Abstract
Objectives:
To investigate the protective effect of betulinic acid (BA) on endothelium-dependent relaxation (EDR) in rat aortas exposed to pyrogallol-produced superoxide anion and its underlying mechanism.
Materials and Methods:
The thoracic aorta of male Sprague-Dawley rats was isolated to mount in the organ bath system and the effect of BA on acetylcholine (ACh)-induced EDR, nitric oxide (NO) level, reactive oxygen species (ROS) level, nitric oxide synthase (NOS) activity, and superoxide dismutase (SOD) activity of aortic rings exposed to pyrogallol (500 μM) for 15 min were measured.
Results:
BA evoked a concentration-dependent EDR in aortas, and pretreatment with EC50 (2.0 μM) concentration of BA markedly enhanced ACh-induced EDR of aortas exposed to pyrogallol-produced superoxide anion (Emax rose from 23.91 ± 5.41% to 42.45 ± 9.99%), which was markedly reversed by both Nw -nitro-L-arginine methyl ester hydrochloride (L-NAME) and methylene blue, but not by indomethacin. Moreover, BA significantly inhibited the increase of ROS level, as well as the decrease of NO level, the endothelial NOS (eNOS) activity, and the SOD activity in aortas induced by pyrogallol-derived superoxide anion.
Conclusion:
These results indicate that BA reduces the impairment of EDR in rat aortas exposed to exogenous superoxide anion, which may closely relate to the reduction of oxidative stress and activation of eNOS–NO pathway.
KEY WORDS: Betulinic acid, endothelium-dependent relaxation, nitric oxide oxidative stress, rat thoracic aorta
Introduction
Endothelial cells regulate vascular tone by releasing various relaxing and contracting factors including nitric oxide (NO), endothelium-derived hyperpolarizing factors, arachidonic acid metabolites, reactive oxygen species (ROS), and other vasoactive peptides. [1] Obviously, the mechanisms of endothelium-dependent responses are complex, and the modulation by age and disease leads to endothelial dysfunction, which has become a predictor of major cardiovascular diseases. [2] Endothelium-dependent relaxation (EDR) impairment is usually the initial event of endothelial dysfunction. Though the etiology of EDR impairment is multi-factorial and not fully elucidated, overproduction of ROS under pathophysiologic conditions, alterations of endothelial nitric oxide synthase (eNOS) expression and activity, and decreased bioavailability of NO have all been considered responsible for the impairment of EDR. [3,4]
The physiological NO in the vasculature is mainly produced by eNOS, [5] and interruption of endothelial-derived NO elevates arterial blood pressure accompanied with impaired vascular responses to acetylcholine (ACh). [6,7] Overproduction of superoxide anion (O2-) induced by diabetes, hypercholesterolemia, and other diseases quickly scavenges the bioactivity of NO by generating peroxynitrite (ONOO-), a potent cytotoxic ROS. [8] Furthermore, ONOO- itself causes uncoupling of nitric oxide synthase (NOS) to produce superoxide instead of NO, [9] which exacerbates the oxidative stress and impairment of EDR leading to endothelial dysfunction. [10] So it is suggested that steps improving endothelial-derived NO bioavailability in oxidative stress may have potential protection against endothelial dysfunction.
Zizyphi Spinosi semen (ZSS), a traditional Chinese herb for treating neurasthenia, [11] is the dried seed of Zizyphus jujuba var. spinosa, and has been demonstrated being beneficial to the cardiovascular system by modern pharmacological studies, such as reducing myocardial ischemia injury and enhancing endothelium-derived NO bioavailability. [12,13] Betulinic acid (BA), the key active constituent of ZSS, may mediate such cardiovascular effects through upregulation of eNOS and downregulation of NADPH oxidase. [13] Recently, BA was reported to reduce cerebral ischemia-reperfusion injury in mice by decreasing oxidative stress and nitrosative stress, as well enhancing blood flow. [14] We thereby hypothesized that BA may attenuate the impairment of EDR induced by exogenous oxidants via modulating the bioavailability of endothelium derived NO.
Therefore, the aim of this work was to explore the effect of BA on ACh-induced EDR in rat thoracic aortas exposed to pyrogallol-produced O2-. To clarify the underlying mechanism, NO level, ROS production, activity of NOS, and superoxide dismutase (SOD) in aortas were measured.
