Abstract
Aim:
Scopolamine is known to produce amnesia due to blockade of the cholinergic neurotransmission. The present study investigated the potential of Convolvulus pluricaulis (CP) to attenuate scopolamine (2 mg/kg, i.p) induced increased protein and mRNA levels of tau, amyloid precursor protein (AβPP), amyloid β (Aβ) levels and histopathological changes in rat cerebral cortex.
Materials and Methods:
The study was conducted on male Wistar rats (250 ± 20 g) divided into four groups of eight animals each. Groups 1 and 2 served as controls receiving normal saline and scopolamine for 4 weeks, respectively. Group 3 received rivastigmine (standard) and group 4 received aqueous extract of CP simultaneously with scopolamine. Western blot and RT-PCR analysis were used to evaluate the levels of protein and mRNA of amyloid precursor protein (AβPP) and tau in rat cortex and ELISA was used to measure the amyloid β (Aβ) levels. Histopathology was also performed on cortical section of all groups.
Result:
Oral administration of CP extract (150 mg/kg) to scopolamine treated rats reduced the increased protein and mRNA levels of tau and AβPP levels followed by reduction in Aβ levels compared with scopolamine treated group. The potential of extract to prevent scopolamine neurotoxicity was reflected at the microscopic level as well, indicative of its neuroprotective effects.
Conclusion:
CP treatment alleviated neurotoxic effect of scopolamine reflects its potential as potent neuroprotective agent.
KEY WORDS: Alzheimer's disease, amyloid beta, amyloid precursor protein, scopolamine, tau
Introduction
Research on Alzheimer's disease (AD) has established that genesis of β-amyloid (Aβ) derived from amyloidogenic processing of β-amyloid precursor protein (AβPP) is the key event in AD pathophysiology.[1] The main targets of Aβ include cholesterol dyshomeostasis,[2] severe degeneration of the cholinergic neurons, projecting from the basal forebrain to cortical and hippocampal areas,[3] which subsequently leads to imbalance of signaling molecules vital for learning and memory.[4] These events have been found to cause accumulation of neurofibrillary tangles (NFTs), which consist of hyperphosphorylated tau. The NFTs are known to aggregate in paired helical filaments (PHF),[5] thus confirming amyloid cascade hypothesis of tau phosphorylation.
So far, the most effective pharmacological strategies in AD have aimed at reducing amyloid by immunization strategies, restoring cholesterol equilibrium with the use of cholesterol-lowering drugs (statins), revitalizing cholinergic deficits by employing cholinergic enhancers (tacrine, donepezil, and rivastigmine), and by regulating neuroinflammation with the use of nonsteroidal antiinflammatory drugs [NSAIDs (aspirin, ibuprofen, and indomethacin), COX-2 inhibitors (celecoxib, rofecoxib)]. However, the benefits are generally modest due to associated side effects.[6]
Recent studies have shown that natural compounds have an advantage over conventional drugs as being constituents of living system; they may be less toxic and hence more acceptable for human application.[7] Ayurveda, an Indian system of medicine, contains herbal remedies for a variety of diseases. One such alternative is Convolvulus pluricaulis (CP) (English name: Bindweed, Hindi: Shankhpushpi) belongs to the family Convolvulaceae. The plant contains carbohydrates[8,9] (D-glucose, maltose, rhamnose, sucrose, and starch), proteins, amino acids, and the alkaloid shankhpushpine (C17H25NO2), having a melting point of 162-164°C. The most notable constituents are tropane alkaloids. Studies have found that ethanolic extract of CP attenuate increased levels of malondialdehyde (MDA) and protein carbonyl. It has also shown beneficial effects on declined glutathione peroxidase (GPx) and reduced glutathione (GSH) levels in hippocampus.[10] The extract has been described as an anthelmintic, a remedy for bowel complaints, brain and hair tonic, cures skin ailments, and reduces high blood pressure. The ethanolic extract of CP has also demonstrated nootropic activity in rats.[11] Previous studies have shown that oral administration of CP at a daily dose of 150 mg/kg for 1 week attenuates scopolamine induced memory impairment in Wistar rats. A decrease in the elevated acetylcholine esterase activity, lowering of lipid peroxidation, protein carbonyl levels as well as restoration of the altered levels of antioxidants associated with scopolamine administration was observed.[12] The present study was undertaken to determine effects of aqueous extract of CP on scopolamine induced increase in protein and mRNA levels of AD related biomarkers namely AβPP and tau. Histopathological studies were also undertaken to determine the morphological alteration in the cerebral cortex.
