Abstract
Previously we proposed that endogenous amphiphilic substances may partition from the aqueous cytoplasm into the lipid phase during dehydration of desiccation-tolerant organ(ism)s and vice versa during rehydration. Their perturbing presence in membranes could thus explain the transient leakage from imbibing organisms. To study the mechanism of this phenomenon, amphiphilic nitroxide spin probes were introduced into the pollen of a model organism, Typha latifolia, and their partitioning behavior during dehydration and rehydration was analyzed by electron paramagnetic resonance spectroscopy. In hydrated pollen the spin probes mainly occurred in the aqueous phase; during dehydration, however, the amphiphilic spin probes partitioned into the lipid phase and had disappeared from the aqueous phase below 0.4 g water g−1 dry weight. During rehydration the probes reappeared in the aqueous phase above 0.4 g water g−1 dry weight. The partitioning back into the cytoplasm coincided with the decrease of the initially high plasma membrane permeability. A charged polar spin probe was trapped in the cytoplasm during drying. Liposome experiments showed that partitioning of an amphiphilic spin probe into the bilayer during dehydration caused transient leakage during rehydration. This was also observed with endogenous amphipaths that were extracted from pollen, implying similar partitioning behavior. In view of the fluidizing effect on membranes and the antioxidant properties of many endogenous amphipaths, we suggest that partitioning with drying may be pivotal to desiccation tolerance, despite the risk of imbibitional leakage.
All dry biological systems release various substances of relatively low Mr when placed in water. This is a clear indication of the increased membrane permeability associated with cellular dehydration. In the case of desiccation-tolerant (anhydrobiotic) organisms, the leakage generally declines with the progression of rehydration, whereas desiccation-sensitive organisms continue to leak. But even in desiccation-tolerant organisms, excessive leakage can ensue, particularly when imbibition occurs at low temperatures and/or when the organism is very dry.
Several hypotheses have attempted to explain the mechanism of leakage during water uptake (Simon, 1974; Crowe et al., 1989; Hoekstra et al., 1992a). The prevailing hypothesis is based on the observation that liposomal membranes become transiently leaky during the phase change from the gel phase to the liquid-crystalline phase (Hammoudah et al., 1981). Defects at the boundary between these phases are held responsible for the increased permeability. In dehydrating and rehydrating organisms membranes can undergo phase changes and thus become transiently leaky (Crowe et al., 1989; Hoekstra et al., 1992a, 1992b). For example, during imbibition of dry pollen the transition of membrane lipids from the gel phase into the liquid-crystalline phase coincides with an instantaneous loss of endogenous solutes, an event that the pollen does not survive.
When pollen membranes are manipulated to assume the liquid-crystalline state before the uptake of liquid water, thus avoiding the phase transition during imbibition, solute leakage is considerably less and viability is preserved. However, even with their membranes in the liquid-crystalline phase, dry anhydrobiotes still leak to a certain extent upon imbibition (Hoekstra et al., 1992a; Tetteroo et al., 1996). This leakage is transient, but nevertheless may constitute 50% of the total amount of endogenous solutes present, usually without affecting viability but with some loss of vigor. The aim of the present work was to elucidate the mechanism of this transient imbibitional leakage that is not associated with a change from the gel phase to the liquid-crystalline phase.
Increased permeability may result from the presence of compounds associated with the membrane surface or located more deeply in the core of the membrane (Maher and Singer, 1984). The production of large amounts of disaccharides during desiccation of anhydrobiotes is well known (Crowe et al., 1984). Hydrogen bonding of these sugars to the polar headgroups of the membrane phospholipids is considered important in the tolerance of desiccation. The spacing of the individual phospholipid molecules that results from this hydrogen bonding prevents the dehydration-induced increase of Tm in liposomes, thereby keeping the membranes in the liquid-crystalline phase (Crowe et al., 1987). It has been argued that this mechanism also works in vivo and plays a key role in desiccation tolerance and prevention of imbibitional leakage (Crowe et al., 1992). However, the content of sugars in dry organisms may not be sufficient to fully protect the membranes, even if all sugars were hydrogen bonded to the phospholipids (Hoekstra et al., 1997).
Flavonols were considered in the search for compounds other than sugars that may depress the dehydration-induced increase of Tm in membranes (Hoekstra et al., 1997). Quercetin, for example, a flavonol with strong antioxidant activity (Terao et al., 1994) that can be found in pollen as 0.2% to 1% of the dry matter (Ylstra et al., 1992; Vogt and Taylor, 1995), decreases the Tm of dry palmitoyl-oleoyl PC liposomes by 30°C (Hoekstra et al., 1997). This occurs at the low molar ratio of quercetin to phospholipid of 1:10. Quercetin is inserted in the more apolar part of the liposomal membrane and increases the disorder of the acyl chains there. The amphipathic behavior of flavonols was suggested to lead to partitioning from the cytoplasm into membranes during dehydration and to prevent gel-phase formation by membrane fluidization (Hoekstra et al., 1997). The increased disorder could cause some transient leakage during rehydration, which could explain the observed initial leakage from imbibing anhydrobiotes. Although partitioning of amphiphilic substances into membranes and the associated physiological consequences have been thoroughly studied (e.g. Rowe et al., 1998), to our knowledge, this phenomenon has never been considered in relation to the desiccation of anhydrobiotic organisms.
To verify our hypothesis we used an EPR spin-probe technique using a number of mostly amphipathic spin probes with different polarities. The parameters of the EPR spectra of these spin probes depend on the polarity of the environment (Marsh, 1981), which allows the location in hydrophilic and hydrophobic environments of the cell to be distinguished. Thus, we studied partitioning of the spin-probe molecules into the lipid phase of the anhydrobiotic pollen of Typha latifolia, with drying and the release of these molecules into the cytoplasm with rehydration to explain imbibitional leakage. The disturbing effect of amphipaths, both synthetic and extracted from pollen, on membranes during drying and rehydration was also verified in a model membrane system.
MATERIALS AND METHODS
Plant Material and Treatments
Mature male inflorescences of Typha latifolia L. were collected from field populations near Wageningen, The Netherlands, in 1995, and allowed to shed their pollen in the laboratory. Pollen was cleaned by sieving through a fine copper mesh, dried in dry air to 0.05 to 0.08 g water g−1 DW, and stored in closed plastic vials (20 mL) at −20°C until use. Before all experiments, the dry pollen was washed three times in hexane to remove apolar hydrocarbons from the pollen wall, and the adhering hexane was removed by evaporation. This treatment is necessary to obtain homogeneous hydration and does not impair viability (Iwanami and Nakamura, 1972).
Elevated moisture content was obtained by exposing the pollen at 12°C to a stream of water vapor-saturated air produced at 24°C. In this humid atmosphere the moisture content of the pollen reached 0.7 g water g−1 DW within a few hours, and 1.5 g water g−1 DW within 20 h. In the course of this vapor hydration, samples of approximately 100 mg were taken for studies of plasma membrane permeability.
