Abstract
Objective:
Obesity plays a central role in the insulin resistance syndrome, which is associated with hyperinsulinemia, hypertension, hyperlipidemia, type 2 diabetes mellitus, and an increased risk of atherosclerotic cardiovascular disease. The present study was done to assess the effect of Gymnema sylvestre extract (GSE) in the high fat diet (HFD)-induced cellular obesity and cardiac damage in Wistar rats.
Materials and Methods:
Adult male Wistar rats (150–200 g body weight) were used in this study. HFD was used to induce obesity. Body mass index, hemodynamic parameters, serum leptin, insulin, glucose, lipids, apolipoprotein levels, myocardial apoptosis, and antioxidant enzymes were assessed. Organ and visceral fat pad weights and histopathological studies were also carried out.
Results:
Oral feeding of HFD (20 g/day) for a period of 28 days resulted in a significant increase in body mass index, organ weights, visceral fat pad weight, cardiac caspase-3, cardiac DNA laddering (indicating apoptotic inter-nucleosomal DNA fragment), and lipid peroxide levels of cardiac tissues of rats. Further, mean arterial blood pressure, heart rate, serum leptin, insulin, LDH, LDL-C, total cholesterol, triglycerides, and apolipoprotein-B levels were enhanced significantly, whereas serum HDL-C, apoliporotein-A1 levels, and cardiac Na+ K+ ATPase, antioxidant enzymes levels were significantly decreased. Furthermore, treatment with standardized ethanolic GSE (200 m/kg/p.o.) for a period of 28 days resulted in significant reversal of above mentioned changes in the obese Wistar rats.
Conclusion:
The present study has demonstrated the significant antiobesity potential of GSE in murine model of obesity.
KEY WORDS: Cardiomyocyte apoptosis, Gymnema sylvestre, insulin, leptin, obesity
Introduction
Obesity, the most common nutritional disorders in humans, is a major problem not only in Asia but also in all over the world.[1] According to World Health Organization (WHO), the prevalence of obesity is rapidly rising at an alarming rate to epidemic proportion globally. The International Obesity Task Force estimates that more than 300 million individuals worldwide are obese with body mass index (BMI) ≥ 30 kg/ m2 and 800 million are overweight (BMI between 25 and 29.9 kg/ m2). Currently, 66% of US adults are overweight or obese, 16% of US children and adolescents are overweight, and 34% are at risk of becoming overweight.[2]
Obesity poses a major risk for serious diet-related chronic diseases, including type 2 diabetes, hyperlipidemia, cardiovascular disease, hypertension and stroke, obstructive sleep apnea, asthma, orthopedic disorders, social and mental health problems, and certain forms of cancer.[3] Cardiac apoptosis plays a critical role in the development and progression of obesity[4] and a significant positive correlation has been observed between mean dietary fat intake and the incidence of obesity and its related complications and risk factors.[5]
Gymnema sylvestre extract (GSE) (Asclepiadaceae), a plant native to the tropical forests of India, has been used frequently in traditional medicine for the treatment of diabetes. Maji et al.[6] have reported that water soluble portion of the alcoholic extract of G. sylvestre leaves has a significant glucose lowering effect compared with standard hypoglycaemic agents. Shanmugasundaram et al.[7] have tested two water soluble extracts, GS3 and GS4, obtained from the leaves of G. sylvestre, in streptozotocin treated rats for their effects on blood glucose homeostasis and pancreatic endocrine tissue. Oral administration of G. sylvestre R. Br. leaf extract has been noted to have hypolipidemic and antiatherosclerotic effect in albino rats fed on high fat diet (HFD).[8] The present investigation was designed to study the effect of GSE in HFD-induced obesity and associated metabolic disorders using normal healthy Wistar rats.
Materials and Methods
Authentication and Extraction of G. Sylvestre Leaves
The leaves of G. sylvestre were purchased locally, air dried, and authenticated by Head, Raw Materials Herbarium and Museum, NISCAIR, New Delhi, India. The specimen voucher (Ref. NISCAIR/RHMD/Consult/2008-09/980/11) was retained in the Department of Pharmacology, Faculty of Pharmacy Jamia Hamdard, New Delhi. Dried leaves of G. sylvestre were extracted with ethanol (70%) in Soxhlet's apparatus. The ethanolic extract was standardized according to the WHO guidelines.