Materials and Methods
Plant Material
Roots of CP were obtained from herbal market in New Delhi. The plant material was identified and authenticated from National Institute of Sciences Communication and Information Resources (Ref: NISCAIR/RHMD/Consult 06/77/4191). The extract was prepared as described previously[11] and contained 50–60% alkaloids estimated as total alkaloids. The estimation method involves total alkaloids precipitated by Dragendorff's reagent in plant materials. It is based on the formation of yellow bismuth complex in nitric acid medium with thiourea, which obeys Lambert–Beer's law in the concentration range of 0.06–50 μg/ml with λmax at 435 nm.[13] The extract was suspended in distilled water to the desired concentration just before use and administered orally.
Animals
Naive male Wistar rats (8 month old), weighing 200 g ± 20 were used in this study. Animals were obtained from the animal house of central animal facility of the Dr B. R Ambedkar Center for Biomedical Research, New Delhi. The experimental animals were randomly assigned to four groups of eight rats each. Group 1 served as control and received saline only. Group 2 received scopolamine (2 mg/ kg, i.p) dissolved in saline daily. Group 3 received rivastigmine tartrate at an oral dose of 1 mg/kg, followed by scopolamine (2 mg/kg, i.p) after 40 min and group 4 was given aqueous extract of CP at an oral dose of 150 mg/kg, followed by scopolamine (2 mg/kg, i.p) after 40 min. The above mentioned treatment was continued for 4 weeks, after which the animals were sacrificed by decapitation. The brain was dissected out on ice and the cerebral cortex was micro-dissected and was stored at -80°C till further use. The animal study was performed in accordance with the guidelines provided by the Institutional Animal Ethics Committee of Dr B. R. Ambedkar Center for Biomedical Research.
Western Blot Analysis
Cortical brain tissues were homogenized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1 SDS, 1 mM EDTA, and 0.1% protease inhibitor cocktail). The homogenates were centrifuged at 10,000 × g for 10 min at 4°C and the supernatants were collected and used for western blot. Protein concentration was determined by bicinchoninic acid assay (BCA) kit (Pierce Biotechnology). A total of 40 μg of total protein from each sample was applied for western blot. Protein samples were denatured and resolved in 8% SDS-PAGE then transferred to PVDF membrane. The membranes were blocked with 5% nonfat milk in Tris buffered saline (TBS, pH 7.4) for 1 h, then incubated overnight at 4°C with Anti-AβPP A4 Antibody, a.a. 66-81 of APP (N-terminus), clone 22C11, mouse monoclonal (Millipore) at a dilution 1:1000 and mouse monoclonal anti tau (Tau- 46, Sigma-Aldrich) at a dilution of 1:1000. On the following day, membranes were washed and exposed to horse radish peroxidase (HRP) conjugated antimouse antibody (diluted at 1:5000, Pierce Biotechnology) for 1 h. The intensities of bands obtained from western blot were estimated with Alpha Imager™ 2200 (Alpha Innotech Corporation, USA). As a control for equal protein loading, membranes were stripped and reprobed with rabbit β-actin antibody (diluted at 1:2500, Sigma-Aldrich) and exposed to HRP conjugated anti-rabbit antibody (diluted at 1:5000, Pierce Biotechnology).
