Abstract
Aims:
The aim of this study was to investigate the effect of ethanolic extract of Asparagus racemosus on urolithiasis in rats.
Materials and Methods:
Thirty-six male Wistar albino rats were randomly divided into six groups (n = 6). Ethylene glycol (EG) 0.75% and ammonium chloride (AC) 2% in drinking water were fed to all groups (Groups II–VI) except normal control (Group I) rats for 10 days to induce urolithiasis. Group III–VI rats were treated with ethanolic extract of Asparagus racemosus at doses 200, 400, 800, and 1600 mg/kg, respectively, for 10 days. Positive control (Group II) rats were treated with EG/AC alone. Group I rats were administered drinking water and distilled water (6 μl/g) by gavage. After 10 days, blood samples were collected and analyzed for serum concentrations of calcium, phosphorus, urea, and creatinine. The kidneys were removed and sectioned for histopathological examination. The data were presented as mean ± standard error of mean and analyzed using one-way analysis of variance and Student's “t”-test. P < 0.05 was considered statistically significant. Conventional windows software was used for statistical analysis.
Results:
The rats treated with ethanolic extract of A. racemosus at doses 800 and 1600 mg/ kg significantly (P < 0.05) reduced the serum concentrations of calcium, phosphorus, urea, and creatinine. Histopathology of the kidneys in Groups V and VI revealed less tissue damage and were almost similar to Group I rats.
Conclusions:
The ethanolic extract of A. racemosus has protective effect against urolithiasis.
KEY WORDS: Ammonium chloride, antiurolithiatic activity, Asparagus racemosus, ethylene glycol
Introduction
Urolithiasis refers to the solid non-metallic minerals in the urinary tract. This is the third most common condition of the urinary tract after urinary tract infection and pathologic condition of prostate.[1] Urolithiasis is a complex process that is a consequence of an imbalance between promoters and inhibitors in the kidneys. The formation of kidney stones involves several physicochemical events beginning with crystal nucleation, aggregation, and end with retention within the urinary tract.[2] Among the several types of kidney stones, the most common are calcium oxalate stones representing up to 80% of the analyzed stones.[3]
Even though surgery is the treatment of choice for urinary stones, medical interventions and lifestyle changes are important, as the recurrence rate is as high as 50% within 5 years after surgery without medical treatment.[1] The formation and growth of calculi continues to trouble mankind as there is no satisfactory drug to treat kidney stones. Several plant extracts have been used to treat kidney stones with promising effect in prevention and treatment.[4–7]
Asparagus racemosus Wild (Liliaceae), commonly called as Satavari (Sanskrit), is a spinous under-shrub, with short rootstock bearing numerous succulent tuberous roots. The plant grows throughout the tropical and subtropical parts of India up to an altitude of 1500 m.[8] The root of the plant has long been used in the traditional system for various clinical conditions such as antiulcer,[9] antitussive,[10] antidiarrhoeal,[11] immunomodulatory,[12] antihepatotoxic,[13] and galactogogue.[14] It also has antioxidant,[15] antibacterial,[16] and diuretic[17] effects. On the basis of these reports, this study has been undertaken to study the antiurolithiatic activity of ethanolic extract of A. racemosus in experimentally induced urolithiasis in rats.
Materials and Methods
Animals
Thirty-six healthy male Wistar albino rats weighing between 220 and 270 g were used for the study. The animals were acclimatized for 7 days before experiments commenced. The animals were housed in polypropylene cages and maintained under standard laboratory conditions. They were fed with standard pellet diet and water ad libitum. The experimental protocols were approved by the Institutional Animal Ethics Committee (7/22/2009/IAEC/SVSMC/MBNR).
Drugs and Chemicals
The sample of the ethanolic extract of A. racemosus was procured commercially from Ayurvedic Extracts Laboratories (Batch No. AHAR 1105, Haryana). Ethylene glycol was purchased from S.D Fine Chemicals Ltd (Mumbai). Ammonium chloride was purchased from Qualgens Fine Chemicals (Mumbai). Diagnostic kits for calcium, phosphorus, urea, and creatinine were purchased from Crest Biosystems (Division of Coral Clinical Systems, Goa).
Acute Toxicity Study
Acute toxicity study was carried out as per Organization for Economic Co-operation and Development guidelines 425.[18] Four arbitrary doses of 200, 400, 800, and 1600 mg/kg were selected for the study, as the extract was found safe even at doses more than 2000 mg/kg without any sign of toxicity or mortality.