Materials and Methods
Animals
Male Sprague-Dawley rats (4–6 months old and weighing 240-270 g) were obtained from the Experimental Animal Center of Zhejiang Academy of Medical Sciences. All procedures were performed according to the protocols approved by the Institutional Committee for Use and Care of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The experiments were approved by the Ethics Committee for the Use of Experimental Animals in Jiaxing University.
Drugs and Chemicals
BA was obtained from Shanghai Tauto Biotech Co., Ltd. (Shanghai, China), and the purity was 98% by high performance liquid chromatography (HPLC). ACh, phenylephrine (PE), Nw -nitro-l-arginine methyl ester hydrochloride (L-NAME), methylene blue (MB), and indomethacin (Indo) were from Sigma-Aldrich Inc. (Saint Louis, MO, USA). 3-amino,4- aminomethyl-2’,7’-difluorescein, diacetate (DAF-FM DA) and 2’,7’-dichlorfluorescin- diacetate (DCFH-DA) were from Molecular Probes (Eugene, OR, USA). The kits for measurement of NOS and SOD were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other reagents were of analytical purity.
Preparation of Rat Thoracic Aortic Rings and Bioassay of Vasoreactivity
Bioassay of vasoreactivity in the organ bath system was based on the methods of Qian et al. with modifications. [15] Briefly, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and killed by cervical dislocation. The descending thoracic aorta was rapidly dissected out, cut approximately 4 mm in length, and mounted in a 10 ml organ bath containing Krebs’ solution at 37°C bubbled with 95% O2 + 5% CO2 (pH 7.4), composed of (mM): NaCl 118; KCl 4.7; KH2 PO4 1.2; MgSO4 1.2; NaHCO3 25; glucose 10; and CaCl2 2.5. Before each experiment, aortic rings were stimulated three times with 60 mM KCl until a reproducible contractile response was obtained. The presence of functional endothelium was verified by the ability of ACh (10 μM) to induce more than 80% relaxation of aortic rings previously contracted by PE (1 μM).
Aortic Endothelial Impairment in Pyrogallol-Produced O2 –
As in the previous studies, [16,17] an arterial injury by O2- was accomplished through the auto-oxidation of pyrogallol added to the organ baths. After 15 min incubation in pyrogallol (500 μM), the cumulative concentration-response curves to ACh (10-9–l0-5M) were generated in rings previously contracted by PE (1 μM) in fresh Krebs’ solution.
Assay of NOS and SOD Activity
As in the previous studies, [18] aortic SOD activity was assayed by the xanthine–xanthine oxidase method, and total NOS (tNOS) (constitutive NOS [cNOS] + inducible NOS [iNOS]) and iNOS activity were assayed following the kit manual. The tNOS activity minus the iNOS activity gave the cNOS activity (in rat aortas the main cNOS is eNOS).
Measurement of ROS Release and NO Release
Determination of NO and ROS was based on the methods of previous studies with modifications. [19] After the supernatant was obtained from the aortic homogenate, it was pipetted into 96-well black plates (49.5 μl/well) loaded with 10 μM DCFH-DA or 10 μM DAF-FM DA for 30 min at room temperature in the dark. Levels of DCF in the supernatant were measured by microplate reader using 488 nm excitation and 530 nm emission. Levels of DAF-FM in the supernatant were measured by microplate reader using 495 nm excitation and 515 nm emission. Background fluorescence was corrected by the inclusion of parallel blanks. ROS or NO production was expressed as percentage compared with the control group.
Protocols of Experiments
The first series of experiments were designed to evaluate the effect of BA on EDR. PE (1 μM) was used to induce steady contraction in endothelium-intact aortic rings, then BA (0.1–100 μM) was added cumulatively. The half-maximum effective concentration (EC50), which was defined as the concentration of BA that induced 50% of maximum relaxation from the contraction elicited by PE, was calculated.
The second series of experiments were designed to evaluate protective effects of BA against the ACh-induced EDR of aortic rings exposed to pyrogallol-produced O2-. Experiments were carried out in six groups with eight endothelium-intact aortic rings from eight rats in each group: (1) Control group (Con), endothelium-intact rings incubated in Krebs’ solution; (2) BA alone (BA), endothelium-intact rings preincubated with BA at the EC50 for 30 min; (3) Pyrogallol, rings preincubated with 500 μM pyrogallol for 15 min; (4) BA+pyrogallol, endothelium-intact rings incubated with BA at the EC50 for 30 min, and 500 μM pyrogallol for the last 15 min; (5) L-NAME+BA+pyrogallol, rings preincubated with 100 μM L-NAME for 30 min before incubating with BA at the EC50 for another 30 min, and 500 μM pyrogallol for the last 15 min; (6) Indo+BA+pyrogallol, rings preincubated with 10 μM Indo for 30 min before incubating with BA at the EC50 for another 30 min, and 500 μM pyrogallol for the last 15 min; (7) MB+BA+pyrogallol, rings preincubated with 10 μM MB before incubating with BA at the EC50 for another 30 min, and 500 μM pyrogallol for the last 15 min. L-NAME, Indo, and MB were washed prior to the incubation of BA. After completion of these treatments, the rings were incubated in normal Krebs’ solution and ACh-induced EDR was performed.
