Abstract
Objectives:
This study was aimed to assess the cardioprotective role of low molecular weight glycosaminoglycans (LMW-GAG) in isoproterenol (ISO)-induced myocardial infarction (MI) in male Wistar rats. Effect of LMW-GAG on biomolecules, lactate dehydrogenase (LDH)-isoenzyme, and electrocardiographic (ECG)-patterns was studied as evidence of cardioprotection.
Materials and Methods:
Male Wistar rats (140 ± 10 g) were divided into four groups; untreated control (group I), LMW-GAG treated (300 μg/day s. c. for 2 weeks—group II), ISO (85 mg s.c. injected on 13th and 14th days—group III), and LMW-GAG plus ISO (300 μg/day s. c. for 12 days followed by 85 mg/kg ISO on the end of 13th and 14th days—group IV). At the end of the experimental period, all animals were terminated.
Results:
Rats treated with LMW-GAG (300 μg/kg) for 12 days showed significant increasing levels of triglyceride (TG) (both serum and heart tissue), low density lipoprotein (LDL), very low density lipoprotein (VLDL), total cholesterol, uric acid, creatinine, and glucose. However, it significantly decreased the levels of high density lipoprotein (HDL) (serum), plasma total protein, and albumin/globulin (A/G) ratio. ISO also adversely affected the LDH-isoenzymes and caused marked elevation in ST segment. Pretreatment with LMW-GAG (300 μg/kg) daily for a period of 2 weeks prevented the ISO-treated changes.
Conclusions:
The results indicate that LMW-GAG exhibits a cardioprotective effect in ISO-induced MI in rats, by maintaining the biomolecules and LDH-isoenzymes.
KEY WORDS: A. pleuronectus, ECG, LDH-isoenzyme, LMW-GAG, myocardial infarction
Introduction
Ischemic heart disease and stroke are the two most common causes of death worldwide. Over 80% of deaths and 85% of disability from cardiovascular disease (CVD) occur in low- and middle-income countries. The Indian subcontinent (India, Pakistan, Bangladesh, Sri Lanka, and Nepal) is home to 20% of the world's population and may be one of the regions with the highest burden of CVD in world.[1] Risk factors are traits and life style that increase a person's chance of having a coronary artery disease. Some risk factors cannot be changed or controlled while others are controllable. The most important risk factors are high blood pressure, high blood cholesterol, diabetes, obesity, aging, physical inactivity, and smoking.[2]
ISO-induced model of MI is one of the most widely used experimental tool. Advantages of this model include, minimal time needed for animal preparation and a high yield of MI. This experimental approach has been utilized frequently to evaluate cardioprotective drugs.[3,4]
In the past 30–40 years, marine plants and animals have been the focus of a worldwide interest. A small number of marine plants, animals, and microbes have already yielded more than 12,000 novel chemicals, with hundreds of new compounds still being discovered every year. These efforts have yielded several bioactive metabolites that have been successfully developed by the pharmaceutical industry.[5] LMW-GAG represents the sulfated carbohydrate moieties of proteoglycans, which occur abundantly in tissues of the cardiovascular system. Many proteins bind specifically to GAG and perform an important role in inflammation, cell proliferation, and blood coagulation processes.[6] We have previously reported the cardioprotective effect of LMW-GAG, extracted from the marine bivalve molluscs Amussium pleuronectus on ISO-induced myocardial injury in rats.[7] The present study was designed to evaluate the influence of LMW-GAG on LDH-isoenzymes, biomolecules, and ECG-patterns in normal and ISO-treated MI in Wistar rats.
Materials and Methods
All experiments were carried out in male albino Wistar rats (140–160 g) obtained from the Central Animal House, Rajah Muthiah Institute of Health Sciences, Annamalai University, Tamil Nadu, India. They were housed in polypropylene cages (47 × 34 × 20 cm) lined with husk, renewed every 24 h, under a 12-h light: 12-h dark cycle at around 22°C and had free access to tap water and food. The rats were fed on a standard pellet diet (Pranav Agro Industries Ltd., Maharashtra, India). The pellet diet consisted of 22.02% crude protein, 4.52% crude oil, 3.25% crude fiber, 7.5% ash, 1.38% sand silica, 0.8% calcium, 0.6% phosphorus, 2.46% glucose, 1.8% vitamins, and 56.17% nitrogen free extract (carbohydrates). The diet provided metabolisable energy of 3000 kcal. The work was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India and approved by the Animal Ethical Committee of Annamalai University (No. 428; dated 21.03.07).