Ethylene Glycol–Ammonium Chloride-Induced Urolithiasis
Ethylene glycol and ammonium chloride–induced urolithiasis model was used for the experiment.[19] Thirty-six rats were divided into six groups of six animals each. The treatment protocol for 10 days for each group was as follows:
Group I: ad libitum access to regular food and drinking water and administered 6 μl distilled water per 1 g of body weight by gavage (normal control).
Groups II, III, IV, V, and VI: ad libitum access to regular food and ad libitum access to drinking water containing 0.75% [v/v] ethylene glycol (EG) and 2% [w/v] ammonium chloride (AC) in order to promote urolithiasis.
Group III, IV, V, and VI rats were also administered test drug, the ethanolic extract of A. racemosus by gavage at the following doses. Group III 200 mg/kg, Group IV 400 mg/kg, Group V 800 mg/kg, and Group VI 1600 mg/kg and served as test groups.
Group II rats were administered 6 μl distilled water per 1 g of body weight by gavage and served as positive control. All rats were weighed daily.
Assessment of Antiurolithiatic Activity
Serum analysis
At the end of 10 days of the experimental period, rats were anaesthetized and blood was collected from the retro-orbital region, centrifuged at 10,000 × g for 10 min. The serum was estimated for calcium, phosphorus, urea, and creatinine using the respective diagnostic kits.
Histopathological studies
The rats were killed by high doses of ether, abdomen was cut opened, and the kidneys were removed. The kidneys were stored in formalin (10%), fixed in bouin liquid, soaked in paraffin, cut at 2–3 μm intervals, and the slices were stained using hematoxylin and eosin. Tissue slices were photographed using optical microscopy under polarized light.
Statistical Analysis
The data were presented as mean ± standard error of mean (SEM) and analysed using one-way analysis of variance (ANOVA) and Student's “t”-test. P < 0.05 was considered statistically significant. Conventional windows software was used for statistical analysis.
Results
The acute toxicity studies showed no adverse effect or mortality in albino rats up to 2000 mg/kg p.o. of ethanolic extract of A. racemosus during the 24-hour observation period. The Group I rats remained active and gained weight. While Group II to VI rats lost weight over the 10 days of treatment. Serum analysis showed that urea and creatinine levels were higher in Groups II, III, IV, V, and VI compared with Group I [Table 1]. The biochemical parameters were significantly (P < 0.05) reduced in rats treated with 800 and 1600 mg/kg ethanolic extract of A. racemosus (Groups V and VI) compared with positive control rats (Group II).
Table 1.
Comparison of effect of EEAR on biochemical parameters in urolithiatic rats
Microscopic examination using polarized light of urolithiatic kidney sections showed intratubular and interstitial crystal deposits in Group II rats. However, rats treated with ethanolic extract of A. racemosus had far less kidney calcification. Histopathological examination of normal control Group I showed normal size tubules with single epithelial lining along the margin. In the positive control rats, there was marked dilatation of the tubules and total degeneration of the epithelial lining with infiltration of the inflammatory cells into the interstitial space. In Groups V and VI, the specimen showed characters similar to normal control Group I rats [Figure 1].
Figure 1.
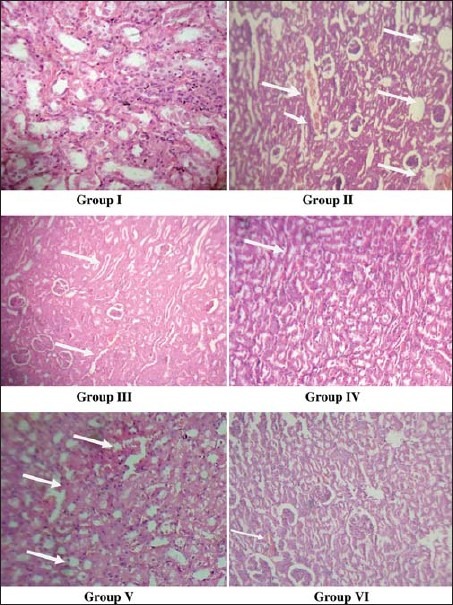
Histology of kidney sections. There is marked dilatation of tubules and crystal deposition in Group II rats. Histology of Group V and IV rats are almost similar to Group I
Discussion
This study examined the antiurolithiatic effect of ethanolic extract of A. racemosus in experimentally induced urolithiasis in rats. Rat models of calcium oxalate urolithiasis induced by either ethylene glycol (EG) alone or in combination with ammonium chloride (AC) are most commonly used to study the pathogenesis of urolithiasis. This study is an accelerated model, where rats are treated with 0.75% EG and 2% AC for 10 days.[19]
In this study, body weight, serum concentrations of calcium, phosphorus, urea, creatinine, and the histopathology of the kidney are analyzed. The possible mode of action of A. racemosus may be due to excessive secretion or decrease in the urinary concentration of the urinary salts that prevent super saturation of the crystallizing salts. Earlier studies have demonstrated the diuretic property of A. racemosus.[17] This property favours antiurolithiasis by hastening the process of dissolving or by flushing of the preformed stones or by preventing the new stone formation in urinary system on prophylactic treatment.
