Abstract
Objectives:
To evaluate the effect of aqueous extract of stem of Tinospora cordifolia (TC) on hyperalgesia in streptozotocin induced diabetic rats and in- vitro aldose reductase inhibition.
Materials and Methods:
Wistar albino rats, rendered diabetic with streptozotocin, were divided into 5 groups, namely the diabetic control treated with vehicle (DC), standard control which received glibenclamide+metformin (SC), test groups treated with 100, 200and 400 mg/kg b.w. of Tinospora cordifolia (TC1, TC2 and TC3 respectively). A group of five normal animals served as normal control (NC). Fasting blood glucose, body weight and reaction time to tail flick were measured one week after induction of diabetes. The animals were then treated orally for two weeks after which the same parameters were repeated. In-vitro aldose reductase inhibition assay was carried out at concentrations of 5, 10, 25, 50, 100 and 200 mcg/ml of Tinospora cordifolia using rat lens from normal rats. The in-vivo results were analysed with Mann Whitney test.
Results:
The DC group demonstrated a decrease in the reaction time (hyperalgesia) compared to NC while a significant increase in the reaction time was observed with SC, TC2 and TC3 groups (p<0.05) as compared to the DC group. TC1 and TC2 showed a significant reduction in body weight compared to their baseline values (p<0.05). There was no significant change in the fasting blood glucose (FBS) in any of the groups. In-vitro aldose reductase inhibition was observed with TC with an IC50 of 103 mcg/ml.
Conclusions:
Tinospora cordifolia prevents the hyperalgesia in experimental diabetic neuropathy. It has an aldose reductase inhibitory activity in-vitro which may contribute to the beneficial effects.
KEY WORDS: Aldose reductase, hyperalgesia, neuropathy, Tinospora cordifolia
Introduction
Diabetic neuropathy (DN) is an important complication of diabetes mellitus resulting in a great deal of morbidity. The prevalence of diabetic neuropathy is about 26.1% in Indian population.[1] Peripheral diabetic neuropathy is characterised by symptoms such as tingling and numbness, sharp pains or insensitivity to pain, motor incoordination, loss of sense of vibration etc. Untreated, it may lead to loss of reflexes and deformities that may progress to gangrene. The condition is characterised by peripheral demyelination, decrease in the nerve conduction and degeneration of myelinated and demyelinated sensory nerve fibres.[2]
Oxidative stress as a result of glucose oxidation and the subsequent formation of advanced glycation end (AGE) products, disruption of polyol pathway, altered glucose metabolism and decreased anti-oxidant defences are some of the implicated mechanisms in the pathogenesis of neuropathy.[3] Aldose reductase plays an important role as it diverts excess of glucose in hyperglycemic states to sorbitol by polyol pathway which is responsible for diabetic complications including neuropathy. Chronic use of aldose reductase inhibitors have shown beneficial effect on peripheral sensory neuropathy of experimentally induced diabetic rats as well as in clinical trials.[4,5]
There is no definitive treatment for DN at present. Tricyclic antidepressants, SNRIs, anticonvulsants, opioids and topical capsaicin have been tried in the management of painful neuropathy of which duloxetine and pregabalin have been approved by the US FDA.[6] The use of these drugs is limited by their cost and side effects. The only aldose reductase inhibitor approved is Epalrestat which is marketed in Japan.
Herbal medicines may be used as an alternative therapy in this condition as they are effectively used for the treatment of diabetes in Ayurveda and are generally well tolerated. Tinospora cordifolia (TC) is recommended for diabetes, burning sensation, fever, edema etc. It has been scientifically validated in various animal models for hypoglycemic, immunomodulatory, anti-inflammatory, antioxidant and other pharmacological activities.[7] The aqueous and alcoholic extract of the plant has been shown to improve glucose tolerance in diabetic rats.[8] A study by Grover et al showed amelioration of experimental diabetic neuropathy by TC at a dose of 400mg/kg b.w.[9]
It was hypothesised that TC may reduce the symptoms of DN since it has hypoglycemic, anti-oxidant and analgesic activity and reduces burning sensation as reported in Ayurvedic literature. Hence the present work aimed at studying the effect of three different doses of standardized aqueous extract of stem of TC in animal model of diabetic neuropathy. Our primary objective was to evaluate the effect of TC extract on hyperalgesia in diabetic rats. We have also evaluated the effect of TC on aldose reductase inhibition in-vitro, to explore its probable mode of action.