The germination medium used for imbibition consisted of 1.6 mm H3BO3, 1.3 mm Ca(NO3)2·4H2O, 0.8 mm MgSO4·7H2O, 1.0 mm KNO3, and 0.2 m Suc in 2 mm sodium-phosphate-citrate buffer, pH 5.9.
For every sample taken for EPR, two samples (50–80 mg) were taken for determination of moisture content. Water content was analyzed by weighing the samples before and after heating for 4 h at 105°C and calculating the water loss.
EPR Measurements
EPR spectra were obtained at room temperature with an X-band EPR spectrometer (model 300E, Bruker Analytik, Rheinstetten, Germany). Microwave power was 2 mW, and the modulation amplitude was 1 G. Four spectra were accumulated to improve the signal-to-noise ratio.
Determination of Plasma Membrane Permeability
Plasma membrane permeability of the pollen was determined by an EPR technique using the water-soluble nitroxide radical TEMPONE (1 mm) as the spin probe and ferricyanide (120 mm) as the broadening agent (Golovina and Tikhonov, 1994; Golovina et al., 1997). TEMPONE easily penetrates through the plasma membrane into cells because of its amphipathic nature and relatively small size. In contrast, the charged ferricyanide ions cannot penetrate through intact membranes. Inside cells TEMPONE partitions between hydrophilic (cytoplasmic water) and hydrophobic (membranes and oil bodies) compartments.
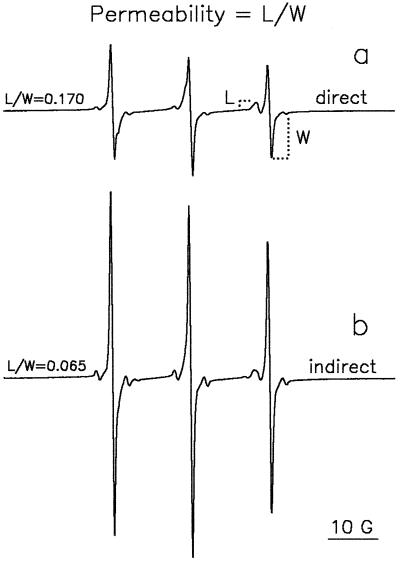
The EPR spectrum of TEMPONE in pollen (Fig. 1) is the result of the superposition of two spectra, slightly different in shape, arising from TEMPONE molecules in the polar cytoplasm and in the apolar membranes and/or lipid bodies. The superposition of these two spectra can be resolved in the high-field spectral region only. The TEMPONE signal from outside the grains is fully broadened by ferricyanide. Because ferricyanide cannot penetrate through an intact plasma membrane, the signal from TEMPONE inside the aqueous cytoplasm is not broadened. If there are defects in the plasma membrane, some ferricyanide ions will be able to penetrate into the cell and to some extent broaden the signal arising from TEMPONE located in the cytoplasm. This has no influence on the lipid signal, because the lipid environment is inaccessible to ferricyanide ions, allowing the use of the apolar peak as the internal standard for the amount of pollen examined. Thus, the line-height ratio of the lipid peak to the water peak (L/W) depends only on the number of ferricyanide ions that have penetrated the cell through the plasma membrane and can be used to characterize plasma membrane permeability (Fig. 1).
Figure 1.
EPR spectra of TEMPONE in T. latifolia pollen. Pollen of approximately 0.37 g water g−1 DW was incubated in a solution of 1 mm TEMPONE and 120 mm ferricyanide directly (a) and indirectly (b) after a previous 5 min of rehydration in germination medium. The TEMPONE at the outside of the pollen was fully broadened by ferricyanide. The permeability of the plasma membrane to ferricyanide can be calculated as the ratio between the line heights of the lipid fraction (left) and the aqueous cytoplasmic fraction (right) in the high-field region of the spectrum (L/W).
The following protocols were carried out to discriminate between the plasma membrane permeability at the onset of imbibition and after completion of rehydration (5 min; F.A. Hoekstra and E.A. Golovina, unpublished data). One sample was directly incubated in a 10-mL tube containing 5 mL of a 1 mm TEMPONE/120 mm ferricyanide (K3Fe[CN]6) solution at 22°C (the direct method). After 5 min of gentle shaking the sample was recovered by filtration (plasma/serum filters, Greiner, Alphen a/d Rijn, The Netherlands), and then loaded into a capillary (2 mm diameter, filled for 1 cm). A very small amount of the solution was injected on top of the sample to prevent drying, and the EPR spectra of TEMPONE were recorded. A second sample was incubated in a 10-mL tube containing 5 mL of liquid germination medium for 5 min at 22°C with gentle shaking, after which the medium was filtered off (the indirect method). Subsequently, the pollen was incubated in 5 mL of the TEMPONE/ferricyanide solution. After 5 min the pollen was recovered by filtration, loaded in the capillary as described above, and EPR spectra were recorded.
Determination of Partitioning
To study the in situ partitioning of amphiphilic substances during drying and rehydration, an EPR spin-probe technique was also used. For this purpose TEMPONE was used as the model amphiphilic compound. Because of differences in the isotropic hyperfine splitting constants of EPR spectra in hydrophilic and hydrophobic environments, the location of TEMPONE in these environments could be easily determined.
The loading of the spin probe into the pollen was as follows: Pollen (3 g) was prehydrated in water vapor to about 0.8 g water g−1 DW and then mixed at 25°C with approximately 6 mL of the liquid germination medium. After a few minutes another 20 mL of germination medium was added and the pollen was recovered by filtration. The restricted addition of liquid kept leakage of endogenous solutes to a minimum (F.A. Hoekstra and E.A. Golovina, unpublished results). The pollen was then mixed with 20 mL of a solution containing 1 mm spin probe and 120 mm ferricyanide (necessary for quenching of the EPR signal from TEMPONE in the adhering extracellular solution). After 5 min the pollen was recovered by filtration, spread out in a large Petri dish, and allowed to slowly dry on the laboratory bench at room temperature. At intervals, samples were loaded into capillaries (2 mm diameter) for EPR analysis. The nitroxide spin probes TEMPO and TEMP-amine were used in addition to TEMPONE.
For repartition experiments during rehydration of dry pollen in humid air, the same procedures of spin-probe loading were followed as outlined above, except that the 120 mm ferricyanide was replaced by 10 mm. Because ferricyanide caused some membrane deterioration during the long interval between loading and analysis, the concentration had to be less than 120 mm. In fact, the lack of extracellular EPR signal in the vapor-rehydration experiments made the use of ferricyanide as a quencher superfluous. However, some ferricyanide (10 mm) was necessary as an external electron acceptor to prevent reduction of TEMPONE to a non-free-radical form (Kaplan et al., 1973; Miller, 1978). The filtered pollen sample loaded with the spin probe was rapidly dried overnight in a flow of dry air (3% RH) in a dry box, and retained good viability during the procedure. The dried pollen was rehydrated in vapor-saturated air at 12°C, and at intervals samples were loaded into the capillaries under humid conditions.