Drugs and Chemicals
Isoproterenol hydrochloride, (Sigma Chemical Company, St. Louis, MO, USA) and LMW-GAG from A. pleuronectus[7] were used. Thiobarbituric acid and reduced glutathione were purchased from Sisco Research Laboratories, Mumbai, India. Glucose, uric acid, total protein, and A/G ratio kits were purchased from Qualigens Diagnostics, Mumbai, India. All other chemicals used in this study were of analytical grade.
Induction of Experimental Myocardial Infarction
ISO (85 mg/kg) was dissolved in normal saline and injected subcutaneously to rats at an interval of 24 h for 2 days to induce experimental MI.[7]
Experimental Design
The animals were grouped as six rats in each group—Group I: normal control rats; Group II: LMW-GAG 300 μg/ day s.c. for 2 weeks; Group III: ISO treated rats; Group IV: LMW-GAG 300 μg/day s.c. for 12 days and ISO (85 mg/kg) for last 2 days of experimental period. All rats were anesthetized with sodium pentobarbital (35 mg/kg, i.p.) and sacrificed by cervical decapitation. Serum samples were prepared for enzyme assay. The excised heart tissues were rinsed in ice-cold saline and homogenized in 100 mM Tris–HCl buffer (pH 7.4) to give a 10% homogenate. Aliquots of the tissue homogenate were suitably processed for biochemical assays.
Biochemical Assays
TG (S 13C), HDL, and cholesterol (S 06) were estimated by using a commercial kit procured from Beacon Diagnostics (Navsari, India). VLDL and LDL fractions were calculated as VLDL = TG/5 and LDL = total cholesterol – (HDL cholesterol + VLDL cholesterol), respectively. Glucose levels were estimated by glucose oxidase/peroxidase method using a commercial kit. Serum uric acid (S 15E), total proteins (Z 17A), and A/G ratio (Z 07A) were estimated using commercial kits obtained from Beacon Diagnostics (Navsari, India).
Separation and Quantification of LDH-Isoenzymes
LDH-isoenzymes were separated by agarose gel electrophoresis.[8] Agarose gel (1%) was prepared and applied immediately to the glass slide. After polymerization, 15 μl of serum samples were applied into wells. After the run, the gels were removed and stained. The staining solution contained 1 ml of 1M lithium lactate, 1 ml of 1M sodium chloride, 1 ml of 5 mM magnesium chloride, 2.5 ml of 0.1% nitro blue tetrazolium, 0.25 ml of 0.1% phenazine methosulphate, 2.5 ml of 0.5M phosphate buffer (pH 7.5), and 10 mg of nicotinamide adenine dinucleotide in a total volume of 10 ml. They were incubated with the staining solution at 37°C in the dark for a suitable period, washed with acetic acid (7.5%) and preserved in 5% acetic acid. Finally, the gels were quantified using a Sebia, scanning densitometer, France.
Electrocardiography
The ECG-patterns were recorded by Polyrite-4 (Recorders and Medicare Systems, Chandigarh, India). ECG-recordings were made in anesthetized (pentobarbital sodium 35 mg/kg, i.p.) animals for 5 min. The types of alterations (ST-segment elevation or depression) in normal and experimental animals were documented.
Results
The heart weight of ISO-treated rats was found to be significantly higher when compared with control rats. The heart weights were significantly reduced in rats treated with LMW-GAG+ISO as compared with those treated with ISO alone [Figure 1]. Rats treated with ISO showed a significant (P < 0.05) increase in LDL and VLDL levels, with a significant (P < 0.05) decrease in HDL level when compared with the control rats.
Figure 1.