Experimental Design
The study was approved by the Institutional Animal Ethics Committee (IAEC) of Hamdard University, New Delhi, which is registered with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, (Registration No. 173/CPCSEA, dated January 28, 2000). Male Wistar albino rats, weighing 150–200 g, were procured from the Central Animal House Facility, Hamdard University, New Delhi and acclimatized under standard laboratory conditions at 25 ± 2°C, relative humidity (50 ± 15%).
Obesity was induced by oral feeding of HFD for a period of 28 days. HFD was purchased from National Centre for Laboratory Animal Sciences (NCLAS), National Institute of Nutrition (NIN), Hyderabad, Andhra Pradesh, India. One kilo of HFD contains (g/kg) casein 342 g, cystine 3 g, starch 172 g, sucrose 172 g, cellulose 50 g, groundnut oil 25 g, tallow 90 g, mineral oil 37 g, and vitamin mix 10 g.
After acclimatization, the animals were randomly divided into five groups of eight animals each and treated as follows: group I (normal healthy control)—fed with standard diet for 28 days; group II (HFD control)—fed with HFD for 28 days; group III (ethanolic GSE treated group)—fed with HFD for 28 days + from 8th day, ethanolic GSE (200 mg/kg/p.o.) to 28th day; group IV (rimonabant treatment group)—fed with HFD for 28 days + from 8th day, rimonabant (10 mg/kg/p.o.) to 28th day; group V (per se group)—fed with normal diet for 28 days + from 8th day ethanolic GSE, 200 mg/kg/p.o. to 28th day.
Measurement of Body Weight Gain and BMI
The increase in body weight was calculated as a difference between final body weight and initial body weight. The BMI was calculated by measurement of body weight (kg) divided by square of naso-anal length of rat [BMI = weight (kg) / length (m)2].
Measurement of Hemodynamic Parameters
Hemodynamic parameters (systolic, diastolic, mean arterial blood pressure, and heart rate) were measured by noninvasive blood pressure recorder using rat tail-cuff method (Kent Scientific Corporation, USA).
Measurement of Biochemical Parameters in Serum
The concentrations of lactate dehydrogenase (LDH) and high density lipoprotein-cholesterol (HDL-C) (Reckon Diagnostics Pvt. Ltd., Baroda, Gujarat, India), glucose, total cholesterol (TC), and triglycerides (TGs) (all the three were from Span diagnostics Ltd., Surat, Gujarat, India), in serum were measured with commercial kits. The concentrations of insulin, leptin, and apolipoprotein-A1 and B in the serum were measured respectively with rat insulin ELISA kit (Alpco Diagnostics, Salem, NH, USA), rat leptin ELISA kit (BioVendor, Brno, Czech Republic), and apo-A1 and B, immunoturbidimetric immunoaasay kit (Randox Laboratories Ltd., Antrim, UK).
Measurement of Organs and Visceral Fat Pad Weights
Different organs (heart, liver, and kidney) and visceral fat pads (epididymal, perirenal, and mesenteric) were removed, washed with normal saline and weighed.
Measurement of Antioxidant Parameters and Lipdperoxidation in Heart Tissue
All antioxidant enzyme activities were determined after the tissue was homogenized with phosphate buffered saline (PBS) at a pH of 7·0. Glutathione was measured according to the Ellman method.[9] Homogenized cardiac tissue was used for the assay of glutathione peroxidase (GPx) activity,[10] glutathione reductase (GR) activity,[11] glutathione S transferase (GST) activity,[12] superoxide dismutase (SOD) activity[13] , and catalase activity.[14] Lipid peroxidation was determined with spectrophotometric measurement of the amount of malondialdehyde equivalents with thiobarbituric acid and expressed as thiobarbituric acid reactive substances (TBARS; nmol malondialdehyde/mg protein), according to the method of Ohkawa et al.[15]
