Abstract
Aim:
Plant Clitoria ternatea L. is claimed to possess a wide range of activities including antiinflammatory, local anesthetic and antidiabetic effect, etc. The aim of the present study was to evaluate the wound healing potential of standardized C. ternatea leaf extract in terms of different enzymatic models, which are mostly associated with skin wound.
Materials and Methods:
The methanol extract and fractions were screened for its hyaluronidase, elastase, and matrix metalloproteinase-1 (MMP-1) inhibitory activity compared with standard oleanolic acid. The activity was rationalized through reverse phase high performance liquid chromatography (RP-HPLC) standardization of the extract and fractions with respect to its isolated biomarker taraxerol (yield 5.27% w/w).
Results:
The extract showed significant (P < 0.001) hyaluronidase (IC50 18.08 ± 0.46 μg/ ml) and MMP-1 (P < 0.05) inhibition, but the elastase inhibition was insignificant (IC50 42.68 ± 0.46 μg/ml). Among the fractions, ethyl acetate fraction showed significant (P < 0.001) inhibition of hyaluronidase (IC50 28.01 ± 0.48 μg/ml) and MMP-1 (P < 0.01). The HPLC analysis revealed that the extract and the ethyl acetate fraction are enriched with taraxerol (5.32% w/w and 4.55% w/w, respectively).
Conclusions:
The experiment validated the traditional uses of C. ternatea and may be recommended for use in the treatment of different types of skin wounds, where taraxerol may be a responsible biomarker.
KEY WORDS: Clitoria ternatea, elastase, hyaluronidase, matrix-metalloproteinase-1, taraxerol
Introduction
Wound of human skin is the most often dermatological concern, which involves decline of cell functions during the chronic exposures of environmental and other factors.[1] Factors responsible for skin wounds, for example, trauma, thermal injury, UV irradiation from sun light, toxic chemical exposure, surgery, and microbial infection cause generation of reactive oxygen species (ROS), which trigger the inflammation and tissue damage. This inflammatory response accelerates the synthesis of dermal enzymes (elastase, hyaluronidase, matrix metalloproteinases, etc.) leading to degradation of extracellular matrix (ECM). ECM is constructed of different fibers such as fibrin, elastin, hyaluronan (glycosaminoglycan), and type I and type III collagen, which together form a fiber network to maintain the structural integrity. Elastase hydrolyzes fibrin and elastin fibers; hyaluronan depolymerised by hyaluronidase and matrix metalloproteinases-1 particularly breaks the type I collagen. ROS also cause the synthesis of inflammatory cytokines such as interleukin-6 and -8, which are similarly responsible for inflammation. The overall effects are loss of skin elasticity, transepidermal water, and tensile strength. These evidences suggest that the expression of dermal enzymes and the downregulation of fiber synthesis play a major role in the process of skin wound.[2] Most promising treatment of skin wound includes herbal extract, vitamins, and antioxidant food supplements, which have been accepted widely to scavenge free radicals and reduces the level of dermal enzymes to restore the normal skin function and slowdown the process of inflammation.[3]
Clitoria ternatea L. (family: Fabaceae) commonly known as ‘Butterfly pea’, is an indigenous herbal medicine claimed to treat central nervous systems (CNS) problems; stress, anxiety, depression and convulsions, etc. The plant has been reported to contain wide range of secondary metabolites including taraxerol, taraxerone, delphinidin 3, 3, 5-triglucoside, p-hydroxycinnamic acid, acylated anthocyanins, aparajitin, clitorin, and several others, which are claimed to possess a number of neuropharmacological actions and antipyretic, antiinflammatory, analgesic, local anesthetic, and antidiabetic activities.[4] These reports lead us to evaluate the wound healing property of C. ternatea leaf extract in terms of different enzymatic models, which are mostly associated with the skin wounds. The methanolic extract and fractions were screened for hyaluronidase, elastase, and MMP-1 inhibitory activity compared with standard oleanolic acid. The activity was rationalized through RP-HPLC standardization of the extract and fractions with respect to its isolated biomarker taraxerol [Figure 1].