In urolithiasis, the glomerular filtration rate decreases due to the obstruction to the outflow of urine by stones in the urinary system and also due to the damage to renal parenchyma. Due to this, the waste products, particularly nitrogenous substances such as urea, creatinine, and uric acid, get accumulated in the blood. The decrease in the serum levels of these are due to the antiurolithiatic effect of A. racemosus.
The other possible mode of action of A. racemosus may be due to its antioxidant effect. There is in vivo evidence that hyperoxaluria-induced per-oxidative damage to the renal tubular membrane surface provides a favorable environment for individual calcium oxalate crystal attachment and subsequent development of kidney stones. The study done on lemon juice in rat urolithiatis model has shown that it has protective activity against urolithiasis due to its high antioxidant property due to the presence of vitamin E and vitamin C.[19] Several studies have reported the antioxidant activity of A. racemosus.[9,20] Thus, it is possible that ethanolic extract of A. racemosus also prevents stone formation via its antioxidant effects. Moreover, the antibacterial activity of the A. racemosus probably contributes for its antiurolithiasis activity as bacterial infection also promotes urolithiasis.
The photochemical studies done earlier have demonstrated that flavanoids, saponins, polyphenols, asparagamine a polycyclic alkaloid, racemosol a cyclic hydrocarbon (9,10-dihydrophenantherene), and polysaccharides are the active principles.[17,21–25] From the earlier studies it has been reported that flavanoids and saponins have diuretic activity.[17] Flavanoids and polyphenols also have antioxidant effect.[25] The active principle 9,10-dehydrophenantherene has antibacterial effect.[25] All these chemical constituents may be responsible for the antiurolithiatic property of ethanolic extract of A. racemosus.
Conclusion
Thus, it can be concluded that the administration of ethanolic extract of A. racemosus significantly decreased the development of urolithiasis in rats.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Marshall L, Stoller MD. Urinary stone disease. In: Tanagho EA, McAninch JW, editors. Smith's General Urology. 16th ed. New York: McGraw-Hill Lange Medical Books; 2004. pp. 256–91. [Google Scholar]
- 2.Khan SR. Interactions between stone-forming calcific crystals and macromolecules. Urol Int. 1997;59:59–71. doi: 10.1159/000283025. [DOI] [PubMed] [Google Scholar]
- 3.Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsute on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92:137–40. doi: 10.1046/j.1464-410x.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- 4.Ashok P, Koti BC, Vishwanathswamy AH. Antiurolithiatic and antioxidant activity of Mimusops elengi on ethylene glycol-induced urolithiasis in rats. Indian J Pharmacol. 2010;42:380–3. doi: 10.4103/0253-7613.71925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suman KM, Satyaranjan M, Sabuj S, Prasannakumar P. Antiurolithiatic activity of Cratavera manga Lour.bark. Indian J Nat Prod Resour. 2011;2:28–33. [Google Scholar]
- 6.Soundararajan P, Mahesh R, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981–6. [PubMed] [Google Scholar]
- 7.Hosseinzadeh H, Khooei AR, Khashayarmanesh Z, Motamed-Shariaty V. Antiurolothiatic activity of Pinus eldarica medw: Fruits aqueous extract in rats. Urol J. 2010;7:232–7. [PubMed] [Google Scholar]