Statistical Analysis
All data are expressed as mean ± SD. The BA/ACh-induced maximal relaxation (Emax) in aortic rings was calculated as a percentage of the contraction elicited by PE (1 μM). The half-maximum effective concentration (EC50) was defined as the concentration of BA or ACh that induced 50% of maximum vasorelaxation of the contraction elicited by PE, which was calculated from the concentration-response curve by nonlinear regression (curve fit) using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA), and pD2 is the negative logarithm of the EC50. Statistical comparisons were made using the one-way analysis of variance (ANOVA) followed by the Newman–Keuls test. A value of P < 0.05 was considered statistically significant.
Results
Effect of BA on Relaxation in Aortas Previously Contracted by PE
In the endothelium-intact aortic rings previously contracted by PE, BA (0.1–100 μM) evoked a concentration-dependent EDR [Figure 1], the Emax reached 79.06 ± 12.18%, and the pD2 was 5.80 ± 0.10, EC50 was 1.58 μM. We chose the approximate value of EC50 (2.0 μM) as the experimental concentration of BA for subsequent experiments.
Figure 1.
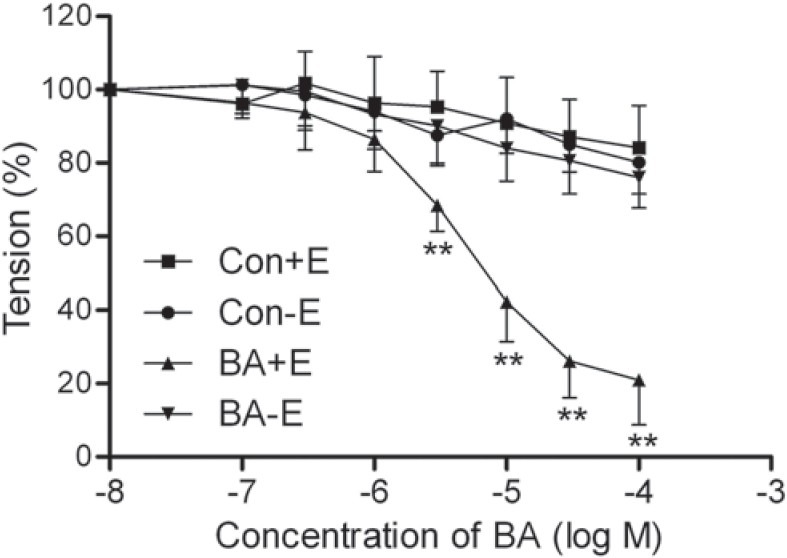
Effect of betulinic acid (BA, 0.1–100 μM) on vasorelaxation of endothelium-intact (+E) and -denuded (-E) aortic rings p reviously contracted by 1 μM phenylephrine (PE). Tension was measured and calculated as a percentage of the contraction elicited by PE. All data are expressed as mean ± SD; n = 7 rings from the seven rats per group. **P < 0.01 vs. Con
In the endothelium-denuded aortic rings previously contracted by PE, BA (0.1–100 μM) did not evoke obvious vasorelaxation, the Emax reached 13.99 ± 8.29% [P > 0.05 vs. the endothelium-denuded control group, Figure 1].
Effect of BA on ACh-Induced EDR in Aortas Preincubated with Pyrogallol and Previously Contracted by PE
The ACh-induced EDR in the aortic rings exposed to pyrogallol was significantly impaired, and the Emax decreased to 23.91 ± 5.41% [P < 0.01 vs. Con, Figure 2a]. However, pretreatment with BA attenuated the dysfunction of relaxation induced by exposure to pyrogallol, and the Emax increased to 42.45 ± 9.99% [P < 0.01 vs. pyrogallol, Figure 2a], which was markedly reversed by both L-NAME, the inhibitor of NOS, and MB, a guanylyl cyclase inhibitor, but not by pretreatment with Indo, a cyclooxygenase inhibitor Figure 2b]. Without exposure to pyrogallol, preincubation with BA also enhanced the ACh-induced relaxation, the Emax increased to 74.61 ± 4.75% in BA group [P < 0.05 vs. Con, Figure 2a].