Reverse Transcriptase Polymerase Chain Reaction
Total RNA was isolated from cerebral cortex (100 mg) using Trizol reagent.[14] Single strand cDNA was synthesized from 2 μg of total RNA, using Moloney murine leukemia viruses (M-MuLV) reverse transcriptase. The total RNA from control and exposed cells was extracted with the TRIzol reagent (Invitrogen). First-strand cDNA was synthesized from 1.5 μg of total RNA using the iScript cDNA kit (Bio-Rad) and then amplified using real-time polymerase chain reaction (PCR). The cDNA for Tau, AβPP, and glyceraldehyde 3 phosphate dehydrogenase (GAPDH) were PCR amplified using the following specific primers: AβPP: forward primer 5’- TGA TCT ACG AGC GCA TGA AC-3’, reverse primer 5’- AGA AGG CAT GAG AGC ATC GT -3’; Tau: forward primer 5’- ACG ATT TCT GCT CCA TGG TC -3’, reverse primer 5’- AAG GTG ACC TCC AAG TGT GG - 3’; GAPDH: forward primer 5’- TTC ACC ACC ATG GAG AAG GC- 3’, reverse primer 5’- GGC ATG GAC TGT GGT CAT GA -3’. Amplified products were resolved by 1% agarose gel electrophoresis, stained with ethidium bromide.
ELISA Aβ (1–40) and Aβ (1–42) Assay
The levels of Aβ were measured using human Aβ (1–40) and Aβ (1–42) assay kits. These kits are solid-phase sandwich ELISA with two kinds of highly specific antibodies, which are 100% reactive with rodent Aβ1-40, and 70% reactive with rodent Aβ1-42. The assay was conducted following the manufacturer's instructions (Immuno-Biological Laboratories, Gunma, Japan) with minor modifications.[15] Around 100 μg of protein samples in 100 μl of EIA buffer and assay standards were added to a 96-well plates precoated with antihuman Aβ mouse IgG mAb. The plates were incubated overnight at 4°C, and were washed seven times using the 40× diluted wash buffer supplied with the kit (0.05% Tween 20 in phosphate buffer). Around 100 μl of labeled antibody was then added to the samples and standards followed by incubation at 4°C for 1 h. After incubation the plates were washed nine times followed by addition of 100 μl of tetramethylbenzidine as a coloring agent. The plates were incubated in the dark for 30 min at room temperature. Finally 100 μl of 1N H2SO4 was added to stop the reaction, and absorbance was measured at 450 nm using Spectra Max UV/Vis Spectrometer (GMI, Inc., Ramsey, MN, USA). The concentration of Aβ in unknown samples was obtained as pg/ml after plotting the absorbance of standards against the standard concentrations.
Histopathology
The histopathological studies were performed in the cerebral cortex of rats as previously described by Nakayama et al.[16] The brain of control and experimental groups were perfusion-fixed, with a mixture of formaldehyde (40%), glacial acetic acid, and methanol (1:1:8, by volume). The tissue was cut 3 mm thick, and its blocks were embedded in paraffin. Sections of 4–5 μm thickness were cut in the coronal plane and stained with hematoxylin and eosin.
Densitometric and statistical analysis
The intensities of bands obtained from western blot and RT-PCR were estimated with Alpha Imager TM 2200 (Alpha Innotech Corporation, USA). All the measurements were made in triplicate and all values are represented as mean ± SEM. The significance of difference between means of two groups was obtained with one-way analysis of variance (ANOVA), Tukey–Kramer multiple comparisons posttest, and student Newman–Keuls comparison posttest, using Graph pad Prism 3.0 computer software. P < 0.05 was considered statistically significant.
Results
Effect of CP Extract on Protein Expression of AβPP and Tau
Microsomal fraction prepared from the cerebral cortex of each experimental group was subjected to immunoblotting with an antibody specific for AβPP and Tau protein. Figures 1a and 2a shows a significant increase in the protein expression of AβPP (P < 0.01) and tau (P < 0.01) in group B, which was visualized by an increase in the immunoreactivity as compared with the control (group A). In animals receiving CP (150 mg/ kg) and scopolamine (group D) registered a significant decrease in the protein expression of AβPP and tau as compared with scopolamine treated group (group B). Group C (rivastigmine tartrate + scopolamine) also significantly (P < 0.05) reduced the protein expression of tau and AβPP as compared with group B.
Figure 1.
