *P < 0.05, **P < 0.01 in comparison to normal control, aP < 0.05 and bP < 0.01 in comparison to NeC
Figure 2.
Effect of methanolic extract of K crenata on blood osmolality (a), urine osmolality (b), and urine/plasma osmolality ratio (c) in adriamycininduced nephropathic rats. NC = normal control, NeC = nephropathic control, NeK50 = K crenata extract 50 mg/kg b.w., NeK68 = K crenata extract 68 mg/kg b.w., NeL10 = losartan 10 mg/kg b.w. Data shown are mean ± SEM (n = 5). *P < 0.05, **P < 0.01 in comparison to normal control, aP < 0.05 and bP < 0.01 in comparison to NeC
Figure 3.
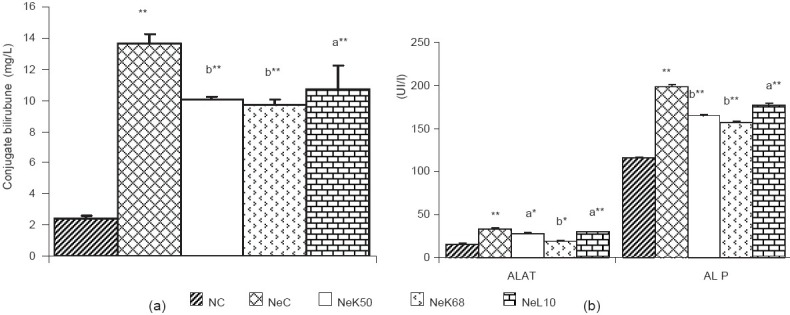
Effect of methanolic extract of K crenata on conjugate bilirubin (a), alanine aminotransferase ALAT and alkaline phosphatase ALP (b), in adriamycin-induced nephropathic rats. NC = normal control, NeC = nephropathic control, NeK50 = K crenata extract 50 mg/kg b.w., NeK68 = K crenata extract 68 mg/kg b.w., NeL10 = losartan 10 mg/kg b.w. Data shown are mean ± SEM (n = 5). *P < 0.05, **P < 0.01 in comparison to normal control, aP < 0.05 and bP < 0.01 in comparison to NeC
The MEKC 50 and 68 mg/kg b.w. and the 10 mg/kg b.w. losartan after 6 weeks of treatment have significantly (P < 0.05 and P < 0.01) reduced the blood creatinine, urea, uric acid, conjugate bilirubin, ALAT, ALP levels, and the urinary volume, protein, urea, uric acid, and glucose levels in urine of nephropathic rats. The proteinemia and the creatinuria were enhanced (P < 0.01). The extract and losartan have not changed the glycemia after 6 weeks treatment. In nephropathic rats the MEKC and losartan have markedly (P < 0.01) increased the blood sodium and the urinary potassium level and decreased the blood potassium, urinary sodium level, as well as the Na+/K+ ratio was also decreased [Figure 1].
K. crenata extract and losartan, after 6 weeks of administration, significantly (P < 0.01) reduced urine osmolality and urinary osmolality/blood osmolality ratio and enhanced the blood osmolality in nephropathic rats.