Partitioning of the preloaded spin probes during drying and vapor rehydration of the pollen was estimated from the line-height ratios in the high-field region of the spectrum in two different ways: with the amplitude of the lipid peak as a percentage of the sum of the amplitudes of the lipid and the aqueous peaks (L/[L + W]), and as the partition parameter P (W/L) (Fig. 2).
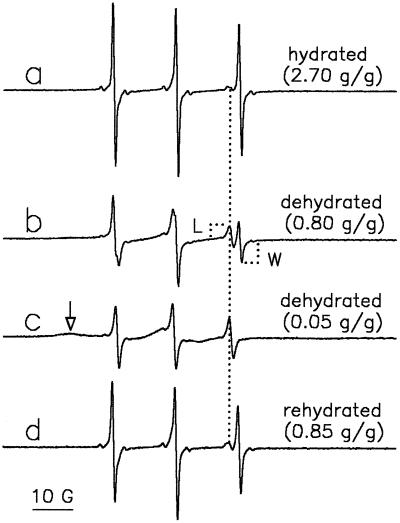
Figure 2.
EPR spectra of preloaded TEMPONE in T. latifolia pollen at different levels of dehydration and rehydration. Vapor-prehydrated pollen was incubated for 5 min in germination medium that was replaced by TEMPONE/ferricyanide. After 5 more min, the pollen was filtered and EPR spectra were recorded in the hydrated state (2.70 g water g−1 DW) (a); after 2 h of exposure to laboratory air (0.80 g water g−1 DW) (b); after 5 h of exposure to laboratory air (0.05 g water g−1 DW) (c); and after forced rehydration of dried pollen in humid air (0.85 g water g−1 DW) (d). The arrow in c points to the anisotropic spectrum, representing slow-moving TEMPONE molecules trapped in a solid environment. The dotted line represents the position of the lipid peak of the spectra.
Extraction of Amphipathic Compounds from Pollen
Hexane-washed pollen (2.5 g) was suspended in 10 mL of deionized water and rapidly frozen. The frozen material was pressed through the orifice of a homogenizer (AB-Biox, Järfälla, Sweden) at −20°C at 20,000 p.s.i. The crushed pollen was mixed with 100 mL of chloroform:methanol (1:1, v/v), followed by 5 min of mild ultrasonic treatment. After low-speed centrifugation (1000g) the supernatant was mixed with 15 mL of a 0.9% NaCl solution. After phase separation the chloroform layer at the bottom of the glass tube was collected and dried by passage over a column of anhydrous Na2SO4. After volume reduction, the material was loaded on a BioSil A (Bio-Rad) column (18 cm in length, 16 mm in diameter) that was prewashed with 110 mL of chloroform. After elution of neutral lipids with 150 mL of chloroform, the amphipathic substances were recovered with 100 mL of acetone. After removal of the acetone the material was suspended in 0.5 mL of water for use in the liposome experiments.
Alternatively, amphipathic substances were recovered from an aqueous pollen extract. After homogenization as described above, the homogenate was thawed and subsequently centrifuged for 10 min at 30,000g, and the supernatant was recentrifuged at 150,000g for 30 min. The supernatant was led over three coupled Sep-Pak C18 cartridges (no. 51910, Waters), and the cartridges were washed with 10 mL of water. Amphipathic substances were eluted from the cartridges by 10 mL each of increasing concentrations of methanol in water (v/v): 20%, 50%, 80%, and 100%. After vacuum evaporation of the methanol and freeze drying of the aqueous extract, the solid material was thoroughly mixed with 250 μL of water for use in the liposome experiments.
Liposome Studies
EggPC (100 mg) in chloroform, purchased from Fluka, was used without further purification. The chloroform was evaporated in a stream of nitrogen and, after the sample was dried under vacuum for at least 2 h, 1 mL of water containing 500 mg of Suc (Pfanstiehl, Inc., Waukegan, IL) was added (Suc:eggPC = 5:1, w/w). After five freeze-thaw cycles and vortexing, liposomes were produced by extrusion through one 100-nm pore-size polycarbonate filter (Nuclepore Corp., Pleasanton, CA) 35 times using the LiposoFast extruder (Avestin, Inc., Ottawa, Canada) according to the method of MacDonald et al. (1991). These liposomes were used for EPR studies.
To produce liposomes with encapsulated CF, the same procedure was followed, but the dried eggPC (20 mg) was mixed with 2 mL of 100 mm CF (purified according to the method of Klausner et al. [1981]), and 0.25 m Suc in 1 mm Tes buffer, pH 7.5. The suspension was then layered onto a Sephadex G-50 column (50–80 mesh). Elution was with 1 mm Tes buffer, pH 7.5, to separate the vesicles from excess Suc and CF. In the opened lid of an Eppendorf tube, 10 μL of the liposome suspension was mixed with another 20 μL of water and Suc and/or amphipaths as desired. Drying of the 30-μL droplets in the opened lids was carried out by exposure to a stream of dry air (RH < 3%) in a dry box for at least 3 h at 25°C in the dark (0.02 g water g−1 DW). One milliliter of 1 mm Tes buffer, pH 7.5, was then added and after closure of the lids the lipid mixture was suspended by vortexing. Leakage of CF from the vesicles was assessed with a fluorometer (excitation wavelength = 490 nm and emission wavelength = 515 nm; Aminco, Silver Spring, MD) according to methods described previously (Hoekstra and Van Roekel, 1988).
RESULTS AND DISCUSSION
Decline of Membrane Permeability with Rehydration
Figure 1 (spectrum a) shows an EPR spectrum of TEMPONE in pollen (initial moisture content of 0.37 g water g−1 DW) that was allowed to imbibe in the presence of TEMPONE/ferricyanide (1:120 mm) solution. This rehydration is referred to as direct incubation. Spectrum b shows such a spectrum after first allowing the pollen to imbibe in germination medium for 5 min, followed by the addition of TEMPONE/ferricyanide. We refer to this treatment as indirect incubation. From the spectra it becomes clear that the aqueous contribution to the spectrum (W) has increased with the previous 5 min of rehydration in germination medium. Apparently, more ferricyanide ions could enter pollen to broaden the water signal if ferricyanide was present at the onset of imbibition rather than being added after 5 min. Because the spin-probe method provides information on membrane permeability (L/W) at the moment of addition of the spin probe, the difference between the spectra shows that plasma membranes are more permeable at the onset of imbibition than after rehydration is complete. After 5 min the permeability did not continue to decrease with time of rehydration (data not shown). Full rehydration in medium apparently led to minimum permeability values. Thus, it is expected that with increasing initial moisture content of the pollen, the permeability of plasma membranes at the onset of imbibition decreases and finally approaches this minimum value (if plasma membranes are not irreversibly disrupted).