Measurement of Myocardial Apoptosis
Sodium potassium ATPase activity was measured in heart tissue by the method reported by Bonting.[16] Caspase-3 activity was measured using Caspase-3/CPP32 Colorimetric Assay Kit (BioVision, USA). Around 50 μl supernatant from homogenized tissue with cooled lysis buffer was taken from each sample and 50 μl of 2× reaction buffer (containing 10 mM DTT) was added. Then, 5 μl of the 4 mM DEVD-pNA substrate (200 μM final concentration) was added and incubated at 37°C for 1–2 h to allow dissociation of p-nitroanilide (pNA) from the conjugate DEVD-pNA. CPP-32 activity was measured spectrophotometrically at 405 nm. Caspase-3 activity was calculated as nmol/h/mg protein.[17]
Apoptosis was evaluated by examining the characteristic pattern of DNA laddering generated in the apoptotic myocardium using gel electrophoresis. Myocardial samples were homogenized in solution containing 50 mmol/l Tris–HCl (pH 8.0), 100 mmol/l EDTA, 100 mmol/l NaCl, and 1% sodium dodecyl Sulfate. The tissue homogenate was digested with 5 μl of proteinase K (stock solution 20 mg/ml) at 56°C for 2 h and incubated with RNase A (1 μl/ml) at 37°C for 1 h. After that phenol/chloroform (1:1) extraction was performed twice. The tubes were shaken and kept at room temperature (5–10 min). The tissues were then precipitated and centrifuged at 4°C and 10,000 rpm for 10 min. Supernatant containing DNA were precipitated with 600 μl isopropanol and the resulting DNA pellets after centrifugation were washed with 75% chilled ethanol and dissolved in 100 μl of TE buffer solution (10 mmol/l Tris-HCl [pH 8.0], 1 mmol/l EDTA). DNA samples (5 μl DNA + 1 μl gel loading dye) were subjected to electrophoresis on 2% agarose gel, stained with ethidium bromide. DNA laddering, an indicator of tissue apoptotic nucleosomal DNA fragmentation was visualized and photographed under ultraviolet transilluminator.[18]
Statistical Analysis
Statistical analysis was carried out using Graphpad Prism 3.0 (Graphpad software; San Diego, CA, USA). All results were expressed as mean ± standard error of mean (S.E.M.). Groups of data were compared with analysis of variance (ANOVA) followed by Dunnett's t-test. P < 0.05 was considered statistically significant.
Results
Effect of G. Sylvestre Ethanolic Extract (GSE) on BMI, Body Weight Gain, Food Intake, and Hemodynamic Parameters
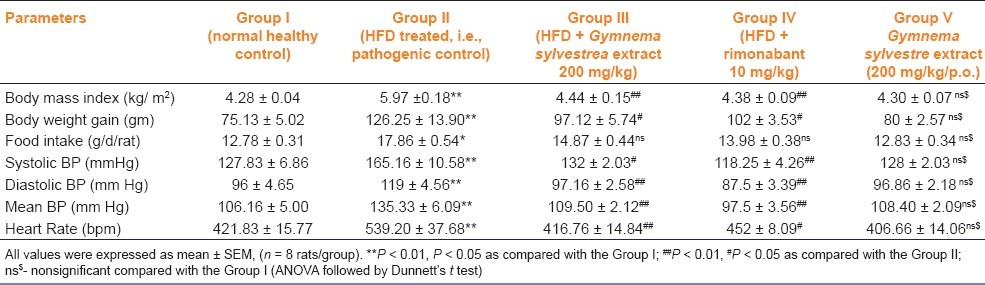
After 4 weeks of treatment, BMI, weight gain, food intake, and all hemodynamic parameters (systolic, diastolic, mean arterial BP and heart rate), were significantly (P < 0.01) increased in HFD treated group (i.e., group II) as compared with the normal control group (i.e., group I). All hemodynamic parameters, BMI were significantly decreased (P < 0.01) in group III (GSE 200 mg/ kg/p.o.) and group IV (rimonabant 10 mg/kg/p.o.) treated rats as compared with the group II treated rats. There were no significant changes in the hemodynamic parameters in G. sylvestre per se group (i.e., group V) as compared with group I [Table 1].
Table 1.