Materials and Methods
The study was carried out after the approval of the Institutional Animal Ethics Committee (Reg No. 1133/AC/07/CPCSEA/2008). Streptozotocin was obtained from Sigma Chemicals and glibenclamide (Bristol Mayer's squibb) with metformin (Franco Indian pharmaceuticals) were used as standard control. A standardised aqueous extract of Tinospora cordifolia was prepared.
Extraction of Tinospora cordifolia and Analysis
Coarse ground stem bark of Tinospora cordifolia (authenticated at the National Institute of Science Communication and Information Resources, New Delhi) was charged into a stainless steel jacketed extractor fitted with a reflux condenser. Distilled water was added to the extractor and refluxed for 3 hours by providing steam in the jacket and then filtered through a filtration cloth. The extraction of marc with water was carried out thrice. The liquid aqueous extract filtered was combined and charged to a concentrator and then subjected to distillation under vacuum (at <75°C) until the total solid content in the liquid reached about 15-20% (w/v). The concentrated liquid was then spray dried to get the water extract.
The water extract was subjected to physico-chemical and phytochemical analysis. The physicochemical analysis revealed that the moisture content of 4.0% w/w acid insoluble ash was 0.3%w/w. The sample was also analyzed for heavy metals, pesticide and microbial count. The lead, cadmium and arsenic content was less than 0.2 ppm and mercury was less than 0.1 ppm. The total viable microbial count was less than 1000 cfu/g and no growth of Salmonella, E.coli and Staphylococcus were observed. The phytochemical analysis of the extract sample was analyzed for Bitters content (by gravimetry) and was found to contain 4.8%. The pesticide residues were also within the United States Pharmacopoeia (USP) limits. The analysis was carried out as per USP 2009 protocols and all the parameters were within the limits specified by USP.
Experimental Evaluation of Diabetic Neuropathy
Adult female Wistar albino rats, aged 10-12 weeks, weighing 130-200 g were obtained from Venkateshwara industries (Reg. No. 237/CPCSEA/2000). They were acclimatised to room temperature with a relative humidity 55±10% for 2 weeks during which time they were provided standard feed (from Hindustan lever) and filtered water ad libitum. They were housed in propylene cages.
An initial fasting blood sugar (FBS) was done by glucose oxidase method with the help of a well calibrated glucometer (Accucheck) after 14 hours of fasting. All the tests were carried out between 9.30 -10.30 a.m. We also recorded a reaction time to radiant heat using techno-analgesiometer (Incorp, India). The animal was put into a rat restrainer which had an opening for the tail at the rear wall and holes on the front wall for easy breathing. The restrainer was placed on the techno-analgesiometer. The tail was held gently by the investigator so that the middle portion of proximal one third was placed on the metal base, belowwhich was the heating element (nichrome wire). The temperature was preset to allow a flow of 1.5 amperes of current. After allowing the animal to acclimatise in this position for 2-3 minutes, once the tail was found to be in a relatively stable position, the switch was put on. The time taken for the rat to flick its tail in response to radiant heat was considered as the reaction time. Animals showing FBS of 60-90mg/dl and reaction time of 7-12 seconds were selected for the final experiment.
The animals were made diabetic by an intraperitoneal injection of freshly prepared streptozotocin at a dose of 90 mg/kg b.w. after overnight fasting. Streptozotocin was prepared in ice-cold citrate buffer (pH 4.0) as per the international protocol of animal models of Diabetes Complications Consortium.[10] The animals with FBS more than 200 mg/dl after 48 hours of injecting streptozotocin, were randomized into 5 groups, with a minimum of 6 animals each, and were given the following treatment.
NC -Normal control -Vehicle only
DC- Diabetic control-Vehicle only
SC-Standard control- Glibenclamide 5mg/kg body weight+metformin 25 mg/kg
TC1-Tinospora cordifolia aqeous extract 100mg/kg
TC2-Tinospora cordifolia aqeous extract 200mg/kg
TC3- Tinospora cordifolia aqeous extract 400mg/kg
One week after the induction of diabetes, FBS was repeated and the medications were administered once a day orally for 15 days starting from the eighth day. The FBS, body weight and the reaction time were repeated once again on the 15th day of dosing.