Plasma Membrane Permeability at the Onset of Imbibition as Influenced by Initial Moisture Content
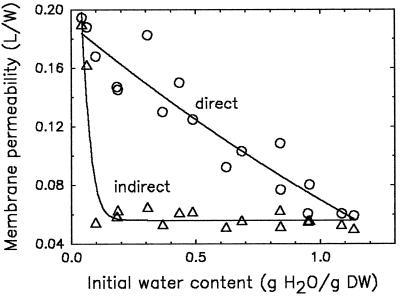
Plasma membrane permeability at the onset of imbibition decreased more or less linearly with increasing initial moisture content of the pollen and reached a minimum value at approximately 1.2 g water g−1 DW (direct rehydration in TEMPONE/ferricyanide solution; Fig. 3). When the TEMPONE/ferricyanide solution was added after completion of rehydration (Fig. 3, indirect), low permeability was observed for moisture contents above 0.06 water g−1 DW. The elevated permeability at the onset of imbibition (Fig. 3, direct) is reduced to the same low value upon completion of rehydration, indicating that the structural defects of the dried plasma membrane have been restored upon rehydration. In contrast, the high permeability at initial moisture contents below 0.06 g water g−1 DW is of a more drastic nature, because membranes were still permeable after 5 min of preincubation in germination medium (indirect curve). The membrane phospholipids of pollen with moisture content below 0.06 g water g−1 DW were in the gel phase (Crowe et al., 1989; Hoekstra et al., 1992a). As a consequence of imbibition, a transition to the liquid-crystalline phase occurred, which is supposed to cause the complete loss of endogenous solutes and viability.
Figure 3.
Permeability of plasma membranes of T. latifolia pollen of different initial moisture contents upon direct hydration in TEMPONE/ferricyanide (1 mm:120 mm) solution (direct incubation) or after a previous hydration in liquid germination medium for 5 min (indirect incubation), followed by substitution for TEMPONE/ferricyanide solution. Imbibition temperature was 22°C. Plasma membrane permeability was expressed as L/W, in arbitrary units.
The region of initial moisture contents just above 0.06 g water g−1 DW is characterized by a rather high initial permeability of the plasma membranes (Fig. 3, direct). Although membranes are already in the liquid-crystalline phase before imbibition (Crowe et al., 1989; Hoekstra et al., 1992a) and therefore no phase change is involved, approximately 40% to 50% of the endogenous solutes may leak out during this rehydration period. This may reduce vigor but generally does not impair germination capacity (Hoekstra et al., 1992a; F.A. Hoekstra and E.A. Golovina, unpublished results). Furthermore, high initial moisture contents of approximately 1.1 to 1.2 g water g−1 DW result in less leakage and improved pollen vigor.
Because the high initial leakage during rehydration is not associated with a membrane phase transition, another mechanism for this phenomenon was sought. High membrane permeability can be caused by fluidizing membrane perturbants (Maher and Singer, 1984; Casal et al., 1987). We previously raised the possibility that endogenous amphipaths may partition from the aqueous phase into the lipid phase during dehydration and vice versa during rehydration (Hoekstra et al., 1997). Accordingly, the imbibitional leakage may stem from the perturbing effect of such compounds. To study this possibility in more detail we conducted experiments with the spin probe TEMPONE as a model amphipathic compound loaded into pollen to follow its partitioning with drying and rehydration.
Partitioning of Amphipaths during Dehydration and Rehydration
EPR spectra of TEMPONE in hydrated pollen show a considerable contribution from the aqueous cytoplasm (Fig. 2, spectrum a), characterized by a hyperfine splitting constant of about 17 G (distance between peaks measured in magnetic field units). The small peak directly to the left of the water line in the high-field region of the spectrum (Fig. 2, spectrum a) is typical of TEMPONE in a lipidic environment (hyperfine splitting of 14.0–14.5 G; for review, see Marsh, 1981). The distribution of TEMPONE over the aqueous and lipidic environments depends on the partitioning coefficient of this spin probe and the volume of both environments available. Lipid is present in T. latifolia pollen as approximately 13.3% of the dry matter and consists of 8.0% neutral lipids and 5.3% polar lipids (Hoekstra et al., 1991). Drying of the pollen to 0.8 g water g−1 DW caused a considerable decrease in the polar peak and an increase in the lipid peak (Fig. 2, spectrum b). Further drying to 0.05 g water g−1 DW resulted in the complete disappearance of the water component from the spectrum (Fig. 2, spectrum c). The lipid character of the remaining lines follows from the value of the hyperfine splitting constant (14.0–14.5 G). Also, a slight sign of an anisotropic spectrum can be observed (minute bulge at the left side of the spectrum), which indicates that some slow-moving TEMPONE molecules are trapped in a solid environment. The considerable increase in the line height of the lipid component with drying shows that partitioning of TEMPONE into the lipid phase has occurred. This phenomenon was described before in lyophilized yeast cells, but to our knowledge has not been discussed in terms of the physiological consequences (Keith and Snipes, 1974).
Vapor rehydration of dry pollen previously loaded with TEMPONE/ferricyanide (1 mm:10 mm) caused the lipid component to decrease and the water component to reappear (Fig. 2, spectrum d). We interpret this to mean that TEMPONE partitioning into the lipid phase is reversible, depending on the volume of water available.
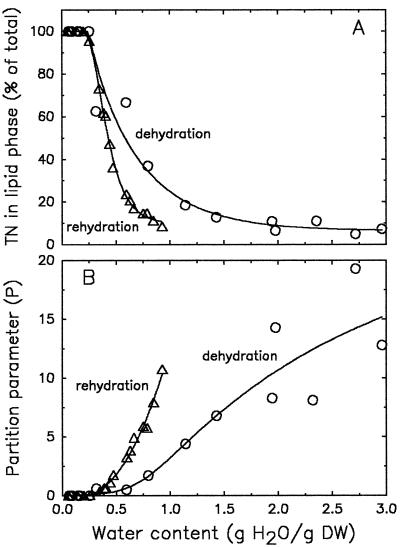
To follow the partitioning of the spin label from a water to a lipid environment with drying, we plotted the amplitude of the lipid peak as a percentage of the amplitudes of both the lipid and the aqueous peaks (Fig. 4A). The product of the amplitude (hi) and the square of the width (ΔsHi2) of a peak is used to approximate the amount of spin probe in each environment (Ai = hiΔsHi2). However, if the peak widths do not change, peak amplitudes can be used instead (Shimshick and McConnell, 1973). Because we did not notice pronounced changes in peak widths with drying, we used amplitude characteristics in Figure 4 to describe partitioning. The amounts of TEMPONE in the lipid component increased slightly with drying of the fully hydrated sample, to increase more rapidly at moisture contents below 1.2 g water g−1 DW. TEMPONE was out of the solution below approximately 0.4 g water g−1 DW. The rehydration curve follows the same pattern but with some hysteresis. The starting point of repartitioning was the same (0.4 g water g−1 DW), but the probe moved into the water earlier with respect to moisture content. We speculate that this was the result of different relative amounts of sorbed and bulk water between samples with the same moisture content when dried or rehydrated, possibly caused by the hysteresis of water sorption by polymers (Slade and Levine, 1991).