Effect of Gymnema sylvestre ethanolic extract on body mass index and hemodynamic parameters in wistar rats
Effects of GSE on Serum Lipid Profile Levels
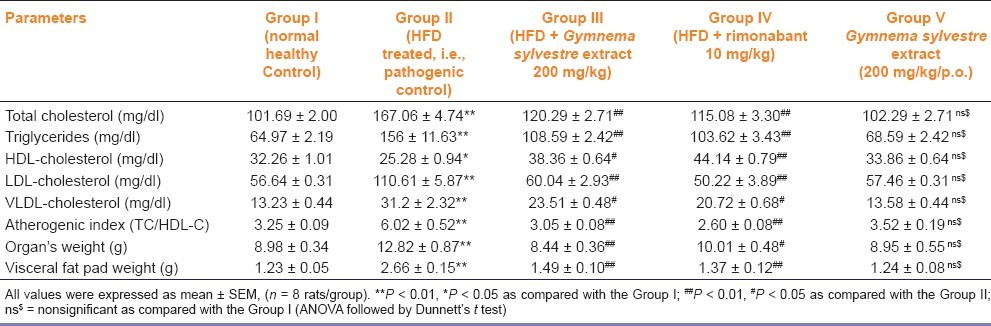
HFD treated group showed significantly (P < 0.01) increased levels of TC, TGs, low density lipoprotein-cholesterol (LDL-C), very low density lipoprotein-cholesterol (VLDL-C), and atherogenic index while HDL-C levels significantly (P < 0.01) decreased as compared with the normal control group after 4 weeks of treatment. All these levels were significantly decreased in group III and group IV as compared with the group II. There was no significant change in the lipid levels in the per se group (i.e., group V) [Table 2].
Table 2.
Effect of Gymnema sylvestre ethanolic extract on serum lipids, atherogenic index, organ, and visceral fat pad weight in wistar rats
Effect of GSE on Serum Leptin, Insulin, Glucose, Apolipoproteins A1 and B, and LDH Levels
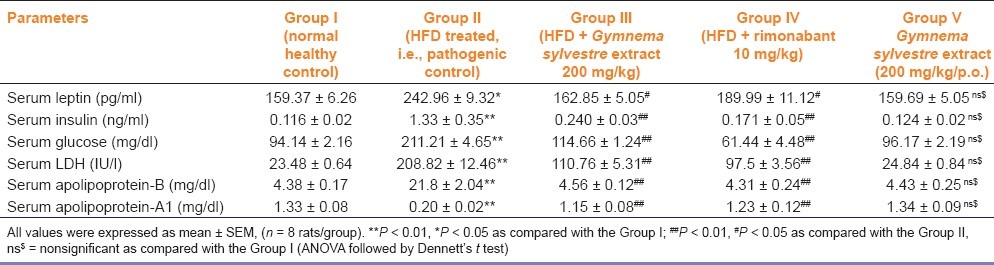
Serum leptin, insulin, glucose, LDH, and apolipoprotein-B levels were significantly (P < 0.01) increased in HFD treated rats as compared with the normal healthy control rats and apolipoprotein-A1 level was significantly decreased in group II as compared with group I. Serum leptin levels were significantly (P < 0.05) decreased in group III as compared with the group II. Serum insulin, LDH, glucose, and apolipoprotein-B levels were significantly (P < 0.01) decreased in group III and group IV as compared with the group II, whereas, levels of apolipoprotein-A1 were significantly increased in group III as compared with the group II. These levels were not significantly changed in group V [Table 3].
Table 3.
Effect of Gymnema sylvestre ethanolic extract on serum leptin, insulin, serum lactate dehydrogenase (LDH), glucose, and apolipoprotein-A1 and B, in wistar rats
Effect of GSE on Cardiac Na+-K+ ATPase, Caspase-3 and DNA Gel Electrophoresis
After 4 weeks of HFD, Na+-K+ ATPase levels in heart and liver tissues were significantly (P < 0.01) decreased in HFD treated group as compared with the normal control group. These levels were significantly (P < 0.01) increased by GSE as compared with the group II. The mean caspase-3 levels were significantly (P < 0.01) increased by 3 to 4 folds in HFD fed rats as compared with normal healthy control rats. There were no significant changes in the Na+-K+ ATPase activity and caspase-3 activity in group V [Table 4]. DNA laddering was more prominent in HFD group as compared the the normal control group while DNA band was preserved in heart tissue of group III [Figure 1].
Table 4.