In-vitro Aldose-reductase Inhibition Assay
Aldose reductase activity was assayed according to the method described by Hayman and Kinoshita[11] with some modifications. The incubation mixture, in a final volume of 250μL, consisted of 67mM potassium phosphate buffer (pH-6.2), 0.4M lithium sulphate, 150μM NADPH, 300μM DL-glyceraldehyde, enzyme (50μL) and various concentrations of the test sample preparations (as mentioned in the table below). Quercetin 0.5μg/mL was used as standard inhibitor. Appropriate blanks were prepared for quercetin and the test samples without DL- glyceraldehyde. The reaction was initiated by adding NADPH. The absorbance was read at 340nm for 20 minutes using FLUOstar microplate reader (BMG Labtech) in a kinetic mode. The percent inhibition by test sample/quercetin was calculated by considering the control value as 100%, using the equation mentioned below. The median inhibitory concentration (IC50) was calculated using Finney software. Similar procedure was carried out with TC at strengths of 5, 10, 25, 50, 100 and 200 mcg/ml. The percentage inhibition was calculated with the following formula:
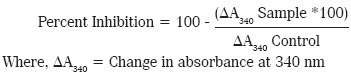
Statistical Analysis
The results for experimental neuropathy are expressed as mean ± standard error of mean and analysed using Mann-Whitney test with SPSS software. P<0.05 was considered as statistically significant. IC50 was calculated for the in-vitro aldose inhibitory activity of TC.
Results
Effect of Tinospora Cordifolia on Reaction Time
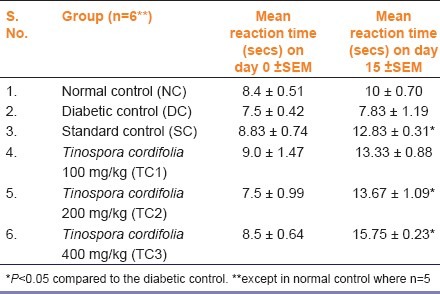
As shown in Table 1, the reaction time on the 15th day was less in the DC group as compared to the normal animals (NC), indicating hyperalgesia. All the other groups showed an increase in the reaction time which was statistically significant in SC, TC2 and TC3 groups as compared to the diabetic group (P<0.05). The percentage increase in reaction tine was calculated with reference to their base line values. The increase in the normal control was found to be 19.04%. This value was deducted from the observed percentage increase in the standard and the test groups. Accordingly, the percentage increase in the SC group was 26% and groups TC1, 2, and 3 showed an increase by 29%, 63% and 61% respectively. The actual values are shown in Table 1.
Table 1.
Effect of Tinospora cordifolia on the tail flick reaction time in Wistar albino rats
Effect of Tinospora Cordifolia on Fasting Blood Glucose
At the end of 15 days of treatment, the FBS in the NC group remained in the range of 63-85mg/dl while the animals in diabetic control showed very high values (>500mg/dl). In the SC group, there was no change in FBS values except in one animal where it was 142mg/dl (from a value of 417gms/ dl). The TC1, TC2 and TC3 groups showed no response except in one animal in the TC2 group, where an improvement from 354mg/ dl to 264 mg/dl was observed.
Effect of Tinospora Cordifolia on Body Weight
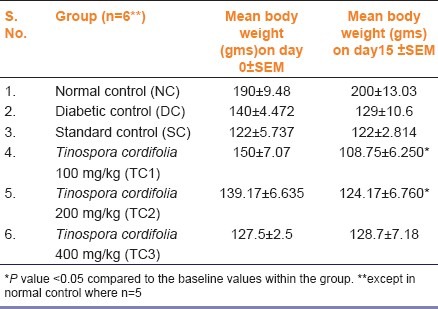
There was weight loss in the DC group as compared to their baseline values (7.8%) which was not statistically significant. The SC group, however, did not show any weight loss. TC1 and TC2 showed significant reduction in body weight compared to their base line values, the mean reduction being 28%, and 10% respectively (P<0.05). There was no reduction in the feed intake in any of the groups [Table 2].
Table 2.