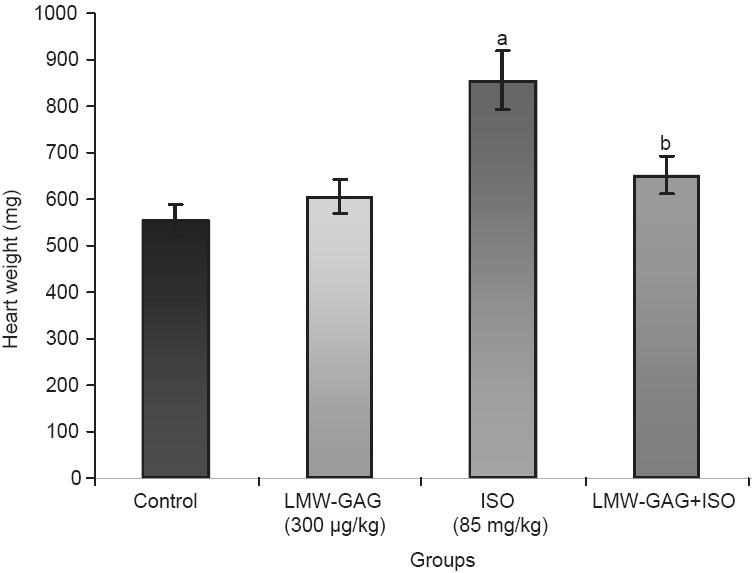
Effects of LMW-GAG on heart weight in experimental rats, Values are expressed as mean ± SD, aP <0.05, as compared with group I, bP <0.05, as compared with group III
Rats treated with LMW-GAG plus ISO showed a significant (P < 0.05) decrease in the levels of LDL and VLDL with subsequent increase in the level of HDL. ISO-treated rats showed a significant (P < 0.05) decrease in the serum levels of total protein and A/G ratio as compared with the control rats [Table 1].
Table 1.
Effect of LMW-GAG on lipid profile and other biochemical parameters in control and experimental group of rats (n = 6 in each group)
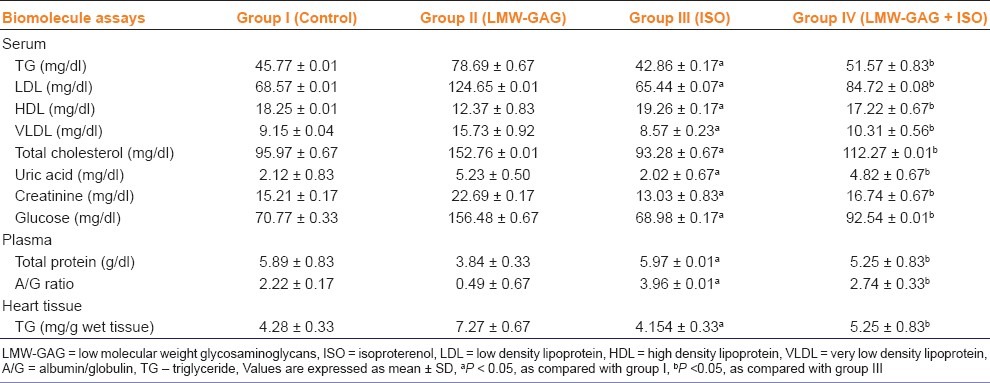
ISO-treatment enhanced the intensity of bands of LDH-isoenzyme and the densitometric scan peaks, mostly LDH1 and LDH2 when compared with the control rats. The dose of LMW-GAG (300 μg/kg) was effective for the study of LDH-isoenzyme pattern. Combination of LMW-GAG and ISO showed a decrease in the intensity of LDH2-band and densitometric scan peaks when compared with those treated with ISO-alone [Figures 2 and 3].
Figure 2.
Agarose gel electrophoresis pattern of effect of LMW-GAG on serum LDH-isoenzyme in control and ISO-treated rats. Lane-1-control; Lane-2-LMW-GAG (300 μg/kg); Lane-3-ISO-treated (85 mg/ kg) Lane- 4- LMW-GAG (300 μg/kg) + ISO
Figure 3.