Figure 1.
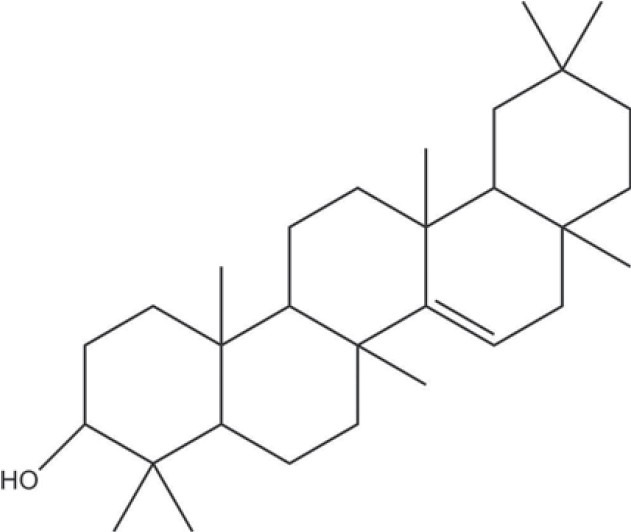
Structure of isolated taraxerol from C. ternatea leaf extract
Materials and Methods
Chemicals and Reagents
Human leukocyte elastase (HLE), hyaluronic acid potassium salt from human umbilical cord, hyaluronidase from bovine testes, N-succinyl-(Ala)3-p-nitroanilide, and oleanolic acid were purchased from Sigma-Aldrich (USA). Type-I collagen fluorescein conjugate (substrate) from bovine skin and MMP-1 were purchased from Invitrogen BioServices India Pvt. Ltd. Acetonitrile was procured from Merck (Darmstadt, Germany). All other solvents and reagents used were of analytical grade.
Apparatus
Waters RP-HPLC system (Milford, MA, USA) consisted of 600 quaternary pump, Rheodyne-7725i injection valve with a sample loop of 20 μl and a UV-Vis dual wavelength detector. A Waters Spherisorb (Ireland) C18 column (250 × 4.6 mm, 5 μm particle size) was used as stationary phase. Spectrophotometric measurement (visible range) was performed by UV-Visible spectrophotometer (CECIL CE7200) and 96 well micro plate reader (BIO-RAD, Model: 680-XR). Fluorescence was measured by 96 well fluorescence microplate reader (BioTek, FLx 800T, USA).
Preparation of Plant Material
C. ternatea leaf was procured locally and authenticated by Dr. S. Rajan, Field Botanist, Ooty, Tamilnadu, India. A voucher specimen (specimen number SNPSJU/2010/1068) was submitted to the School of Natural Product Studies, Jadavpur University, Kolkata, India. About 1 kg of fresh leaves were crushed and kept for cold maceration with 95.5% methanol for 72 h. The extract was filtered and the solvent was recovered using rotary evaporator (EYELA, Tokyo, Japan) at a temperature not exceeding 45°C. The recovered solvent was again mixed with the same plant material and kept for 48 h. The process was repeated for another two times and the combined extract was lyophilized to obtain powder (yield 1.6% w/w). The lyophilized extract was dissolved in water to fractionate successively with ethyl acetate and n-butanol. Then C. ternatea methanol extract (CTMeOH), ethyl acetate fraction (CTEA), n-butanol fraction (CTnB), and aqueous fraction (CTAQ) were used as test sample along with standard oleanolic acid for the enzyme inhibition assay. Taraxerol (yield 5.27% w/w) was obtained as a major bioactive molecule from CTEA by conventional column chromatography. Approximately 5 g of ethyl acetate fraction was subjected to column chromatography using silica gel (mesh size 230–400). The column was gradually eluted with pet ether/chloroform (50:50, 45:55, 40:60, 30:70, and 100% chloroform) solvent mixture. Fractions obtained from the pet ether/chloroform (45:55 and 40:60) were collected together and this fraction gave single spot over TLC (mobile phase was optimized as pet ether/chloroform, 70:30). This fraction was further purified with activated charcoal and recrystallized with methanol at 60°C. The structural characterization of the crystal performed by LC-MS spectra showed M+ ion peak with m/z 449.59 and molecular formula was calculated as C30H50O, which very much reassembled the spectral data of taraxerol isolated earlier from C. ternatea root extract in our laboratory.[5]