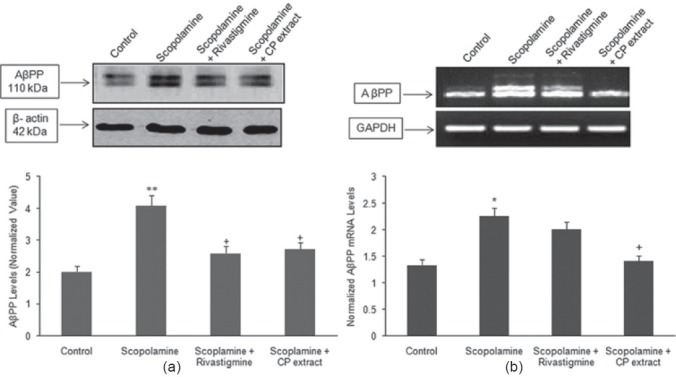
(a, b) Western blotting was performed to examine the effect of CP extract and rivastigmine tartrate on the increased protein and mRNA levels of AβPP induced by administration of scopolamine (2 mg/kg). The western blot and mRNA levels are representative of three independent experiments. The protein expression of AβPP was normalized against β-actin, while the transcriptive levels of AβPP were normalized against GAPDH. The data was expressed as mean ± S.E.M. **P < 0.01 as compared with control group, +P < 0.05 as compared with scopolamine treated group
Figure 2.
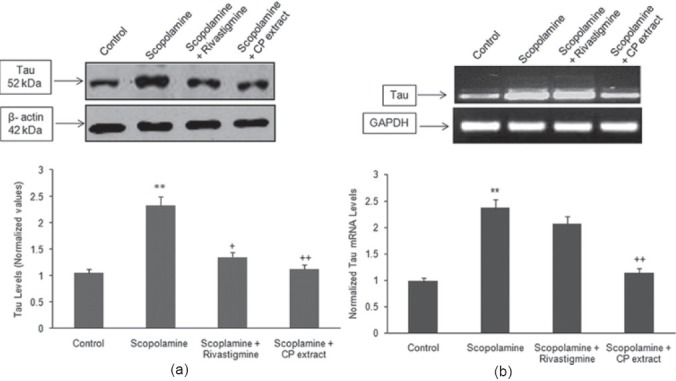
(a, b) Western blotting was performed to examine the effect of CP extract and rivastigmine tartrate on the increased protein and mRNA levels of Tau elevated by administration of scopolamine (2 mg/kg). The western blot and mRNA levels are representative of three independent experiments. The protein expression of Tau was normalized against β-actin, while the transcriptive levels of Tau were normalized against GAPDH. The data was expressed as mean ± S.E.M. **P < 0.01 as compared with control group, +P < 0.05, ++P < 0.01 as compared with scopolamine treated group
Effect of Convolvulus Pluricaulis on mRNA Levels of APP and Tau
Reverse transcriptase-PCR was performed to assess the effect of aqueous extract of CP (150 mg/kg) on the mRNA levels of APP and Tau. Figures 1b and 2b shows that daily intraperitoneal administration of scopolamine for 4 weeks upregulated the mRNA levels of APP and tau as compared with the control group. In animals administered CP extract (group D) registered a significant decreased in the mRNA levels as compared with the scopolamine treated group while no significant change was seen with group C.
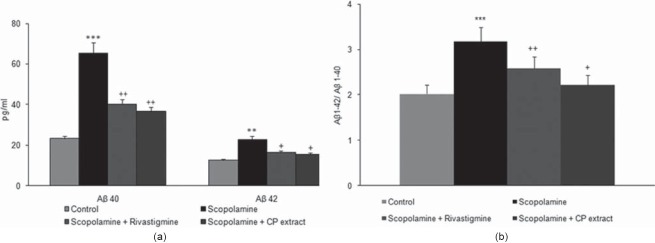
Effect of Convolvulus Pluricaulis on Cerebral Aβ Levels
Scopolamine administration was found to significantly increase the cerebral cortex Aβ load as compared with the control. Oral supplementation of CP (150 mg/kg) during scopolamine treatment was found to decrease the elevated levels of Aβ 1–40 and Aβ 1–42 compared with scopolamine treated groups. Oral treatment with rivastigmine was also found to reduce the levels of Aβ 1–40 and Aβ 1–42 compared with scopolamine treated group [Figure 3].