Figure 4.
The effect of dehydration and rehydration on the percentage of TEMPONE (TN) in the lipid phase of T. latifolia pollen (L × 100/[L + W]) (A), and the partition parameter P (W/L) as calculated from amplitudes of the EPR spectra (B). TEMPONE was preloaded as described in Methods. Dehydration of fully hydrated pollen was performed on the laboratory bench at room temperature; rehydration of dried pollen was in vapor-saturated air at 12°C.
The same data presented as the ratio between the water and lipid components (the partition parameter P) are shown in Figure 4B. This presentation emphasizes the partitioning at high moisture contents, which was less clear in Figure 4A. This parameter is sensitive to slight changes in the amplitude of the lipid component. The scatter of the P values at elevated water contents may be attributable to the calculation of this parameter using the considerable line heights of the water contribution to the spectra divided by the very small line heights of the lipid component. During rehydration, P was 0 until it began to increase rapidly at moisture contents above 0.4 g water g−1 DW. Apparently, partitioning of TEMPONE from the lipidic phase into the aqueous phase had not begun until the water content had reached 0.4 g water g−1 DW. Because of the dehydration curve shown in Figure 4B, we attempted to describe the driving force for the partitioning of TEMPONE from the aqueous phase to the lipid phase.
Supposing that the changes in the size of the water compartment (the cytoplasm and vacuole) are responsible for the partitioning into the lipid compartment, the size of which remained the same, we considered the following: the amount of spin probe in water (Aw) or lipid (Al) compartments can be calculated as the product of concentration (Cw, Cl) and volume (Vw, Vl)
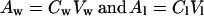 |
1 |
The ratio between the amounts of spin probe in both compartments is:
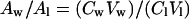 |
2 |
If we express the partition coefficient as:
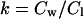 |
3 |
then:
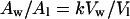 |
4 |
Supposing that the partition coefficient k and the size of the lipid compartment Vl do not change with drying, then:
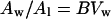 |
5 |
where B = k/Vl, which is constant. According to Equation 5, the partition parameter P, defined as Aw/Al, has a linear dependence on moisture content in the range where bulk water is present. As mentioned above, amplitudes in the high-field region of the spectrum can be used instead of the amounts of spin probe in each environment when the widths do not change (Shimshick and McConnell, 1973). Figure 4B shows the relationship between the partition parameter P and moisture content expressed on a DW basis for dehydrating pollen. Indeed, the dependence is approximately linear over the high range above approximately 1 g water g−1 DW, but deviates from this linearity below this moisture content. The comparatively low values of P may be attributed to the slightly increased viscosity below 1 g water g−1 DW and the steep increase in viscosity below 0.6 g water g−1 DW (Leprince and Hoekstra, 1998). From the data obtained it is not completely clear whether the deviation of P from the expected straight line is caused by water-line broadening as a result of increased viscosity, or whether the increased viscosity of the cytoplasm itself promotes partitioning into the more liquid lipid phase. However, the very slight increase in line width of the water peak between 1.0 and 0.6 g water g−1 DW (data not shown) favors the latter explanation.
Effect of Polarity of Molecules on Dehydration-Induced Partitioning
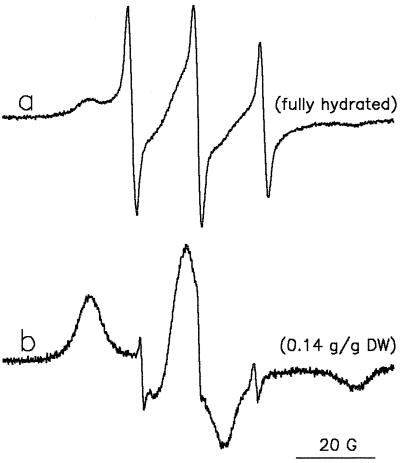
Insight into the partitioning behavior of molecules with different polarity during dehydration was obtained using TEMPamine and TEMPO as model molecules. The EPR spectrum of the more polar, charged TEMPamine in fully hydrated pollen has the typical isotropic shape of a nitroxide spin probe in water solution, with a distance between peaks of 17 G (Fig. 5, spectrum a). There was no sign of TEMPamine in the lipid environment, but a clear indication of an anisotropic spectrum (low-field side) was apparent, which can be explained by the binding of some charged TEMPamine molecules to the surface of immobile structures such as proteins. After dehydration this spectrum disappeared completely, and a new complex spectrum appeared instead (Fig. 5, spectrum b). This spectrum of TEMPamine in dehydrated pollen is characterized by signal contributions from two different environments. The two outer extremes of the spectrum represent the high- and low-field peaks of an anisotropic spectrum characterized by restricted mobility of the spin probe. The two small, narrow lines with a hyperfine splitting of about 14.5 G can be attributed to TEMPamine molecules localized in the lipid environment. The central component of the spectrum was not resolved.
Figure 5.
EPR spectra of preloaded TEMPamine in T. latifolia pollen. Prehydrated pollen was incubated for 5 min in germination medium that was replaced by a TEMPamine/ferricyanide (1 mm:120 mm) solution. After 5 more min, the pollen was filtered and EPR spectra were recorded in the hydrated state in the presence of minute amounts of the solution (a) and after 5 h of exposure of the pollen to air (moisture content = 0.14 g water g−1 DW) (b).
Comparing the spectra of TEMPamine in hydrated and dehydrated pollen (Fig. 5, spectra a and b), we conclude that only a small part of the TEMPamine molecules partitioned into the lipid phase during dehydration. The majority of the TEMPamine molecules were trapped in the dried cytoplasm, their charge apparently preventing their partitioning into the lipid phase during dehydration. The small amount of spin-probe molecules in the lipid phase may be explained by the presence of the neutral form of TEMPamine molecules, possibly due to changes in ion strength and pH of the cytoplasm with drying.
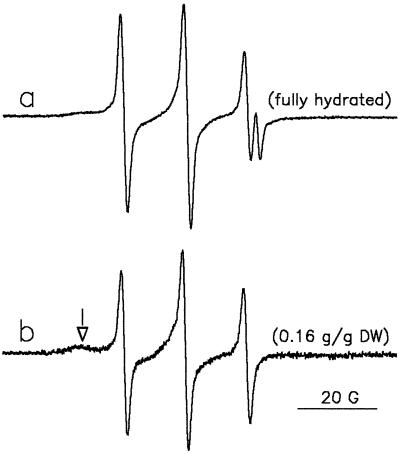
A considerable number of TEMPO molecules were present in the lipid phase of fully hydrated pollen grains (Fig. 6, spectrum a), as resolved in the high-field part of the spectrum. The more apolar nature of TEMPO compared with TEMPONE explains why the aqueous contribution to the spectrum is rather low, even for hydrated pollen. The spectrum of TEMPO in dried pollen is characterized by three narrow lines with a hyperfine splitting of 14.5 G, which is typical of lipid surroundings (Fig. 6, spectrum b). A very slight indication of an anisotropic spectrum (left side) is visible. It is clear that upon drying almost all TEMPO molecules partitioned out of the aqueous environment into the lipid environment, similarly to TEMPONE.