Standardization Through RP-HPLC Analysis
Extract and the fractions were analyzed by means of RP-HPLC with respect to its isolated biomarker taraxerol. Mobile phase composition was acetonitrile:water (86:14, v/v) with isocratic elution at 1 ml/min flow rate and detection at 210 nm. The column was equilibrated for 30–40 min with the mobile phase, prior to the injection of analyte. Taraxerol stock solution (1 mg/ml) was prepared by dissolving it with mobile phase in a volumetric flask by ultra-sonication. Extract and fractions stock solutions (1 mg/ml) were also prepared in mobile phase and filtered through Whatman NYL 0.45 μm syringe filter prior to injection. Calibration curve was plotted in a linearity range of 10–1000 μg/ml of taraxerol.
Hyaluronidase Inhibition Assay
Hyaluronidase inhibitory assay was performed by the method described previously using 96 well microplate.[6] Hyaluronidase reacts with the substrate hyaluronic acid to release N-acetyl glucosamine. In presence of any inhibitor, the release of N-acetyl glucosamine is reduced and it is monitored by measuring the absorbance at 600 nm. The reaction mixture contains 5 μl of test samples (diluted to 1.56, 3.12, 6.25, 12.50, 25, and 50 μg/ml concentrations) in dimethyl sulphoxide (DMSO) preincubated with hyaluronidase (1.50 U in 100 μl), sodium phosphate buffer 20 mM (pH 7.0) with sodium chloride 77 mM, and bovine serum albumin (BSA) 0.01% for 10 min at 37°C. Subsequently hyaluronic acid 100 μl (0.03% in 300 mM sodium phosphate, pH 5.35) is added to the incubation mixture and further incubated for 45 min at 37°C. Hyaluronic acid (undigested) is precipitated with acid albumin solution made up of BSA 0.1% in sodium acetate 24 mM and acetic acid 79 mM, pH 3.75. It is kept at room temperature for 10 min and then absorbance is measured at 600 nm. An absorbance value of intact undigested hyaluronic acid was considered as 100%. For calculation of percentage of enzyme activity, the following formula was used:
% Enzyme activity = (100%) – {A600 nm of hyaluronic acid + hyaluronidase/A600 nm hyaluronic acid × 100}
Elastase Inhibition Assay
The elastase inhibition assay was performed by UV-Visible spectrophotometer according to the method of Nema et al.[6] The release of p-nitro aniline due to proteolysis of N-succinyl-(Ala)3-p-nitroanilide by human leukocyte elastase in the presence or absence of inhibitor was monitored by measuring the absorbance at 410 nm. For the experiment, 1.015 mM N-succinyl-(Ala)3-p-nitroanilide was prepared in a 0.12 mol Tris–HCl buffer (pH 8.0) and this solution (1300 ml) was added to the sample solutions (100 ml). The samples (extract, fractions, and oleanolic acid standard) were diluted to 1.56, 3.12, 6.25, 12.50, 25, and 50 μg/ml concentrations. The reaction mixtures were vortexed and preincubated for 10 min at 25°C and then elastase (0.0375 U/ml) solution (100 ml) was added. Each reaction mixture was placed in water bath for 10 min at 25°C and the absorbance was measured at 410 nm. The percentage inhibition was calculated as:
Inhibition (%) = (1 – B / A) × 100; where A is the enzyme activity without the sample and B is the activity in presence of the sample.