Figure 2.
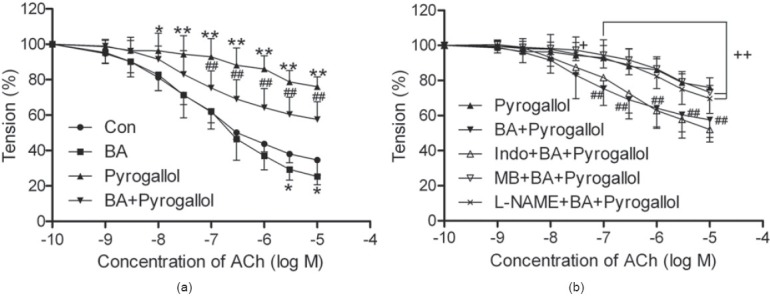
Effect of betulinic acid (BA, 2.0 μM) on acetylcholine (ACh)-induced vasorelaxation in endothelium-intact rat aortic rings previously contracted by 1 μM phenylephrine (PE) after exposure to pyro gallol (500 μM) for 15 min. Tension was measured and calculated as a percentage of the contraction in response to PE. Con: rings without any pretreatments; BA: rings preincubated with BA for 30 min; Pyrogallol: rings preincubated with pyrogallol for 15 min; BA+pyrogallol: rings incubated with BA for 30 min, and pyrogallol for the last 15 min; L-NAME/Indo/MB+BA+pyrogallol: rings preincubated with L-NAME (100 μM), indomethacin (Indo, 10 μM), or methylene blue (MB, 10 μM) for 30 min and then washed before incubating with BA for another 30 min, and pyrogallol for the last 15 min. All data are expressed as mean ± SD; n = 8 rings from the eight rats per group. *P < 0.05, **P < 0.01 vs. Con; ##P < 0.01 vs. pyrogallol; +P < 0.05, ++P < 0.01 vs. BA+pyrogallol
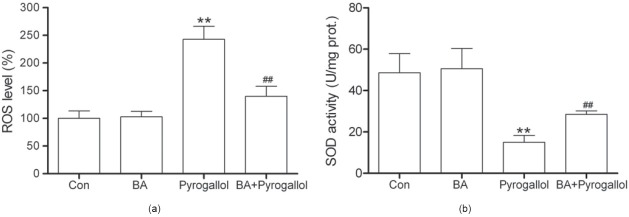
Effect of BA on ROS Level and SOD Activity in Aortas Preincubated with Pyrogallol
As shown in Figure 3a, we found that ROS level was markedly increased to 242.99% in aortas exposed to pyrogallol (P < 0.01 vs. Con), which was attenuated by treatment with BA (P < 0.01 vs. pyrogallol). The decrease of aortic SOD activity induced by pyrogallol was significantly reversed by BA [P < 0.01 vs. pyrogallol, Figure 3b]. Treatment with BA alone had no significant effect on ROS level and SOD activity.
Figure 3.
Effect of betulinic acid (BA, 2.0 μM) on ROS level (a) and activity of SOD (b) in endothelium-intact rat aortas exposed to pyrogallol (500 μM) for 15 min. All data are expressed as mean ± SD; n = 8 aortas fro m the eight rats per group. **P < 0.01 vs. Con; ##P < 0.01 vs. pyrogallol
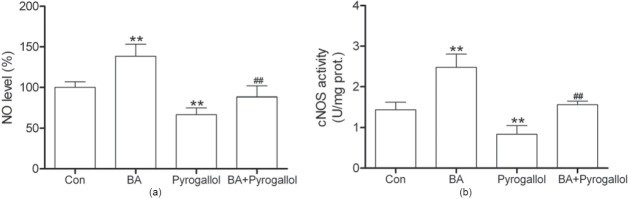
Effect of BA on NO Level and NOS Activity in Aortas Preincubated with Pyrogallol
The decrease of NO level in aortas exposed to pyrogallol was markedly inhibited by BA [P < 0.01 vs. pyrogallol, Figure 4a]. As shown in Figure 4b, BA also inhibited the decrease of cNOS activity in aortas induced by pyrogallol (P < 0.01 vs. pyrogallol). Treatment with BA alone markedly increased NO level and cNOS activity in normal rat aortas.