Figure 6.
EPR spectra of preloaded TEMPO in T. latifolia pollen. Prehydrated pollen was incubated for 5 min in germination medium that was replaced by a TEMPO/ferricyanide (1 mm:120 mm) solution. After 5 more min, the pollen was filtered and EPR spectra were recorded in the hydrated state in the presence of minute amounts of the solution (a) and after 5 h of exposure of the pollen to air (moisture content = 0.16 g water g−1 DW) (b). The arrow in b points to the anisotropic spectrum, representing slow-moving TEMPONE molecules trapped in a solid environment.
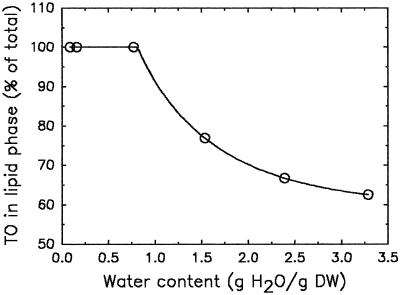
Figure 7 shows the increase in the percentage of TEMPO in the lipid phase during dehydration. At 0.8 g water g−1 DW, all spin-probe molecules had disappeared from the aqueous surroundings and moved to the lipid surroundings. The slight amount of immobilized TEMPO was not accounted for in the calculation of this percentage.
Figure 7.
The effect of dehydration on the percentage of TEMPO (TO) in the lipid phase of T. latifolia pollen, as calculated from the amplitudes of the EPR spectra. The TEMPO was preloaded as described in Methods. Dehydration of the fully hydrated pollen was performed on the laboratory bench at room temperature.
Partitioning of Amphipaths into EggPC Liposomal Membranes upon Dehydration
From the spectra of TEMPONE in dry pollen (Fig. 2, spectrum c) one cannot determine whether the site of partitioning is the membranes, lipid bodies, or both. Because only partitioning into membranes can influence permeability, we investigated whether the spin probe is able to partition into membranes with drying. A mixture of eggPC liposomes, Suc, and TEMPONE plus ferricyanide was allowed to dehydrate. Suc (mass ratio of Suc:eggPC = 5:1) was added to prevent liposome fusion and phase transitions with drying (Crowe et al., 1996), which may mimic the situation in the cytoplasm (pollen contains 23% Suc on a DW basis, which gives a 4:1 mass ratio of Suc:phospholipid [Hoekstra et al., 1992c]). The ferricyanide was used to quench the EPR signal from outside the liposomes.
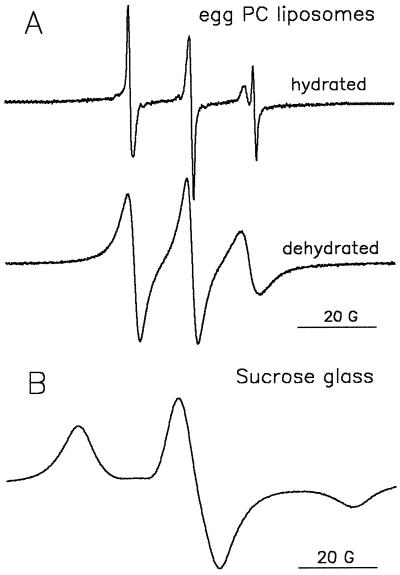
Figure 8A shows EPR spectra of TEMPONE in the liposome suspension before and after drying. In the spectrum of the hydrated liposome sample, the aqueous peak was less pronounced than that in the spectrum of hydrated pollen (Fig. 2, spectrum a). This may be explained by the approximately six-times-higher amount of lipid per trapped volume in liposomes than in pollen grains. Drying of the liposome sample resulted in the disappearance of the water line from the EPR spectrum of TEMPONE. In this dry sample (0.02 g water g−1 DW) only one component with a hyperfine splitting of approximately 14.5 G could be observed, which is typical of a hydrocarbon environment. The shape of the lipid spectrum is attributable to a decreased rotational motion of the spin-probe molecules and indicative of a viscous environment (Marsh, 1981). Compared with the much more fluid character of the environment of the spin probe in dry pollen (Fig. 2, spectrum c), this may indicate that in the latter case a considerable number of TEMPONE molecules had partitioned into lipid bodies. Another possibility could be that membranes are more fluid in dry pollen (mainly 18:2 as the unsaturated fatty acid) than in the dry, lyoprotected eggPC (mainly 18:1) liposomes.
Figure 8.
A, EPR spectra of TEMPONE (1 mm) in suspensions of eggPC liposomes (100 mg/mL) containing 120 mm ferricyanide before and after dehydration for 3 h in a stream of dry air (3% RH) at 24°C. The liposomes were produced in the presence of Suc (mass ratio of Suc:eggPC = 5:1). B, EPR spectrum of TEMPONE in Suc glass.
These changes in EPR spectra unambiguously demonstrate that partitioning of amphipathic molecules such as TEMPONE from the aqueous environment into membranes with drying is possible in principle. There was no indication that TEMPONE was trapped in the Suc glass. The EPR spectrum of TEMPONE in a pure Suc glass (i.e. without liposomes) (Fig. 8B) clearly indicates the solid environment of the TEMPONE molecules typically seen with trapping of the probe in a glass (Marsh, 1981; Dzuba et al., 1993).
TEMPONE-Induced Leakage from Desiccated EggPC Liposomes
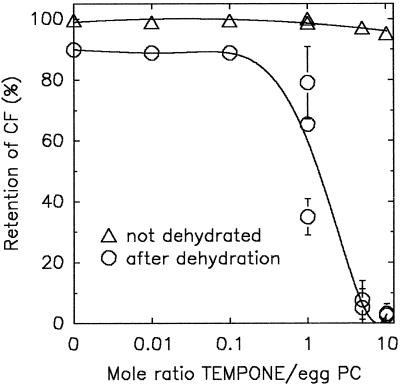
If amphipathic substances in general are responsible for the transient leakage from dry anhydrobiotes during rehydration, then it should be possible to simulate this in liposomes as a model system. It was shown in Figure 8 that TEMPONE can partition into eggPC liposomes upon dehydration. Figure 9 shows the effect of such a partitioning of TEMPONE as a model amphipathic substance on the leakage of CF from eggPC liposomes. When 30 μL of a lyoprotected liposome suspension without TEMPONE was dried for 3 h in a stream of dry air, CF retention was close to 90% upon rehydration. However, when dried in the presence of TEMPONE (at a molar ratio of TEMPONE:eggPC of 1 and higher), CF leakage was considerable. This leakage occurred during rehydration and not during drying, because no green fluorescence was visible in the dried liposomes. The lyoprotective Suc matrix (Suc:eggPC = 5:1) was always present in these experiments to prevent complete leakage (Crowe et al., 1987). Virtually no encapsulated CF was lost from the TEMPONE-treated liposomes when they were kept hydrated for the 3-h period. These data clearly indicate that partitioning of the amphipathic compound into membranes during drying can cause leakage from the liposomes during rehydration.