Assay for MMP-1 Inhibitory Activity
The assay was performed according to a method described previously with minor alteration using 96 well fluorescence microplate.[7,8] When MMP-1 reacts with type-I collagen substrate, it causes collagenolysis but, in presence of inhibitor the reaction slows due to the inhibition of MMP-1. A reaction buffer containing 0.5 mol Tris–HCL, 1.5 mol NaCl, 50 mM CaCl2 and 2 mM sodium azide at pH 7.6 was prepared for all types of dilution. Type-I collagen fluorescein (20 μl) conjugate was mixed with 80 μl of each of diluted (1.56, 3.12, 6.25, 12.50, 25, and 50 μg/ml) inhibitor (extract and fractions). Oleanolic acid was used as reference standard. A 100 μl of diluted (0.2 U/ml) MMP-1 was added to each well and the plate was incubated at room temperature for 1–2 h protected from light. After that fluorescence was measured at excitation maxima at 495 nm and emission maxima at 515 nm. The control used was the reaction buffer with substrate and the inhibitors without MMP-1.
Statistical Analysis
The IC50 values were expressed as mean ± SEM. Statistical analysis was carried out by one way analysis of variance (ANOVA) followed by Dunnett test and P < 0.05 was considered as significant, as compared with the standard oleanolic acid. All calculations were performed using Graph Pad Prism (version 5.0).
Results
The CTMeOH extract, CTEA and CTnB fractions showed significant (P < 0.001, P < 0.01) hyaluronidase (IC50 18.08 ± 0.46, 28.01 ± 0.48, and 38.84 ± 0.41 μg/ml) inhibition, respectively, compared with oleanolic acid (IC50 41.51 ± 0.50 μg/ml) [Figure 2a]. A lesser IC50 value of the test sample as compared with the standard signifies their higher inhibitory activity. However, the elastase inhibition in all test samples was insignificant compared with the oleanolic acid (IC50 9.61 ± 0.36 μg/ml) [Figure 2b]. The CTMeOH extract and CTEA fraction showed significantly (P < 0.05, P < 0.01) higher MMP-1 inhibition with less fluorescence reading compared with oleanolic acid [Figure 2c]. A high collagenolytic activity of MMP-1 is associated with high fluorescence reading and equally a low collagenolytic activity of MMP-1 is associated with low fluorescence reading.[9] The HPLC standardization of the extract and fractions were performed with respect to isolated taraxerol as biomarker. The content of taraxerol in C. ternatea was determined using calibration curve plotted between mean peak area (Y-axis) and concentration (X-axis). Linearity of the calibration curve was tested by regression analysis and found to be linear in the concentration range of 10–1000 μg/ml with coefficient of determination (r2) of 0.9958 and y = 1.01e + 004x + 1.14e + 005, which represents that the data is closest to the line of best fit. The retention time of taraxerol was 14.007 min and content in CTMeOH extract, CTEA and CTnB fractions were found to be 5.32% w/w, 4.55% w/w and 0.43% w/w, respectively. Chromatogram was found to be directly proportional to concentrations of the calibration solutions. Retention times of standard were highly repeatable, with % relative standard deviation (RSD) < 2% even at high concentration.
Figure 2.