Effect of Tinospora cordifolia on body weight in Wistar albino rats
Effect of Tinospora cordifolia on aldose reductase
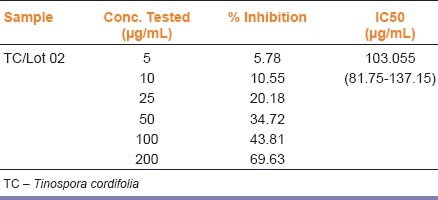
Quercetin at a concentration of 0.5μg/mL exhibited 46.19% inhibition of aldose reductase activity. Percent inhibition of aldose reductase enzyme by the TC samples at different concentrations was calculated and plotted on a graph. IC50 of TC was 103 μg/mL [Table 3].
Table 3.
Effect of Tinospora cordifolia on aldose reductase enyme activity in-vitro
Discussion
The model chosen in the present study was based on the principle that at an increased level of blood sugar in a diabetic rat, hyperalgesia due to the hyperactivity of C nociceptive fibres will be observed. This can be detected as a reduced latent period (reaction time) for tail flick in response to radiant heat by the analgesiometer.[12] A drug beneficial in DN will reverse the hyperalgesia.
The aqueous extract of stem of Tinospora cordifolia (TC), commonly known as Guduchi sattwa in Ayurveda, is recommended for the treatment of diabetes mellitus.[7] We, therefore, preferred the aqueous extract of the stem. The extract met with all the analytical specifications of the standardized herbal extract as per the international standards.
Diabetic neuropathy is characterized by an increased sensitivity to touch and pain due to neuronal loss or alteration in the neurotransmitters. It is reported that significant hyperalgesia i.e. a reduction in the flick tail latency to radiant heat develops 3 weeks after induction of diabetes. A similar observation was made in the present study. Aqueous extract of Tinospora cordifolia reversed the hyperalgesia at all three doses, with significant effects at 200 and 400 mg/kg b.w.. This is in contrast with the study by Grover et al where there was a non significant response at 400 mg/kg.[9] The response increased with increase in the dose although a typical dose response relationship was not observed with the three doses.
S.S. Singh, et al investigated the chemical constituents and the medicinal properties of ethanolic extract of Tinospora cordifolia at a dose of 400mg/kg body weight, which produced a significant reduction of blood sugar in alloxan induced diabetic rats.[13] Grover et al studied the effect of aqueous extract at 400mg/kg body weight on FBS in streptozotocin induced severely diabetic rats when treated for 6 weeks.[14] They concluded that TC is not effective in severe diabetes (FBS above 400 mg/kg). Our observations are in concurrence with this study as far as the improvement in the diabetic state is concerned (the FBS levels in diabetic rats were not reduced). We chose a dose of 90 mg/kg of streptozotocin as we were unable to induce hyperalgesia at lesser doses in our pilot studies.
A significant reduction in the body weight was also observed with TC1 and TC2 inspite of normal feed intake and absence of any behavioural abnormalities. Though Ayurveda mentions the use of Tinospora in obesity, we could not find any study on the anti-obesity effect of TC. On the contrary, Stanley et al have shown a significant reduction in blood glucose and brain lipids and an increase in body weight, total haemoglobin and hepatic hexokinase with T. cordifolia root extract (TCREt).[15] The anti-obesity effect needs further exploration.