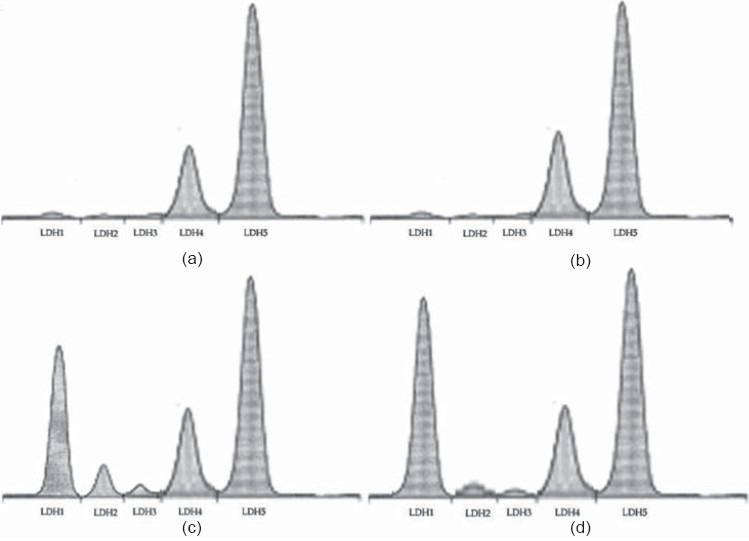
Densitometric scan of LDH-isoenzyme. (a) Control; (b) LMW-GAG treated; (c) ISO-treated; (d) LMW-GAG plus ISO-treated rats
The ECG-pattern of control and ISO-treated rats is shown in Figure 4. Control rats showed a normal ECG-pattern [Figure 4a]. Control rats treated with LMW-GAG (300 μg/kg) did not show any change in ECG-pattern [Figure 4b]. ISO-treated rats showed a marked elevation in ST-segments [Figure 4c]. These changes were restored to normal in LMW-GAG (300 μg/kg) plus ISO-treated rats [Figure 4d].
Figure 4.
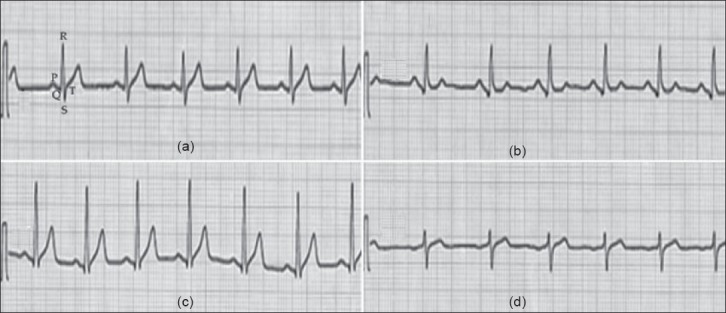
Effect of LMW-GAG on ECG-patterns in control and ISO-treated rats. (a) and (b) Control and LMW-GAG (300 μg/kg) treated rats showing normal ECG-patterns, respectively; (c) ISO (85 mg/kg) - induced rat showing a marked ST-segment elevation; (d) LMW-GAG plus ISO treated rat showing near normal ECG-patterns. LMW-GAG – low molecular weight glycosaminoglycans; ISO – isoproterenol; LDL - low density lipoprotein; HDL – high density lipoprotein; VLDL – very low density lipoprotein; A/G – albumin/globulin; TG – triglyceride; DMRT – Duncan's multiple range test
Discussion
The observed increase in the heart weight of ISO-treated rats may be due to the increased water content, oedematous intramuscular space, and extensive necrosis of cardiac muscle fibers followed by the invasion of damaged tissues by the inflammatory cells as reported earlier.[9] An increase in serum LDL and VLDL fractions along with a decrease in HDL was observed in ISO-treated rats. Pretreatment with LMW-GAG to ISO-treated rats minimized the alterations in serum lipoprotein levels by increasing HDL and decreasing LDL and VLDL cholesterol levels. Similar findings were reported earlier in ISO-treated rats.[10]
LDL formation occurs primarily by the catabolism of VLDL. LDL is capable of carrying the highest concentration of cholesterol.[11] It has also been suggested that oxidative modified LDL rather than unmodified LDL is responsible for atherogenesis. Oxidized LDL promotes the production of several cytokines, immune cell chemoattractant proteins, and growth factors. In addition, they increase platelet aggregation, which aggravates the lesion and causes arterial wall thickening. An increased level of LDL shows a positive correlation with CAD, whereas HDL shows a negative correlation. HDL inhibits the uptake of LDL from arterial wall and facilitates the transport of cholesterol from peripheral tissues to the liver, where it is catabolized and excreted from the body.[12] Hence the beneficial effect of LMW-GAG may be due to the effect on lipoproteins.
LDH is a cytosolic enzyme, which is essentially present in all the tissues involved in glycolysis and exists in five different isoforms designated as LDH1-LDH5. In cardiac tissue, LDH1 and LDH2 are predominating. Hence, detection of elevated concentration of this enzyme released into the blood stream from the damaged tissue has become a definitive diagnostic and prognostic criterion for various diseases and disorders and a study of its isoenzymes has found importance in the location of tissue damage.[13] We observed an increased intensity of LDH1 and LDH2-bands and densitometric scan peaks in ISO-treated rats, which is supported by the earlier findings.[13,14] We observed that the LMW-GAG pretreatment shows a protective effect against ISO-treated MI and altered ECG-patterns perhaps by protecting the cell membranes.