Figure 3.
Cortical Aβ1-40 and Aβ1-42 levels were measured by an ELISA assay according to the procedures illustrated in the methods section. (a) Displays the effect of various treatments groups [Control, Scopolamine treated (2 mg/kg), Scopolamine (2 mg/kg) + Rivastigmine (1 mg/kg), Scopolamine (2 mg/kg) + CP extract (150 mg/kg)] on the levels of Aβ1–40 and Aβ1–42. (b) The ratio of Aβ1–42/Aβ1– 40 following treatment for 4 weeks. Values shown are the mean ± S.E.M. ***P< 0.001 as compared with control group, +P < 0.05, ++P < 0.01 as compared with scopolamine treated group
Effect of Treatment on Histopathological Changes in Cerebral Cortex
The histology of the cerebral cortex was examined under light microscope [Figure 4a–d]. The results show that the daily intraperitoneal administration of scopolamine at a dose regimen of 2 mg/kg for 4 weeks induced alteration at microscopic levels which was manifested by increase in neuronal loss, ghost cells hemorrhage, and vacuolated cytoplasm. Significant reduction in the histopathological alteration in the cerebral cortex was observed in animals, which received oral administration of C.P (150 mg/kg) along with scopolamine, while rivastigmine showed modest beneficial effect.
Figure 4.
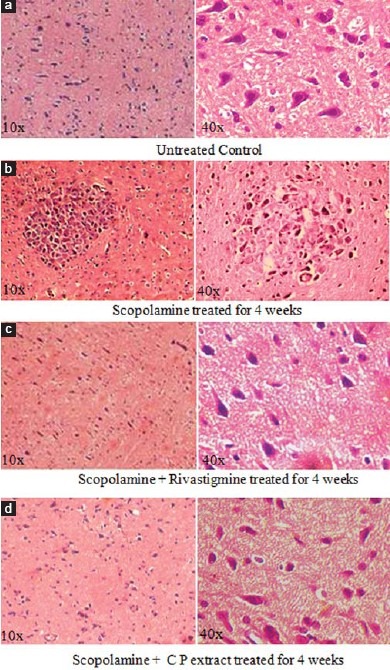
Photographs (a-d) showing histopathological changes in cerebral cortex in different groups. (a) Control group (distilled water), (b) Scopolamine treated (2 mg/kg) treated group, (c) Scopolamine (2 mg/ kg) + rivastigmine tartrate (1 mg/kg), treated group, (d) Scopolamine (2 mg/ kg) + CP extract (150 mg/kg) [H and E 10× and 40×]
Discussion
In the present study we aimed to investigate the effect of aqueous extract of CP on the protein expression of tau, processing of AβPP, and Aβ accumulation in vivo. As the composition of CP may be different depending on geographical locations and plant sources, the main chemical components present in the CP extract were identified. Natural compounds such as alkaloids and flavonoids were seen of which convolvine and convolamine were primary.[17] Studies have found a beneficial effect of these alkaloids on learning and memory via stimulation of cholinergic neuronal transmission.[17] Likewise flavonoids have been shown to possess various biological and pharmacological activities, including antioxidative, antiinflammatory, antitumor, and antiviral effects.[18] Recently, growing evidence suggests that flavonoids have neuroprotective effects in many models of neurodegenerative diseases in-vitro and in-vivo.[19]
To induce a cortical cholinergic dysfunction, the activation of muscarinic acetylcholine receptors was inhibited by systemic intraperitoneal administration of scopolamine, a muscarinic receptor antagonist, capable of penetrating the blood-brain barrier and suppressing central cholinergic function by blockade of the two major forebrain cholinergic systems: septo-hippocampal and nucleus basalis—cerebral cortical pathways.[20] The rats received scopolamine at a daily dosage of 2 mg/kg body weight for 4 weeks, a dose which is known to produce amnestic effects and to impair learning and memory processes in rodents but did not generate any major side effects on the peripheral cholinergic system. The steady state levels of Aβ in the brain are determined by the balance between its synthesis and degradation. Studies have found colocalization of acetylcholinesterase (AChE) with Aβ deposits in brains of Alzheimer patients[21] as well as the capability of AChE to affect AβPP processing[22] and aggregation of Aβ peptides hence emphasizing the role of AChE in AβPP metabolism.[23] Consistent with earlier findings our results showed that daily treatment of Wistar rats with scopolamine for 4 weeks led to increased protein and mRNA levels of AβPP in the cerebral cortex of the rats. These changes were also associated with enhanced level of Aβ (1–40) and Aβ (1–42) as compared with control animals. CP extract administered attenuated the scopolamine induced increased mRNA and protein levels of AβPP, these changes were also visualized by a significant decrease in the elevated levels of Aβ. The protective action of CP against scopolamine induced elevated protein levels of AβPP and Aβ may be mediated either by direct interactions of constituents of the extract with the cholinergic nerve terminal or transsynaptically by mechanisms such as the modulation of AβPP secretion at one side of the synaptic cleft, which in turn can result in activation of neighboring cells and synaptic constituents. Rivastigmine administration was also found to lower the elevated protein levels of AβPP and Aβ.