Discussion
The objective of this work was to assess the effect of the MEKC, an anti-inflammatory and anti-hyperglycemic plant,[8,9] on the renal and hepatic function in adriamycin-induced nephropathic rats. In the acute toxicity study, single oral dose of MEKC up to 2 g/kg was not lethal to both male and female mice. The apparent cause of death in mice at the higher doses may be due to respiratory depression or/and to methanol (solvent) poisoning, since earlier studies with aqueous ethanol extract of K. crenata did not show any overt sign or death in acute toxicity.[8,16] These results suggest that MEKC possesses low toxicity since the LD50 is higher than 2 g/kg and inferior to 5 g/kg and represents 65 and 88 times, respectively, the assay doses.[17]
In adriamycin nephropathic rats, our results demonstrated hyperproteinuria (coupled with hypoproteinemia), hypercreatinemia, and increased urinary excretion of glucose, urea, and uric acid. Normally, the kidney excretes creatinine and only low amount of low-molecular weight protein passes through the glomerulus, whereas glucose, urea, and uric acid are reabsorbed by the proximal tubule.[17] Usually, hypercreatinemia (and hypocreatinuria) observed in nephropathic states are characteristics of glomerular hyperfiltration,[18,19] and increased urinary glucose, urea, and uric acid levels indicate the alteration of tubular reabsorption. The MEKC showed a significant dose-dependent effect on protein excretion in nephropathic rats, similar to that of the losartan. The decrease of urinary level of proteins could be due, when compared with losartan effect, to a potential capability of the plant extract to restore the altered glomerular capillary function in nephropathic rats and may be (like losartan) by the inhibition of angiotensin-converting enzyme or the blockage of angiotensin II receptors that reduce the capillary vessel contraction and hence decrease the retention of water and salt.[20,21] This could also explain the decreased level of urine volume and urinary sodium in nephropathic rats. As losartan, the extract also improved the filtration function and tubular reabsorption by normalizing glycosuria, urinary and blood protein, creatinine, urea, and uric acid level. The extract did not affect normal glycemia which confirmed that the plant has no hypoglycemic activity but an antihyperglycemic effect as described earlier in diabetic rats.[8]
In nephropathic rats, the adriamycin caused a hydroelectrolytic disorder due to the failure of tubular reabsorption causing sodium leak and potassium retention; this could explain the high ratio of Na+/K+.[15] The blood decline and urinary increase of the osmolality (which is a consequence of ion movements) could mainly be related to the change in sodium levels in the two media. Thus, the increased urinary Na+ excretion, which may be due to the decrease of proximal reabsorption, also causes an increased urinary volume.[19] The increase of urinary osmolality might also be linked to the increase of the active osmotic substances: namely, Na+, glucose, and protein, which require important volume of water for their elimination.[19] In the treated rat, the decrease of urinary Na+, urinary osmolality, and urinary volume and the increase of blood potassium could then result from an improvement of the proximal tubular reabsorption by K. crenata. The plant by normalizing the levels of sodium and potassium in the media also normalizes osmolality.
In the nephropathic rats, the enzymes (ALP and ALAT) and conjugate bilirubin level were increased. In normal case, the ALAT is found only in the cytoplasm and its blood level increases when the permeability of hepatic cell is affected. The blood proteins are mostly produced in the liver. In this study, decrease of proteinemia was observed in nephropathic control rat. The hypoproteinemia and the high level of blood enzymes may be the result of malfunctioning of liver cells.[22] Low protein levels could also result due to dysfunctioning of the liver cells and the impaired renal function.[23] The increase of ALP results in the decrease or stoppage of the intestinal secretion of the bile, provoking the increase of blood conjugate bilirubin.[15] In nephropathic rats, the plant extract and losartan significantly increased blood protein and decreased the levels of conjugate bilirubin, ALAT, and ALP. This indicates that K. crenata could prevent the alteration of the liver cell structure and/or function that could be induced by adriamycin.
In conclusion, methanolic fraction of K. crenata has improved the kidney and the liver function in nephropathic rats. K. crenata extract and hence shows a potential use in alternative medicine for the treatment or management of the kidney diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Fogo AB, Kon V. Pathophysiology of progressive renal diseases. An overview. In: Neilson EG, Couser WG, editors. Immunologic Renal Diseases. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 55–72. [Google Scholar]