Figure 9.
Retention of CF by eggPC liposomes that were dried for 3 h in a stream of dry air (3% RH) at 24°C in the presence of Suc (mass ratio of Suc:eggPC = 5:1) and varying amounts of TEMPONE. The CF retention of the nondried controls kept for 3 h at 24°C is also indicated.
Pollen Amphipath-Induced Leakage from Desiccated EggPC Liposomes
The extent to which endogenous amphipaths from anhydrobiotic organisms are also able to cause leakage is shown in Table I. Endogenous amphipaths from T. latifolia pollen were obtained by two different methods: from an organic solvent extract and from an aqueous extract. Aliquots of these extracts were added to eggPC liposomes. Both extracts resulted in leakage of CF during rehydration of the dried liposomes, although there was also some leakage observed without drying. The change of color from yellow-orange to green during drying of the droplets containing the amphipath-treated liposomes indicates that some CF leakage had occurred before the liposomes were dry. These data indicate how effective endogenous amphipaths can be at increasing plasma membrane permeability during dehydration, leading to considerable leakage during rehydration.
Table I.
Retention of CF by eggPC liposomes as influenced by Suc and extracts of amphipaths from T. latifolia pollen
| Addition | CF Retention
|
|
|---|---|---|
| Vesicles dried and rehydrated | Vesicles kept hydrated | |
| % | ||
| Control (1 mm Tes) | 15 | 98 |
| Suc (Suc:eggPC = 5:1) | 86 | 99 |
| Suc plus organic solvent extract | 39 | 91 |
| Suc plus aqueous extract | 35 | 80 |
Liposomes were air dried and rehydrated or kept hydrated. The extracts of amphipaths were obtained by organic solvent extraction or by aqueous extraction, as described in “Materials and Methods,” and suspended in water. The aqueous extract used was the fraction that was eluted from reversed-phase (C18) cartridges by 50% methanol:water (v/v).
Is the TEMPONE-Induced Increase of Liposome Permeability Transient?
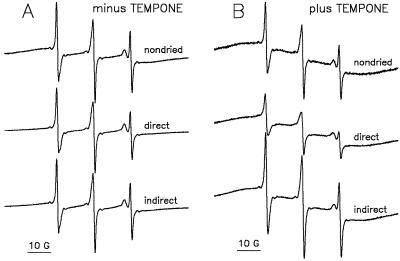
Increased permeability of the pollen plasma membranes during rehydration was shown to be transient (Fig. 3). Therefore, we also expected the high permeability of TEMPONE-treated liposomes at rehydration to be transient after repartitioning of the amphipathic molecules back into the aqueous phase. Figure 10 shows the effects of direct and indirect application of TEMPONE/ferricyanide to the liposomes on the shape of EPR spectra, and indicates that this expectation is correct. In a control experiment (Fig. 10A), the permeability of eggPC liposomes without previous TEMPONE treatment was tested. Compared with the TEMPONE spectrum of the fresh liposome sample, that of the dried sample had a slightly reduced water peak on direct imbibition in TEMPONE/ferricyanide solution, which was restored when rehydration was first in water, and then (after a few minutes) by the addition of a TEMPONE/ferricyanide solution. This slightly reduced water peak resulting from the penetration of some ferricyanide into the liposomes upon rehydration corresponds to the approximately 10% leakage of CF from the control liposomes in Figure 9 (molar ratio of TEMPONE:eggPC = 0).
Figure 10.
EPR spectra of TEMPONE in suspensions of eggPC liposomes containing Suc (mass ratio of Suc:eggPC = 5:1). The suspensions (10-μL droplets) were dried in the absence (A) or presence (B) of TEMPONE (molar ratio of TEMPONE:eggPC = 1:1). Dry samples without previous TEMPONE (A) were rehydrated in TEMPONE/ferricyanide (1 mm:120 mm; direct) or first in water, followed after a few minutes by TEMPONE/ferricyanide (indirect; final concentration 1 mm:120 mm). Samples with TEMPONE administered before drying (B) were diluted in 300 mm ferricyanide (the nondried and the direct treatment) or first in water for a few minutes followed by 600 mm ferricyanide. The final molar ratio of TEMPONE/ferricyanide was in either case approximately 1:120.
When liposomes were dried in the presence of TEMPONE (molar ratio of TEMPONE:eggPC = 1:1), the aqueous contribution to the TEMPONE spectrum was low when the ferricyanide was added directly, and high (comparable with the nondried control) when it was added a few minutes after rehydration (Fig. 10B). We interpret this to mean that permeability of the liposomes for ferricyanide was high in the direct treatment but low in the indirect treatment after partitioning of the majority of TEMPONE molecules back into the aqueous phase. Apparently, after rehydration the TEMPONE-treated liposomes were still intact.
Relevance of Partitioning to the Transient Leakage from Anhydrobiotes during Rehydration
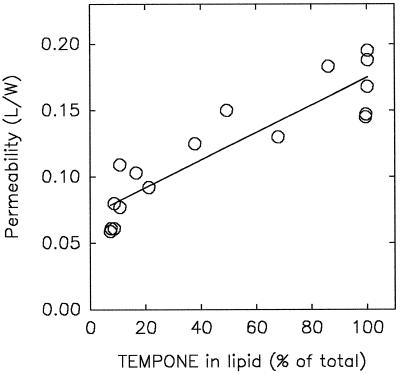
We have made an attempt here to explain the mechanism of the high plasma membrane permeability of anhydrobiotes during rehydration using in situ EPR of stable nitroxide spin probes in pollen as a model organism. The permeability of rehydrating pollen strongly decreases with increasing initial moisture content (Fig. 3), and coincides with repartitioning of the added amphipathic spin probes from the lipid phase into the aqueous cytoplasmic phase during rehydration (Fig. 4). The correlation between plasma membrane permeability and partitioning expressed as percentage of spin probe in the lipid phase is shown in Figure 11.
Figure 11.
Correlation between plasma membrane permeability at the onset of rehydration of T. latifolia pollen having different initial moisture content (data from Fig. 3, direct) and the associated percentage of amphiphilic TEMPONE partitioned in the lipid phase of the pollen. The percentage TEMPONE in the lipid phase was calculated from the equation fitting the rehydration curve of Figure 4A.