Figure 4.
Effect of betulinic acid (BA, 2.0 μM) on NO level (a) and activity of constitutive nitric oxide synthase (cNOS) (b) in aortas exposed to pyrogallol (500 μM) for 15 min. All data are expressed as mean ± SD; n = 8 aortas from the eight rats per group. **P < 0.01 vs. Con; ##P < 0.01 vs. pyrogallol
Discussion
Our current work shows that BA(0.1–100 μM) evoked a concentration-dependent EDR in aortic rings, and demonstrates for the first time that BA improved EDR in aortas exposed to pyrogallol. Moreover, exposure to pyrogallol exacerbated ROS production, weakened NO level and the activities of SOD and cNOS in aorta, all of which were reversed by pretreatment with BA.
NO and prostacyclin (PGI2) are two relaxing factors to modulate EDR in the rat thoracic aorta. [20] NO is mainly formed in the endothelium by the activation of eNOS and diffuses out of the endothelial cell to vascular smooth muscle cells where it activates soluble guanylyl cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP). The rise of cGMP initiates the relaxation of the vascular smooth muscle. [21] Here, we found that the improvement of EDR exerted by BA in aortas subjected to pyrogallol derived O2- was reversed by the NOS inhibitor L-NAME and sGC inhibitor MB, but not by Indo, the inhibitor of cyclooxygenase (the rate-limiting enzyme in PGI2 synthesis), which indicates that the activation of eNOS–NO–cGMP pathway has a close relation to the protective effect of BA on ACh-induced EDR against oxidative stress.
Considerable evidences indicate that increased vascular oxidative stress plays an important role in endothelial dysfunction. [22] Pyrogallol rapidly auto-oxidizes in oxygen-containing aqueous medium to generate O2-[23] and has been used to induce vascular damage from ROS in vitro. In our study, preincubation with pyrogallol (500 μM) for 15 min markedly decreased EDR accompanying the increase of ROS production and the decrease of SOD activity in the isolated rat thoracic aortas, which is consistent with the previous studies and indicates that O2- from the auto-oxidization of pyrogallol elevated enough oxidative stress to damage the endothelial function by disrupting the balance of ROS production and elimination. Excessive ROS alters the selective permeability of plasma membrane, represses the activity of cellular enzymes, and induces cell death through oxidative modifications of proteins, lipids, and DNA, [24] all of which contribute to the weak EDR and hypertension induced by oxidative stress. In addition, the obviously decreased NO level and eNOS (the main cNOS in the rat aorta) activity in pyrogallol treated aortas may be partly responsible for the impairment of EDR in oxidative stress. Pretreatment with BA improved EDR in aortas exposure to pyrogallol derived O2-, which was abolished both by L-NAME and MB. We also found that BA reduced ROS production, reversed the decrease of NO level and eNOS activity induced by O2-. All these facts strengthen the above hypothesis and indicate that the antioxidative activity of BA helps to retain the bioavailability of endothelium derived NO and protects the aortic EDR against oxidative stress.
There are two main events involved in ROS quenching NO as the following. One is that O2- rapidly reacts with NO to produce the strong oxidant ONOO-, which directly aggravates endothelial dysfunction. The other is that ONOO- causes uncoupling of NOS by oxidizing the essential eNOS cofactor tetrahydrobiopterin, to produce ROS instead of NO. [25] Therefore, oxidative stress is exacerbated and NO-related EDR is impaired. Activating the endogenous antioxidant enzymes might be effective in treating oxidative stress-induced endothelial dysfunction, which has been demonstrated by the previous study that SOD gene transfer improves intrahepatic endothelial function and reduces portal pressure,[26] and again by our current results that BA improved the SOD activity, reduced ROS level, as well as ameliorated EDR in aorta subjected to exogenous O2-. Likewise, BA itself induced EDR concentration-dependently and enhanced eNOS-NO in normal aortas, which may also benefit EDR against oxidative stress.
In conclusion, we have shown that BA protected EDR in rat aortas against exogenous O2-, which may closely relate to the reduction of oxidative stress and activation of eNOS-NO pathway. Such endothelial protection conferred by BA may be pivotal to the beneficial effect of ZSS, in which the key active constituent is BA, in the cardiovascular system.
Footnotes
Source of Support: The grant from the Department of Science and Technology of Zhejiang Province, China (2012C33088)
Conflict of Interest: No.