Effect of Gymnema sylvestre ethanolic extract on lipid peroxidation, antioxidant enzymes, Na-K ATPase, and caspase-3 activity in cardiac tissue of wistar rats
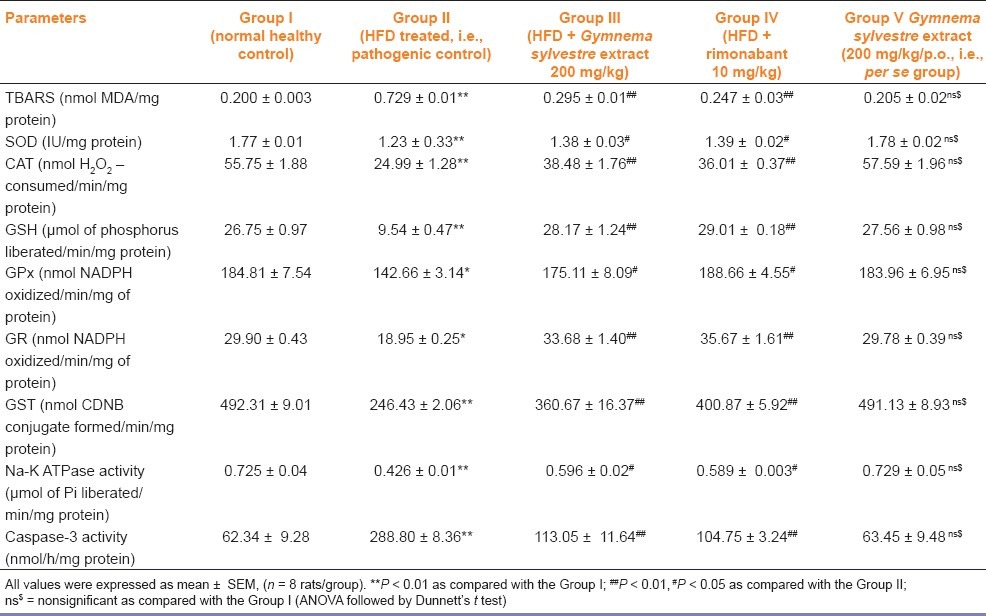
Figure 1.
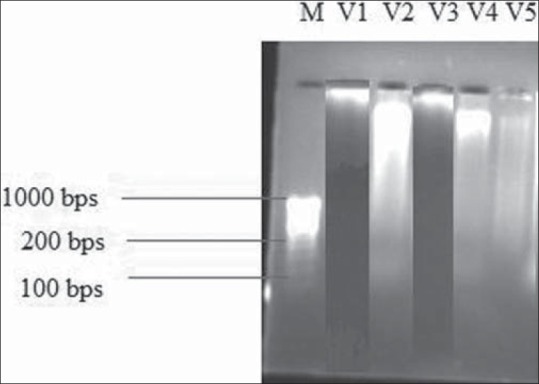
Effect of Gymnema sylvestre ethanolic extract on DNA gel electrophoresis. M-Marker, V1-Normal control group showed no DNA laddering, V2- High fat diet treated group showed DNA laddering band, V3- Gymnema sylvestre ethanolic extract (200 mg/kg/p.o.) treated group restored DNA laddering, V4 = rimonabant (10 mg/kg/p.o.) treated group, V5 = Gymnema sylvestre ethanolic extract (200 mg/kg/p.o., i.e., per se group)
Effect of GSE on Antioxidant Enzymes Levels and Lipid Peroxidation in Cardiac Tissue
All the antioxidant enzymes glutathione (GPx, GR, and GST), SOD, and catalase levels in cardiac tissue were significantly (P < 0.01) decreased in group II as compared with the group I. The antioxidant enzymes levels are increased significantly (P < 0.01) in group III and group IV as compared with those of group II. There were no significant changes in the antioxidant levels in the per se group (i.e., group V) [Table 4]. After 4 weeks of the diet, the level of lipid peroxides, expressed as TBARS, was significantly increased in the cardiac tissue of group II as compared with the group I. TBARS level was significantly decreased (P < 0.01) in group III and group IV as compared with the group II [Table 4].
Effects of GSE on Organs and Visceral Fat Pad Weights
Organs weight (heart, liver, and kidney) and visceral fat pad weights (mesenteric, perirenal, and epididymal) were significantly increased in group II as compared with the group I. The visceral fat pad weights of perirenal fat, mesenteric and epididymal fat in group III were significantly (P < 0·01) decreased as compared with those of group II. There were no significant changes in the visceral fat pad weights and organs weight by the per se group (i.e., group V) [Table 2].