- 2.Schnaper HW. Focal segmental glomerulosclerosis. In: Neilson EG, Couser WG, editors. Immunologic Renal Diseases. 2nd ed. Philadelphia: Lippinott Williams and Wilkins; 2004. pp. 1001–27. [Google Scholar]
- 3.Hovind P, Tarnow L, Rossing P, Eising S, Larsen N, Binder C, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1285–64. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 4.Ritz E, Keller C, Bergis K, Strojek K. Pathogenesis and course of renal disease in IDDM/NIDDM: Differences and similarities. Am J hypertens. 1997;10:202S–7. doi: 10.1016/s0895-7061(97)00154-4. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, et al. Higher incidence of diabetic nephropathy in type 2 than type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–11. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Winc AJ, Brunner FP, Geerlings W, Broyer M, Brynger H, Fassbinder W, et al. Contribution of toxic nephropathies to end stage renal failure in Europe: A report from the EDTA-ERA registry. Toxicol Lett. 1989;46:281–92. doi: 10.1016/0378-4274(89)90136-7. [DOI] [PubMed] [Google Scholar]
- 7.Launches of the fi rst global strategy on the traditional medicine. Vol. 38. WHO Press Release; 2002. WHO; p. 2. [Google Scholar]
- 8.Kamgang R, Mboumi RY, Fondjo AF, Tagne MA, N’dillé GP, Yonkeu JN. Antihyperglycaemic potential of the water-ethanol extract of Kalanchoe crenata (crassulaceae) J Nat Med. 2008;62:34–40. doi: 10.1007/s11418-007-0179-y. [DOI] [PubMed] [Google Scholar]
- 9.Dimo T, Fotio LA, Nguelefack TB, Asongalem EA, Kamtchouing P. Anti-inflammatory activity of leaf extracts of Kalanchoe crenata Andr. Indian J Pharmacol. 2006;28:115–9. [Google Scholar]
- 10.Kim HJ, Ryu JH, Han SW, Park IK, Paik SS, Park MH, et al. Combined therapy of Cilazapril and Losartan has no additive effects in ameliorating Adriamycin-induced glomerulopathy. Nephron Physiol. 2004;97:58–65. doi: 10.1159/000079180. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Wang YP, Tay YC, Harris DC. Progressive adriamycin nephropathy in mice: Sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 12.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. 2nd ed. Ibadan, Nigeria: Spectrum Books Ltd; 1993. pp. 1–153. [Google Scholar]
- 13.Molle J. Amino-peptides-protéines. Cahier N°4 Paris: Ed. A.E.C; 1986. Limites de tolérance et toxicités de quelques amino-acides (formes L et D) pp. 207–32. [Google Scholar]
- 14.Smith JA, Van den Broek FA, Martorell JC, Hackbarth H, Ruksenas O, Zeller W. (A Federation of European Laboratory Animal Science Associations: FELASA, Working Group on Ethical Evaluation of Animal Experiments). Principles and practice in ethical review of animal experiments across Europe: Summary of the report of a FELASA working group on ethical evaluation of animal experiments. Lab Anim. 2007;41:143–60. doi: 10.1258/002367707780378212. [DOI] [PubMed] [Google Scholar]
- 15.Serge B. Biochimie clinique. Instruments et technique de laboratoire. Diagnostics médico-chirurgicaux. Edition Maloine. 1985. [Last accessed on 2011 Sep 15]. p. 392. Available from: https://rosemont.koha.ccsr.qc.ca/cgi-bin/koha/opac-search.pl?q=ccl=su:Diagnostics%20biologiques&sort_by=relevance_az&offset=0&expand=su-to .
- 16.Bruckner JV, Kyle GM, Luthra R, Acosta D, Mehta SM, Sethuraman S, et al. Acute, short-term, and subchronic oral toxicity of 1,1,1-trichloroethane in rats. Toxicol Sci. 2001;60:363–72. doi: 10.1093/toxsci/60.2.363. [DOI] [PubMed] [Google Scholar]
- 17.Viau C, Tardif R. Toxicologie. In: Gérin M, Gosselin P, Cordier S, Viau C, Quénel P, Dewailly E, editors. Environnement et Santé publique. Fondements et pratiques. Acton Vale, Vale: Edisem/Tec & Doc; 2003. [Last accessed on 2011 Sep 15]. pp. 119–43. Available from: http://www.dsest.umontreal.ca/documents/11Chap05.pdf . [Google Scholar]
- 18.Koshikawa M, Mukoyama M, Mori K, Takyoshi S, Kazutomo S, Tetsuro Y, et al. Role of p38 Nitrogen-Activated protein kinase activation in podocyte. Injury and proteinuria in experimental nephritic syndrome. J Am Soc Nephrol. 2005;16:2690–701. doi: 10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- 19.Frey J, Daudon M, Raby N, Augereau C, Dechaux M, Diehl JL, et al. Valeur séméiologique des paramètres biochimiques urinaires. Ann Biol Clin. 2001;59:13–25. [PubMed] [Google Scholar]
- 20.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases in sights from animal models. Kidney Int. 2005;67:404–19. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris RC, Neilson EG. Toward a unified theory of renal progression. Ann Rev Med. 2006;57:365–80. doi: 10.1146/annurev.med.57.121304.131342. [DOI] [PubMed] [Google Scholar]
- 22.Knuckles ME, Inyang F, Ramesh A. Acute and subchronic oral toxicity of benzo[a]pyrene in F-344 Rats. Toxico Sci. 2001;61:382–8. doi: 10.1093/toxsci/61.2.382. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko JJ, Harvey JW, Bruss M. Clinical biochemistry of domestic animal. 5th ed. San Diego, USA: Academic Press; 1997. pp. 117–38. [Google Scholar]