References
- 1.Feletou M, Kohler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: Possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010;12:267–75. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 3.Feletou M, Vanhoutte PM. Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 4.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34:665–73. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 5.Bauer V, Sotnikova R. Nitric oxide--the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys. 2010;29:319–40. [PubMed] [Google Scholar]
- 6.Gkaliagkousi E, Douma S, Zamboulis C, Ferro A. Nitric oxide dysfunction in vascular endothelium and platelets: Role in essential hypertension. J Hypertens. 2009;27:2310–20. doi: 10.1097/HJH.0b013e328330e89a. [DOI] [PubMed] [Google Scholar]
- 7.Nakmareong S, Kukongviriyapan U, Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B, et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:519–29. doi: 10.1007/s00210-011-0624-z. [DOI] [PubMed] [Google Scholar]
- 8.Ferroni P, Basili S, Paoletti V, Davi G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis. 2006;16:222–33. doi: 10.1016/j.numecd.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–23. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–92. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 11.Jiang JG, Huang XJ, Chen J, Lin QS. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat Prod Res. 2007;21:310–20. doi: 10.1080/14786410701192827. [DOI] [PubMed] [Google Scholar]
- 12.Cheng D, Zhu C, Cao J, Jiang W. The protective effects of polyphenols from jujube peel (Ziziphus Jujube Mill) on isoproterenol-induced myocardial ischemia and aluminum-induced oxidative damage in rats. Food Chem Toxicol. 2012;50:1302–8. doi: 10.1016/j.fct.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Steinkamp-Fenske K, Bollinger L, Xu H, Yao Y, Horke S, Forstermann U, et al. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J Pharmacol Exp Ther. 2007;322:836–42. doi: 10.1124/jpet.107.123356. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Xia N, Xu H, Guo L, Wenzel P, Daiber A, et al. Betulinic acid protects against cerebral ischemia-reperfusion injury in mice by reducing oxidative and nitrosative stress. Nitric Oxide. 2011;24:132–8. doi: 10.1016/j.niox.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Qian LB, Wang HP, Chen Y, Chen FX, Ma YY, Bruce IC, et al. Luteolin reduces high glucose-mediated impairment of endothelium-dependent relaxation in rat aorta by reducing oxidative stress. Pharmacol Res. 2010;61:281–7. doi: 10.1016/j.phrs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Jin BH, Qian LB, Chen S, Li J, Wang HP, Bruce IC, et al. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur J Pharmacol. 2009;616:200–5. doi: 10.1016/j.ejphar.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Li YF, Gao Q, Ye ZG, Lu XJ, Wang HP, et al. Inhibition of superoxide anion-mediated impairment of endothelium by treatment with luteolin and apigenin in rat mesenteric artery. Life Sci. 2008;83:110–7. doi: 10.1016/j.lfs.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Iliodromitis EK, Andreadou I, Markantonis-Kyroudis S, Mademli K, Kyrzopoulos S, Georgiadou P, et al. The effects of tirofiban on peripheral markers of oxidative stress and endothelial dysfunction in patients with acute coronary syndromes. Thromb Res. 2007;119:167–74. doi: 10.1016/j.thromres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Gallo MP, Levi R, Ramella R, Brero A, Boero O, Tota B, et al. Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am J Physiol Heart Circ Physiol. 2007;292:2906–12. doi: 10.1152/ajpheart.01253.2006. [DOI] [PubMed] [Google Scholar]
- 20.Bryan RM, Jr, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: A cousin to nitric oxide and prostacyclin. Anesthesiology. 2005;102:1261–77. doi: 10.1097/00000542-200506000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Kim HY, Oh H, Li X, Cho KW, Kang DG, Lee HS. Ethanol extract of seeds of Oenothera odorata induces vasorelaxation via endothelium-dependent NO-cGMP signaling through activation of Akt-eNOS-sGC pathway. J Ethnopharmacol. 2011;133:315–23. doi: 10.1016/j.jep.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Tousoulis D, Briasoulis A, Papageorgiou N, Tsioufis C, Tsiamis E, Toutouzas K, et al. Oxidative stress and endothelial function: Therapeutic interventions. Recent Pat Cardiovasc Drug Discov. 2011;6:103–14. doi: 10.2174/157489011795933819. [DOI] [PubMed] [Google Scholar]
- 23.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 24.Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight. J Mol Cell Cardiol. 2009;47:372–81. doi: 10.1016/j.yjmcc.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 26.Miao L, St Clair DK. Regulation of superoxide dismutase genes: Implications in disease. Free Radic Biol Med. 2009;47:344–56. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]