Similar to Aβ toxicity, aggregation of tau into NFT is another hallmark of AD. Recent studies have provided evidence that downstream mechanism of Aβ-induced toxicity has been linked to phosphorylated tau and the disruption of microtubule network.[24] Basal forebrain pretangles and tangles have been observed prior to the pathology in the entorhinal/perirhinal cortex indicating that abnormalities in cortical cholinergic axons and tauopathy within the basal forebrain cholinergic system occur very early in the course of life, and increase in frequency in old age and AD.[25] Studies have revealed that Aβ triggered protease activity mediates tau fragmentation, producing potentially toxic tau species.[26,27] It is known, however, that Aβ induces increased activity of several tau-targeting kinases, including GSK3β and Cdk5.[28] Gene expression studies undertaken by Hsieh et al. have provided convincing evidence that scopolamine treatment can result in the increased expression AD related genes including tau.[29] Consistent with earlier findings, our results also revealed an increase in the tau protein and mRNA levels in rat cortex. This increase were significantly lowered by the oral administration of CP. Rivastigmine was also able to reduce the protein levels of Tau, however, had insignificant effect on mRNA levels which indicates that it does not have any effect on transcriptional levels.
Administration of scopolamine for 4 weeks led to marked histopathological alterations in the cerebral cortex, including neuronal degeneration as cytoplasmic vacuolization, haemorrhage, ghost cells, and gliosis. Recent studies from our laboratory have shown beneficial effect of CP extract (150mg/g) on the histopathological changes in rat cerebral cortex induced by aluminium exposure,[30] consistent with the earlier reports that the CP extract (150 mg/kg) was able to significantly reduce the scopolamine induced histopathological changes in the cerebral cortex of rats brain.
In conclusion the aqueous extract of CP was found to reduce the biomarkers of AD, that is, AβPP, tau, and Aβ. It also improved the histological changes seen with AD. Hence, its use in AD can be further studied. Additionally, in vivo activity of the active compounds needs to be determined in animal models and humans, so as to determine their environmental efficacy.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 2.Koudinov AR, Koudinova NV. Cholesterol homeostasis failure as a unifying cause of synaptic degeneration. J Neurol Sci. 2005;229-230:233–40. doi: 10.1016/j.jns.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: Relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–45. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 4.Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, et al. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory. J Neurosci. 2003;23:6304–14. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maccioni RB, Munoz JP, Barbeito L. The molecular bases of Alzheimer's disease and other neurodegenerative disorders. Arch Med Res. 2001;32:367–81. doi: 10.1016/s0188-4409(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan NB. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer's transgenic model Tg2576. J Ethnopharmacol. 2006;108:385–94. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock M. The pharmacotherapy of Alzheimer's disease based on the cholinergic hypothesis: An update. Neurodegeneration. 1995;4:349–56. doi: 10.1006/neur.1995.0042. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande SM, Srivastava DN. Chemical studies of Convolvulus pluricaulis. J Indian Chem Soc. 1969;46:759–60. [Google Scholar]
- 9.Deshpande SM, Srivastava DN. Chemical examination of the fatty acids of Convolvulus pluricaulis. Indian Oil Soap J. 1969;34:217–8. [Google Scholar]
- 10.Bardgett ME, Henry JD. Locomotor activity and accumbens fos expression driven by ventral hippocampal stimulation require D1 and D2 receptors. Neuroscience. 1999;94:59–70. doi: 10.1016/s0306-4522(99)00303-6. [DOI] [PubMed] [Google Scholar]