Clinical trials with aldose reductase inhibitor, Sorbinil (Pfizer-CP45634) have demonstrated significant improvement in the pain relief, motor and sensory nerve conduction velocities with minimum toxicity in patients with DN. These observations suggest that aldose reductase inhibitors may be important in the treatment of symptomatic, somatic and autonomic neuropathies complicating diabetes.[16] Hence the in-vitro effect of TC on aldose reductase inhibition was observed. It showed an inhibitory effect although the IC50 was higher as compared to the standard (quercetin). Thus, the beneficial effect of TC on diabetic neuropathy appears to be due to its analgesic effect and unrelated to its anti-hyperglycemic effect. Aldose reductase inhibition may contribute to this action. An anti-oxidant role of TC cannot be ruled out as oxidative damage contributes to the causation of diabetic neuropathy and TC is proven to be an anti-oxidant in experimental models.[17]
Limitations of the study: The study gives only preliminary evidence about the role of TC in diabetic neuropathy. Experiments involving nerve conduction studies would be more confirmatory. Moreover, a true dose response relationship of the action of TC on hyperalgesia could not be demonstrated, which may be because of a small sample size.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: The Chennai Urban Rural Epidemiology Study (CURES-55) Diabet Med. 2008;25:407–12. doi: 10.1111/j.1464-5491.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JR, Stittsworth JD, Jr, Kadir A, Fisher MA. Conduction velocity versus amplitude analysis: Evidence for demyelination in diabetic neuropathy. Muscle Nerve. 1998;21:1228–30. doi: 10.1002/(sici)1097-4598(199809)21:9<1228::aid-mus20>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Tawakoli M, Mojadidi M, Fadavi H, Malik RA. Pathophysiology and treatment of painful diabetic neuropathy. Current Pain Headache Rep. 2012;3:192–7. doi: 10.1007/s11916-008-0034-1. [DOI] [PubMed] [Google Scholar]
- 4.Yagihashi S, Kamijo M, Ido Y, Mirrlees DJ. Effects of long-term aldose reductase inhibition on development of experimental diabetic neuropathy.Ultrastructural and morphometric studies of sural nerve in streptozocin-induced diabetic rats. Diabetes. 1990;39:690–6. doi: 10.2337/diab.39.6.690. [DOI] [PubMed] [Google Scholar]
- 5.Schemmel KE, Padiyara RS, D’Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J Diabetes Complications. 2010;24:354–60. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Heubeck E. New Treatment Options for Diabetic Neuropathy DOC NEWS. [Last accessed on 2010 Dec 02];2006 3(2):8–9. http://docnews.diabetesjournals.org/content/3/2/8.full . [Google Scholar]
- 7.Krishna KL, Jigar B, Jagruti P. Guduchi (Tinospora cordifolia): Biological and Medicinal properties: A review. Internet J Altern Med. 2009;6:2. [Google Scholar]
- 8.Gupta SS, Verma SC, Garg VP, Mahesh R. Antidiabetic effect of Tinospora cordifolia Part I. Effect on fasting blood sugar level, glucose tolerance and adrenalin induced hyperglycaemia. Indian J Exp Biol. 1967;55:733–45. [PubMed] [Google Scholar]
- 9.Grover JK, Rathi SS, Vats V. Amelioration of experimental diabetic neuropathy and gastropathy in rats following oral administration of plant (Eugenia jambolana, Mucuna pruriens and Tinospora cordifolia) extracts. Indian J Exp Biol. 2002;40:273–6. [PubMed] [Google Scholar]
- 10.Low dose streptozotocin Induction protocol(mouse);Animal models of diabetes complications consortium. [Last accessed on 2009 Apr 15th]. Available from: http://www.amdcc.org/
- 11.Hayman S, Kinoshita JH. Isolation and properties of lens aldose. J Biol Chem. 1965;240:877–82. [PubMed] [Google Scholar]
- 12.Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, et al. “Poly(ADP-Ribose) Polymerase Inhibition Alleviates Experimental Diabetic Sensory Neuropathy. Diabetes. 2006;55:1686–94. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC. Chemistry and Medicinal Properties of Tinospora cordifolia (Guduchi) Indian J Pharmacol. 2003;35:83–91. [Google Scholar]
- 14.Grover JK, Vats V, Rathi SS. Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol. 2000;73:461–70. doi: 10.1016/s0378-8741(00)00319-6. [DOI] [PubMed] [Google Scholar]
- 15.Stanely P, Prince M, Menon VP. Hypoglycaemic and other related actions of Tinospora cordifolia roots in alloxan-induced diabetic rats. J Ethnopharmacol. 2000;70:1–9. doi: 10.1016/s0378-8741(99)00136-1. [DOI] [PubMed] [Google Scholar]
- 16.Jaspan J, Herold K, Maselli R, Bartkus C. Treatment of severely painful diabetic neuropathy with an aldose reductase inhibitor: Relief of pain and improved somatic and autonomic nerve function. Lancet. 1983;322:758–62. doi: 10.1016/s0140-6736(83)92296-1. [DOI] [PubMed] [Google Scholar]
- 17.Stanely M, Prince P, Menon VP. Antioxidant activity of Tinospora cordifolia roots in experimental diabetes. J Ethnopharmacol. 1999;65:277–8. doi: 10.1016/s0378-8741(98)00164-0. [DOI] [PubMed] [Google Scholar]