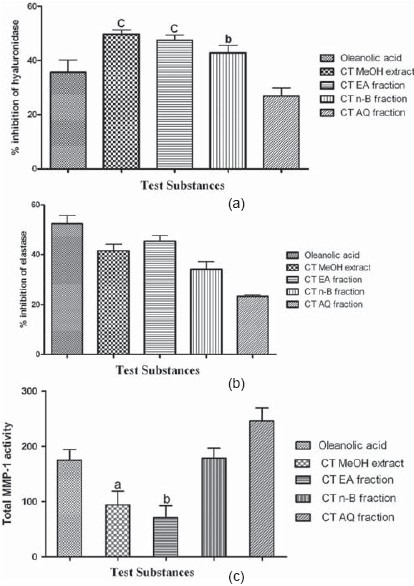
Hyaluronidase (a), elastase (b) and MMP-1 (c) inhibitory activity of C. Ternatea methanol extract, ethyl acetate, n-butanol, and aqueous fractions; [values expressed are mean ± SEM (n = 3)]. Comparisons are made between the oleanolic acid vs. CTMeOH, CTEA, CTnB, and PBAQ. All the test substances (extract, fractions, and oleanolic acid standard) were diluted to 1.56, 3.12, 6.25, 12.50, 25, and 50 μg/ml concentrations for each assay. aP < 0.05, bP < 0.01, and cP < 0.001 were considered as indicative of signifi cance difference, as compared with the oleanolic acid (Dunnett test)
Discussion
Proteolytic degradation of ECM is a characteristic of skin wound and the evidence suggests that it is significantly associated with increased dermal enzymatic activity.[7] Skin-care substances of natural origin have the potential to inhibit these enzymes and may also be used as wound healing agent.[10] C. ternatea extract and fractions were diluted at different concentrations and screened in triplicate (n = 3) for the inhibitory activities on elastase, hyaluronidase, and MMP-1 compared with standard oleanolic acid, which is a pentacyclic triterpene found in several botanical extracts and is also responsible to inhibit the dermal enzymatic process in skin wounds.[11,12] The inhibition of each enzyme due to the test substance was calculated. The hyaluronidase, elastase, and MMP-1 inhibitory activity of CTMeOH extract and the CTEA fraction were found to be more than oleanolic acid. These enzymes are the prime targets in screening of new leads, which could inhibit the process of skin wound through the modulation of wide variety of signaling pathways and pathological processes by these enzymes. The inhibition of MMP-1 is the most effective therapy to improve the structure of type I collagen in ECM and to alleviate active inflammation in the process of wound. Therefore, control of MMP-1 activities may help increase ECM turnover and reduces the level of dermal enzymes to restore skin function and thereby slowdown the process of skin wound. MMP-1 inhibitory activity of CTEA fraction was more, because the fluorescence reading was less compared with the CTMeOH extract and COnB fraction. COnB fraction showed almost similar activity like oleanolic acid. It may be due to the presence of different compounds in the extract and fractions, which may have synergistic effect. Taraxerol from other plant has already been reported to have plenty of pharmacological activities like antitumor, antiinflammatory, antiproliferative, and antioxidant etc.[13–16] These reports suggest that C. ternatea may also have potential therapeutic application over skin wound due to the presence of taraxerol. A better separation of the taraxerol marker in the extract was noted by the peak purity analysis. Robustness of the experimental procedure was found to be in the range of acceptability as there was not much deviation. It can be conclude that the method of quantitative standardization of C. ternatea using taraxerol was properly validated. The proposed RP-HPLC method is simple, accurate, specific, precise, and reproducible. The specificity test of the proposed method demonstrate that other constituent present in the methanol extract of the C. ternatea do not interfere with peak of interest identified as taraxerol. Furthermore, well shaped peaks indicate the specificity of the method and have wide scope for separation and quality assessment of this botanical. Thus, this experiment reveal that C. ternatea has effective inhibitory potential against hyaluronidase, elastase, MMP-1, and establishes C. ternatea as wound healing agent, which may be further explored in higher experimental models for the treatment of skin wounds.
Footnotes
Source of Support: Department of Science and Technology, Drug and Pharmaceutical Research Programme [DST-DPRP, File No. VI-D and P/287/08-09/TDT], Government of India, New Delhi and Parker Robinson Pvt. Ltd., Kolkata
Conflict of Interest: No.