- 11.Nahata A, Patil UK, Dixit VK. Effect of Convulvulus pluricaulis choisy. on learning behaviour and memory enhancement activity in rodents. Nat Prod Res. 2008;22:1472–82. doi: 10.1080/14786410802214199. [DOI] [PubMed] [Google Scholar]
- 12.Bihaqi SW, Singh AP, Tiwari M. In vivo investigation of the neuroprotective property of Convolvulus pluricaulis in scopolamine-induced cognitive impairments in Wistar rats. Indian J Pharmacol. 2011;43:520–5. doi: 10.4103/0253-7613.84958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sreevidya N, Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. J AOAC Int. 2003;86:1124–7. [PubMed] [Google Scholar]
- 14.Dignam JD, Martin PL, Shastry BS, Roeder RG. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–98. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- 15.Morishima-Kawashima M, Oshima N, Ogata H, Yamaguchi H, Yoshimura M, Sugihara S, et al. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–9. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama H, Ginsberg MD, Dietrich WD. (S)-emopamil, a novel calcium channel blocker and serotonin S2 antagonist, markedly reduces infarct size following middle cerebral artery occlusion in the rat. Neurology. 1988;38:1667–73. doi: 10.1212/wnl.38.11.1667. [DOI] [PubMed] [Google Scholar]
- 17.Mirzaev YR, Aripova SF. Neuro and psychopharmacological investigation of the alkaloids convolvine and atropine. Chem Nat Compounds. 1998;34:56–8. [Google Scholar]
- 18.Ross JA, Kasum CM. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, et al. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003;17:1943–4. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- 20.Dunnett SB. Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology (Berl) 1985;87:357–63. doi: 10.1007/BF00432721. [DOI] [PubMed] [Google Scholar]
- 21.Moran MA, Mufson EJ, Gomez-Ramos P. Colocalization of cholinesterases with β-amyloid protein in aged and Alzheimer's brain. Acta Neuropathol. 1993;85:362–9. doi: 10.1007/BF00334445. [DOI] [PubMed] [Google Scholar]
- 22.Lahiri DK, Farlow MR, Nurnberger JJ, Greig NH. Effects of cholinesterase inhibitors on the secretion of beta-amyloid precursor protein in cell cultures. Ann N Y Acad Sci. 1997;826:416–21. doi: 10.1111/j.1749-6632.1997.tb48495.x. [DOI] [PubMed] [Google Scholar]
- 23.Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, Linker C, et al. Acetylcholinesterase accelerates assembly of amyloid-β peptides into Alzheimer's fibrils: Possible role of the peripheral site of the enzyme. Neuron. 1996;16:881–91. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 24.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–22. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 25.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–63. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 26.Park SY, Ferreira A. The generation of a 17 kDa neurotoxic fragment: An alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci. 2005;25:5365–75. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–7. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reifert J, Hartung-Cranston D, Feinstein SC. Amyloid β-mediated cell death of cultured hippocampal neurons reveals extensive Tau fragmentation without increased full-length tau phosphorylation. J Biol Chem. 2011;286:20797–811. doi: 10.1074/jbc.M111.234674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh MT, Hsieh CL, Lin LW, Wu CR, Huang GS. Differential gene expression of scopolamine-treated rat hippocampus-application of cDNA microarray technology. Life Sci. 2003;73:1007–16. doi: 10.1016/s0024-3205(03)00372-2. [DOI] [PubMed] [Google Scholar]
- 30.Bihaqi SW, Sharma M, Singh AP, Tiwari M. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J Ethnopharmacol. 2009;124:409–15. doi: 10.1016/j.jep.2009.05.038. [DOI] [PubMed] [Google Scholar]