Discussion
Obesity results from an imbalance between food intake and energy expenditure, culminating in excessive accumulation of fat in adipose tissue, liver, muscle, pancreatic islets, and other organs involved in metabolism. Obesity increases the risk of diabetes, coronary artery disease, fatty liver, gall stones, sleep apnea, arthritis, and cancer and may shorten the lifespan.[19] A model of HFD-induced obesity in rats is well controlled, shares many features with human obesity and is advantageous in studying obesity-related cardiovascular abnormalities.[20] Because diets high in fat are usually energy dense and palatable, a diet relatively high in fat leads to an increase in energy intake. HFD intakes lead to weight gain and obesity.
In this study, BMI increased in rats fed with HFD as compared with the normal healthy control rats. Altunkaynak reported that BMI was significantly increased in rats with HFD fed for 8 weeks as compared with the control group.[21] GSE (200 mg/kg/p.o.) treatment significantly (P < 0.01) decreased the BMI as compared with the HFD treated group. This may be due to the decrease in food intake that leads to decrease in calorie intake.[22] The results were comparable to the standard drug, that is, rimonabant (10 mg/kg/p.o.) treatment.
In the present study, HFD + GSE group decreased significantly (P < 0.01) the hemodynamic changes as compared to the group II. Kaufman et al.[23] investigated the effect of HFD on blood pressure (BP) and sympathetic nervous activity (SNA) and suggested that BP and urinary norepinephrine (NE) excretion were higher in HFD-fed rats than in low-fat diet-fed rats. In the present study, treatment with GSE for 3 weeks suppressed the increase in body weight, organ weight (liver, heart, and kidney), and weights of perirenal, mesentric fat and epididymal fat induced by a HFD.
Lavie and Milani[24] indicated that obesity adversely affects plasma lipids, especially by increasing TC, LDL-C, VLDL-C, TGs, and decreasing the level of HDL-C. Levels of TGs, TC, LDL-C, VLDL-C, and atherogenic index were significantly decreased in HFD + GSE group. Hyson et al.[25] observed that the blood level of LDL-C and its oxidation are related to cardiovascular risk. Serum insulin and leptin levels in the HFD + GSE group were significantly decreased as compared with those in the HFD group. Fried et al.[26] indicated that the levels of leptin positively correlated with body fat on a HFD. Leptin might contribute to hepatic steatosis by promoting insulin resistance and also by altering insulin signaling in hepatocytes, so as to promote increased intracellular lipids.[27] Therefore, GSE prevents the increase of leptin levels due to its decrease of the body fat content of rats fed with HFD.
Apolipoprotein A1 (Apo-A1) is associated with HDL, having several antiatherogenic properties.
Apolipoprotein B (Apo-B) is associated with low density lipoprotein (LDL), intermediate density lipoprotein (IDL), very low-density lipoprotein (VLDL), and chylomicrons.[28] Apo-B secretion by the liver is regulated by factors such as rate of cholesterol biosynthesis, availability of TGs and cholesterol esters.[29] Serum Apo-B levels were significantly increased in HFD rats as compared with the normal healthy control rats while serum Apo-A1 levels significantly decreased in HFD rats as compared with the normal healthy control rats. G. sylvestre extract significantly (P < 0.01) decreased Apo-B levels as compared with the group II.
In the current study, cardiac Na+ K+ ATPase activity was significantly increased in the GSE (200 mg/kg/p.o.) treatment group as compared with the HFD fed group. The Na-K-ATPase is a membrane enzyme that energizes the Na-pump by hydrolyzing adenosine triphosphate and wasting energy as heat, hence playing a role in thermogenesis and energy balance. Animal and human obesity is associated with reduction of tissue Na+ K+ ATPase, linked to hyperinsulinemia.[30]
Wang et al.[31] reported that the apoptotic hepatocytes were significantly greater in livers of rats fed HFD than in those fed control diet, and these were associated with a higher level of cleaved caspase-3. Apoptosis results from the activation of caspases that cleave various subcellular cytoplasmic proteins and fragment nuclear DNA.[32] In the current study, the caspase dependent apoptotic pathway was significantly increased in cardiac tissues of HFD-induced obese rats, as evidenced by increase in cardiac activated caspase-3 levels in obese rats’ hearts. GSE (200 mg/kg/p.o.) significantly reduced the caspase-3 levels as compared with the HFD fed group.