- 8.Bopana N, Saxena S. Asparagus racemosus-ethanopharmacological evaluation and conservation needs. J Ethnopharmacol. 2007;110:1–15. doi: 10.1016/j.jep.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Singh KP, Singh RH. Clinical trial on Satavari (Asparagus racemosus Willd.) in duodenal ulcer disease. J Res Ay Sid. 1986;7:91–100. [Google Scholar]
- 10.Mandal SC, Kumar CK, Mohana Lakshmi S, Sinha S, Murugesan T, Saha BP, et al. Antitussive effect of Asparagus racemosus root against sulfur dioxide-induced cough in mice. Fitoterapia. 2000;71:686–9. doi: 10.1016/s0367-326x(00)00151-9. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesan N, Thiyagarajan V, Narayanan S, Arul A, Raja S, Vijaya K, et al. Anti-diarrhoeal potential of Asparagus racemosus wild root extracts in laboratory animals. J Pharm Pharm Sci. 2005;8:39–46. [PubMed] [Google Scholar]
- 12.Rege NN, Nazareth HM, Isaac A, Karandikar SM, Dahanukar SA. Immunotherapeutic modulation of intraperitoneal adhesions by Asparagus racemosus. J Postgrad Med. 1989;35:199–203. [PubMed] [Google Scholar]
- 13.Muruganadan S, Garg H, Lal J, Chandra S, Kumar D. Studies on the immunostimulant and antihepatotoxic activities of Asparagus racemosus root extract. Int J Med Arom Plants. 2000;22:49–52. [Google Scholar]
- 14.Sharma S, Ramji S, Kumari S, Bapna JS. Randomized controlled trial of Asparagus racemosus (Shatavari) as a lactogogue in lactational inadequacy. Indian Pediatr. 1996;33:675–7. [PubMed] [Google Scholar]
- 15.Kamat JP, Boloor KK, Devasagayam TP, Venkatachalam SR. Antioxident properties of Asparagus racemosus against damage induced by gamma-radiation in rat liver mitochondria. J Ethnopharmacol. 2000;71:425–35. doi: 10.1016/s0378-8741(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 16.Mandal SC, Nandy A, Pal M, Saha BP. Evaluation of antibacterial activity of Asparagus racemosus wild. Root. Phytother Res. 2000;14:118–9. doi: 10.1002/(sici)1099-1573(200003)14:2<118::aid-ptr493>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Gaitondé BB, Jetmalani MH. Antioxytocic action of saponin isolated from Asparagus racemosus Willd (Shatavari) on uterine muscle. Arch Int Pharmacodyn Ther. 1969;179:121–9. [PubMed] [Google Scholar]
- 18.Organization for economic Co-operation and Development(OECD) Section 4, Health Effects: Test No. 425: Acute oral toxicity: Up-and-down procedure. France: OECD Publishing; 2006. OECD Guidelines for Testing of Chemicals; pp. 1–27. [Google Scholar]
- 19.Touhami M, Laroubi A, Elhabazi K, Loubna F, Zrara I, Chait A, et al. Lemon juice has protective activity in a rat urolithiasis model. BMC Urol. 2007;7:18. doi: 10.1186/1471-2490-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visavadiya NP, Narasimhacharya RL. Hypolipidemic and antioxidant activities of Asparagus racemosus in hypercholesteremic rats. Indian J Pharmacol. 2005;37:376–80. [Google Scholar]
- 21.Joshi JD. Chemistry of ayurvedic crude drugs: Part VIII: Shatavari: 2.Structure elucidation of bioactive shatavarin I and other glycosides. Indian J Chem Sect B Org Chem Incl Med Chem. 1988;27:12–6. [Google Scholar]
- 22.Potduang B, Meeploy M, Giwanon R, Benmart Y, Kaewduang M, Supatanakul W. Biological activities of Asparagus racemosus. Afr J Tradit Complement Altern Med. 2008;5:230–7. doi: 10.4314/ajtcam.v5i3.31278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oketch-Rabah HA. Phytochemical constituents of the Genus Asparagus and their biological activities. Hamdard. 1998;41:33–43. [Google Scholar]
- 24.Amit C, Payal C, Mangalesh, Roy RC. Asparagus racemosus (Wild): Biological activities & its active principles. Indo-Glob J Pharm Sci. 2011;1:113–20. [Google Scholar]
- 25.Michael T. Herbal Monograph – Asparagus racemosus. Phytomedicine. 2000;38:119–28. [Google Scholar]