References
- 1.Lansdown AB. Wound healing in the skin-an interaction between environmental conditions and intrinsic factors. Srinagarind Med J. 1995;10:43–55. [Google Scholar]
- 2.Mukherjee PK, Maity N, Nema NK, Sarkar BK. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19:64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Kim KS, Han CS, Yang HC, Park SH, Ko KI, et al. Inhibitory effects of natural plants of Jeju island on elastase and MMP-1 expression. J Cosmet Sci. 2007;58:19–33. [PubMed] [Google Scholar]
- 4.Mukherjee PK, Kumar V, Kumar NS, Heinrich M. The ayurvedic medicine Clitoria ternatea-from traditional use to scientific assessment. J Ethnopharmacol. 2008;120:291–301. doi: 10.1016/j.jep.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Kumar V, Mukherjee K, Kumar S, Mal M, Mukherjee PK. Validation of HPTLC method for the analysis of taraxerol in Clitoria ternatea. Phytochem Anal. 2008;19:244–50. doi: 10.1002/pca.1042. [DOI] [PubMed] [Google Scholar]
- 6.Nema NK, Maity N, Sarkar B, Mukherjee PK. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch Dermatol Res. 2011;303:247–52. doi: 10.1007/s00403-010-1103-y. [DOI] [PubMed] [Google Scholar]
- 7.Losso JN, Munene CN, Bansode RR, Bawadi HA. Inhibition of matrix metalloproteinase-1 activity by the soybean Bowman–Birk inhibitor. Biotechnol Lett. 2004;26:901–5. doi: 10.1023/b:bile.0000025900.33812.7c. [DOI] [PubMed] [Google Scholar]
- 8.Maity N, Nema NK, Abedy MK, Sarkar BK, Mukherjee PK. Exploring tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J Ethnopharmacol. 2011;137:1300–5. doi: 10.1016/j.jep.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 9.Knight CG, Willenbrock F, Murphy G. A novel coumarins labeled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992;296:263–6. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- 10.Chiu A, Kimball AB. Topical vitamins, minerals and botanical ingredients as modulators of environmental and chronological skin damage. Br J Dermatol. 2003;49:681–91. doi: 10.1046/j.1365-2133.2003.05540.x. [DOI] [PubMed] [Google Scholar]
- 11.Wei YJ, Yan XQ, Ma L, Wu JG, Zhang H, Qin LP. Oleanolic acid inhibits hypertrophic scarring in the rabbit ear model. Clin Exp Dermatol. 2011;36:528–33. doi: 10.1111/j.1365-2230.2010.04012.x. [DOI] [PubMed] [Google Scholar]
- 12.Facino RM, Carini M, Stefani R, Aldini G, Saibene L. Anti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: Factors contributing to their efficacy in the treatment of venous insufficiency. Arch Der Pharmazie. 1995;328:720–4. doi: 10.1002/ardp.19953281006. [DOI] [PubMed] [Google Scholar]
- 13.Tan B, Shi HL, Ji G, Xie JQ. Effects of taraxerol and taraxeryl acetate on cell cycle and apoptosis of human gastric epithelial cell line AGS. Chinese J Integr Med. 2011;9:638–42. doi: 10.3736/jcim20110610. [DOI] [PubMed] [Google Scholar]
- 14.Csupor-Löffler B, Hajdú Z, Zupkó I, Molnár J, Forgo P, Vasas A, et al. Antiproliferative constituents of the roots of conyza canadensis. Planta Med. 2011;77:1183–8. doi: 10.1055/s-0030-1270714. [DOI] [PubMed] [Google Scholar]
- 15.Khiev P, Oh SR, Chae HS, Kwon OK, Ahn KS, Chin YW, et al. Anti-inflammatory diterpene from thyrsanthera suborbicularis. Chem Pharm Bull. 2011;59:382–4. doi: 10.1248/cpb.59.382. [DOI] [PubMed] [Google Scholar]
- 16.Correia Da Silva TB, Souza VK, Da Silva AP, Lyra Lemos RP, Conserva LM. Determination of the phenolic content and antioxidant potential of crude extracts and isolated compounds from leaves of Cordia multispicata and Tournefortia bicolor. Pharm Biol. 2010;48:63–9. doi: 10.3109/13880200903046146. [DOI] [PubMed] [Google Scholar]