In the present study, apoptotic death of myocardial cells was demonstrated in the HFD-induced obesity in Wistar rats by the DNA agarose-gel electrophoresis. DNA electrophoresis demonstrates the presence of small DNA fragments in the form of a DNA ladder in the HFD fed group. The finding is in accordance with that of Li et al.[33] who reported that HFD feeding significantly elevated cytoplasmic DNA fragmentation in the heart and liver samples of HFD fed rats compared with those from low-fat diet fed group, while in the GSE treatment (200 mg/kg/p.o.) group, DNA laddering was preserved. DNA laddering is an index of myocyte apoptosis.[32]
In animal and human studies, obesity is associated with a decrease in tissue or plasma antioxidant capacity.[34] The present data indicates that glutathione (GSH) content was depleted in the rats with obesity induced by a HFD and were restored after the treatment of G. sylvestre extract. Enzymatic antioxidants, such as SOD, catalase or GPx, can scavenge reactive oxygen species and free radicals or prevent their formation. The present results show that antioxidant enzyme activities (GPx, GRd, and GST) in the HFD group were significantly decreased, while HFD + G. sylvestre extract group had significantly increased activities of antioxidant enzymes in the liver and heart. Liou et al.[35] have shown that hyperlipidemia reduces the strength of the antioxidative defence system. Mehta et al.[36] indicated that HFD leads to liver injury and insulin resistance through oxidative stress.
Thus, we conclude that the possible mechanism for an antiobesity effect of G. sylvestre extract may be via suppression of levels of leptin, insulin, dyslipidemia, apolipoproteins, lipids, visceral fat pad weights, and oxidative stress in obese rats fed with HFD. Further, it prevents myocardial apoptosis by decreasing cardiac caspase-3 levels, cardiac DNA fragmentation, and increasing the cardiac Na-K ATPase levels. Hence, it can be further investigated as a potential antiobesity drug.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Marinou K, Tousoulis D, Antonopoulos AS, Stefanadi E, Stefanadis C. Obesity and cardiovascular disease: From pathophysiology to risk stratification. Int J Cardiol. 2010;138:3–8. doi: 10.1016/j.ijcard.2009.03.135. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Obesity and overweight: Global strategy on diet, physical activity and health. WHO; 2003. World Health Organization. [Google Scholar]
- 4.Lee SD, Kuo WW, Lin JA, Chu YF, Wang CK, Yeh YL, et al. Effects of long-term intermittent hypoxia on mitochondrial and Fas death receptor dependent apoptotic pathways in rat hearts. Int J Cardiol. 2007;116:348–56. doi: 10.1016/j.ijcard.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH. Dietary fat and chronic diseases: Epidemiologic overview. J Am Diet Assoc. 1997;97:S9–15. doi: 10.1016/s0002-8223(97)00724-4. [DOI] [PubMed] [Google Scholar]
- 6.Maji BK, Roy S, Gupta SK. Antihyperglycaemic effect of Gymnema sylvestre (Meshasringee) leaf extract in rats. Ind J Physiol Allied Sci. 2000;54:47–54. [Google Scholar]
- 7.Shanmugasundaram ER, Gopinath KL, Shanmugasundaram KR, Rajendran VM. Possible regeneration of the islets of langerhans in streptozotocin- diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol. 1990;30:265–79. doi: 10.1016/0378-8741(90)90106-4. [DOI] [PubMed] [Google Scholar]
- 8.Bishayee A, Chatterjee M. Hypolipidemic and antiatherosclerotic effects of Gymnema sylvestre leaf extract in albino rats fed on high fat diet. Phytother Res. 1994;8:118–20. [Google Scholar]
- 9.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 10.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984;44:5086–90. [PubMed] [Google Scholar]
- 11.Calberg I, Mannerviek B. Glutathione reductase levels in rat brain. J Biol Chem. 1975;250:5475–80. [PubMed] [Google Scholar]
- 12.Habig WH, Pabst MJ, Jakoby WB. Glutthione-S-transferase. The first enzymatic step in mercaptouric acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 13.Marklund S, Marklund G. Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 14.Claiborne A. Catalase activity. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Bonting SL. Sodium potassium activated adenosine triphosphatase and cation transport. In: Bitler EE, editor. Membrane and ion transport. Vol. 1. London: Interscience Wiley; 1970. pp. 257–63. [Google Scholar]