In conclusion the results observed from our study specify that LMW-GAG from A. pleuronuctus offers protection to the myocardium by maintaining the biomolecules, cardiac isoenzyme, and ECG-patterns to near normal in rats.
Acknowledgments
R. Saravanan gratefully acknowledge the facilities provided by the Dean, CAS in Marine Biology, Faculty of Marine Sciences, Annamalai University and the financial assistance awarded by the Indian Council of Medical Research, New Delhi, India in the form of Senior Research Fellow (SRF).
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Goyal A, Yusuf S. The burden of cardiovascular disease in the Indian subcontinent-review. Indian J Med Res. 2006;124:235–44. [PubMed] [Google Scholar]
- 2.Lakatta EG. Deficient neuroendocrine regulation of the cardiovascular system with advancing age in healthy humans. Circul. 1993;87:631–6. doi: 10.1161/01.cir.87.2.631. [DOI] [PubMed] [Google Scholar]
- 3.Wexler BC. Prolonged protective effects following propranolol withdrawal against isoproterenol-induced myocardial infarction in normotensive and hypertensive rats. Br J Exp Pathol. 1985;66:143–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm D, Elsner D, Schunkert H, Pfeifer M, Griese D, Bruckschlegel G, et al. Development of heart failure following isoproterenol administration in the rat: Role of rennin-angiotension system. Cardiovasc Res. 1998;37:91–100. doi: 10.1016/s0008-6363(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 5.Donia M, Hamann M. Marine natural products and their potential applications as anti-infective agents-review. Lancet Infect Dis. 2003;3:338–48. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walenga JM, Michal K, Hoppersteadt D, Wood JJ, Robinson JA, Bick RL. Vascular damage correlates between heparin-induced thrombocytopenia and anti-phospholipid syndrome. Clin Appl Thromb Hemost. 1999;5:76–84. [PubMed] [Google Scholar]
- 7.Saravanan R, Shanmugam A. Preventive effect of low molecular weight gycosaminoglycan from Amussium pleuronectus (Linne) on oxidative injury and cellular abnormalities in isoproterenol-induced cardiotoxicity in Wistar rats. Appl Biochem Biotechnol. 2010;162:43–51. doi: 10.1007/s12010-009-8750-5. [DOI] [PubMed] [Google Scholar]
- 8.Mckenzie D, Henderson AR. Electrophoresis of lactate dehydrogenase isoenzymes. Clin Chem. 1983;29:18995. [PubMed] [Google Scholar]
- 9.Nirmala C, Puvanakrishnan R. Effect of curcumin on certain lysosomal hydrolases in isoproterenol-induced myocardial infarction in rats. Biochem Pharmacol. 1996;51:47–51. doi: 10.1016/0006-2952(95)02118-3. [DOI] [PubMed] [Google Scholar]
- 10.Plaa GL, Zimmerson HJ. Evaluation of hepatotoxicity: Physiological and biochemical measures of hepatic function. In: Mc Cuskey RS, Earnest DL, editors. Comprehensive toxicology. Cambridge: Cambridge University Press; 1997. pp. 97–109. [Google Scholar]
- 11.Sheela SC, Devi CS. Effect of abana pretreatment on isoproterenol-induced hyperlipidaemia in rats. Indian J Pharmacol. 2001;63:1014. [Google Scholar]
- 12.Sathish S, Ebenezar KK, Devaki T. Synergistic effect of nicorandil and amlodipine on tissue defense system during experimental myocardial infarction in rats. Mol Cell Biochem. 2003;243:133–8. doi: 10.1023/a:1021612230000. [DOI] [PubMed] [Google Scholar]
- 13.Levinson SS, Hobbs GA. Usefulness of various lactate dehydrogenase isoenzyme profiles after myocardial infarction. Ann Clin Lab Sci. 1994;24:364–70. [PubMed] [Google Scholar]
- 14.Voet JG, Donald V. Textbook of Biochemistry. 2nd ed. New York: John Wiley; 1995. ATP driven active transport; p. 52435. [Google Scholar]