- 17.Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem. 1997;251:98–102. doi: 10.1006/abio.1997.2220. [DOI] [PubMed] [Google Scholar]
- 18.Ling H, Lou Y. Total flavones from Elsholtzia blanda reduce infarct size during acute myocardial ischemia by inhibiting myocardial apoptosis in rats. J Ethnopharmacol. 2005;101:169–75. doi: 10.1016/j.jep.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Carroll JF, Zenebe WJ, Strange TB. Cardiovascular function in rat model of diet-induced obesity. Hypertension. 2006;48:65–72. doi: 10.1161/01.HYP.0000224147.01024.77. [DOI] [PubMed] [Google Scholar]
- 21.Altunkaynak Z. Effects of high fat diet induced obesity on female rat livers. Eur J Gen Med. 2005;2:100–9. [Google Scholar]
- 22.Nakamura Y, Tsumura Y, Tonogai Y, Shibata T. Fecal steroid excretion is increased in rats by oral administration of gymnemic acids contained in Gymnema sylvestre leaves. J Nutr. 1999;129:1214–22. doi: 10.1093/jn/129.6.1214. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman LN, Peterson MM, Smith SM. Hypertension and sympathetic hyperactivity induced in rats by high-fat or glucose diets. Am J Physiol. 1991;260:E95–100. doi: 10.1152/ajpendo.1991.260.1.E95. [DOI] [PubMed] [Google Scholar]
- 24.Lavie CJ, Milani RV. Obesity and cardiovascular disease: The Hippocrates paradox? J Am Coll Cardiol. 2003;42:677–9. doi: 10.1016/s0735-1097(03)00784-8. [DOI] [PubMed] [Google Scholar]
- 25.Hyson DA, Schneeman BO, Davis PA. Almonds and almond oil have similar effects on plasma lipids and LDL oxidation in healthy men and women. J Nutr. 2002;132:703–7. doi: 10.1093/jn/132.4.703. [DOI] [PubMed] [Google Scholar]
- 26.Fried SK, Ricci MR, Russell CD, Laferrere B. Regulation of leptin production in humans. J Nutr. 2000;130:3127S–31. doi: 10.1093/jn/130.12.3127S. [DOI] [PubMed] [Google Scholar]
- 27.Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, et al. Serum leptin levels in patients with nonalcoholic steatohpatitis. Am J Gastroenterol. 2000;95:3584–9. doi: 10.1111/j.1572-0241.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- 28.D’Souza T, Mengi SA, Hassarajani S, Chattopadhayay S. Efficacy study of the bioactive fraction (F-3) of Acorus calamus in hyperlipidemia. Indian J Pharmacol. 2007;39:196–200. [Google Scholar]
- 29.Dixon J, Furukawa S, Ginsberg HN. Oleate stimulates apolipoprotein B containing lipoproteins from HepG2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991;266:5080–6. [PubMed] [Google Scholar]
- 30.Iannello S, Milazzo P, Belfiore F. Animal and human tissue Na, K-ATPase in normal and insulin-resistant states: Regulation, behaviour and interpretative hypothesis on NEFA effects. Obes Rev. 2006;8:231–51. doi: 10.1111/j.1467-789X.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD. Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J Nutr. 2008;138:1866–71. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi PS, Barouch LA. Cardiomyocyte apoptosis in animal models of obesity. Curr Hypertens Rep. 2008;10:454–60. doi: 10.1007/s11906-008-0085-z. [DOI] [PubMed] [Google Scholar]
- 33.Li SY, Liu Y, Sigmon VK, McCort A, Ren J. High-fat diet enhances visceral advanced glycation end products, nuclear O-Glc-Nac modification, p38 mitogen-activated protein kinase activation and apoptosis. Diabetes Obes Metab. 2005;7:448–54. doi: 10.1111/j.1463-1326.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- 34.Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes Relat Metab Disord. 2002;26:1159–64. doi: 10.1038/sj.ijo.0802066. [DOI] [PubMed] [Google Scholar]
- 35.Liou W, Chang LY, Geuze HJ, Strous GJ, Crapo JD, Slot JW. Distribution of Cu Zn superoxide dismutase in rat liver. Free Radic Biol Med. 1993;14:201–7. doi: 10.1016/0891-5849(93)90011-i. [DOI] [PubMed] [Google Scholar]
- 36.Mehta K, VanThiel DH, Shah N, Mobarhan S. Nonalcoholic fatty liver disease: Pathogenesis and the role of antioxidants. Nutr Rev. 2002;60:289–93. doi: 10.1301/002966402320387224. [DOI] [PubMed] [Google Scholar]