Liposome experiments were conducted to extend the evidence of the relationship between partitioning and permeability from a correlative level to a more causal level. It was shown that when used as a model amphipath, TEMPONE causes considerable leakage (Fig. 9) from rehydrating eggPC liposomes in which it had partitioned during drying (Fig. 8). However, the leakage occurred only at a high molar ratio of TEMPONE:eggPC (1:1 and higher). Nondried liposomes did not leak. We have demonstrated here that the leakage was caused solely by the presence of TEMPONE during drying. After full rehydration the liposomal membranes closed up again (Fig. 10) in a manner similar to that which occurred in fully rehydrated pollen, as concluded from the reduced permeability of the plasma membranes (Fig. 3). It is tempting to assume that the partitioning behavior of the spin probes reflects similar behavior of endogenously occurring amphipaths in the pollen, which is responsible for imbibitional leakage.
Indeed, endogenous amphipaths extracted from the pollen also caused leakage at the appropriate dilution, much more during drying and rehydration than when the liposomes were kept hydrated (Table I). An important question that arises is whether sufficient endogenous amphipaths are available to generate the imbibitional leakage in situ. At a phospholipid content of approximately 6% on a DW basis in the pollen (Hoekstra et al., 1992c), the amphipaths would have to be present at a few percent of the DW (depending on their Mr). This is entirely feasible, considering the large quantities of amphipaths such as flavonoids that are present in pollens and seeds (Gorobets et al., 1982; Wiermann and Vieth, 1983; Ceska and Styles, 1984; Liao et al., 1989; Zerback et al., 1989; Rizk et al., 1992; Ylstra et al., 1992; Vogt and Taylor, 1995).
Amphipathic substances may fluidize membranes, disturb the packing order of membrane components, and increase permeability (Maher and Singer, 1984; Casal et al., 1987). There is circumstantial evidence that such fluidization occurs when anhydrobiotic organisms are desiccated. Previously, we used Fourier-transformed IR data to describe the phase behavior of membranes in dehydrating seeds (Hoekstra et al., 1993) and leaves of the resurrection plant Craterostigma plantagineum (Hoekstra et al., 1997), and these data may support the fluidization hypothesis. The peak position of the CH2 symmetric stretching vibration in the gel phase at low temperature was approximately 1 wave number higher in the dry specimens than in the hydrated controls (Table II). Consequently, the change in wave number with the phase transition was less extensive in organisms when they were dry than when they were hydrated. This means that the vibrational freedom of the CH in the acyl chains is higher in the dry state than in the hydrated state in the gel phase. We interpret this as fluidization of the dry bilayer in situ caused by partitioning. In contrast, there was no difference between hydrated and lyophilized isolated membranes with respect to band position (wave number) in the gel phase, nor between hydrated and lyophilized eggPC liposomes (Table II). During the isolation of membranes the amphipathic substances are likely to have partitioned to the isolation medium.
Table II.
Band position of the CH2 symmetric stretching vibration in Fourier-transformed IR spectra of hydrated and dried specimens at −30°C (below the onset of the phase transition)
| Specimen | Band
Position
|
|
|---|---|---|
| Hydrated (fresh) | Dried or freeze dried | |
| cm−1 | ||
| Alfalfa | 2851.9 | 2853.0 |
| C. plantagineum | ||
| Intact leaf | 2851.8 | 2852.5 |
| Isolated membranes | 2851.4 | 2851.4 |
| EggPC liposomes | 2851.3 | 2851.1 |
Specimens used were leaves and isolated membranes of the desiccation-tolerant resurrection plant C. plantagineum (Hoekstra et al. 1997) and defatted, desiccation-tolerant alfalfa somatic embryos (Hoekstra et al., 1993). As a comparison, IR data are given for eggPC liposomes (F.A. Hoekstra, unpublished results). Dehydration of isolated membranes and liposomes was by freeze drying; dehydration of leaves and somatic embryos was by air drying.
Consequences of Partitioning of Endogenous Amphipaths for Desiccation Tolerance and Storage Stability
The transient leakage of solutes during rehydration may not be the only consequence of amphipath partitioning into membranes with dehydration. Very early during dehydration the decrease in the volume of the aqueous compartment leads to partitioning into the lipid environment (Fig. 4B). This may provide an early signaling of the imminent drought/desiccation stress by membrane fluidization and the associated changes in ion pump activity (for review, see Shinitzky, 1984). We believe that similar signaling may apply to osmotic and freezing stress. During dehydration TEMPO and TEMPONE completed partitioning into the lipid phase at relatively high moisture contents (0.8 and 0.4 g water g−1 DW, respectively; Figs. 4 and 7). Under these conditions there is still freezable water (Buitink et al., 1996), albeit of high viscosity (Leprince and Hoekstra, 1998).
If endogenous amphipaths have a partitioning behavior similar to TEMPONE/TEMPO, which is likely considering the mechanism discussed above, this could have important consequences for the storage of anhydrobiotes at slightly elevated moisture contents (e.g. between 0.2 and 0.7 g water g−1 DW). Under these conditions cells would have increased membrane permeability. This may be one of the reasons for the strongly reduced longevity of seeds in this moisture-content range (Priestley, 1986). It is reasonable to suppose that not only the plasmalemma but also the internal membranes have increased permeability, because amphipathic compounds may partition into any membrane type. Lipid bodies could then have a protective role in that they can harbor a considerable amount of the more apolar amphiphilic compounds.
The beneficial effect of endogenous amphipaths in anhydrobiotes may include the ability to depress the transition temperature of dry membranes and, thus, to prevent the formation of a gel phase (Hoekstra et al., 1997) and to act as antioxidants inside of membranes (Wang and Zheng, 1992; Terao et al., 1994; Saija et al., 1995). A partitioning behavior as outlined above would then be most effective to give automatic antioxidant protection to membranes in dehydrating organisms and, thus, to extend storage longevity. That these membrane-fluidizing compounds also increase permeability may be an inevitable disadvantage. Because of this dual effect of amphipath partitioning, we suggest that there is tight control of this phenomenon in desiccation-tolerant organisms.
Abbreviations:
- CF
5(6)carboxyfluorescein
- DW
dry weight
- EPR
electron paramagnetic resonance
- PC
phosphatidylcholine
- TEMPamine
4-amino-2,2,6,6-tetramethyl-1-piperidinyloxy
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxy
- TEMPONE
4-oxo-2,2,6,6-tetramethyl-1-piperidinyloxy
- Tm
gel phase to liquid-crystalline phase transition temperature
Footnotes
This work was supported by stipends from the Wageningen Agricultural University and the Netherlands Organization for Scientific Research to E.A.G.
LITERATURE CITED
- Buitink J, Walters-Vertucci C, Hoekstra FA, Leprince O. Calorimetric properties of dehydrating pollen. Analysis of a desiccation-tolerant and an intolerant species. Plant Physiol. 1996;111:235–242. doi: 10.1104/pp.111.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal HL, Martin A, Mantsch HH. Infrared spectroscopic characterization of the interaction of lipid bilayers with phenol, salicylic acid and o-acetylsalicylic acid. Chem Phys Lipids. 1987;43:47–53. doi: 10.1016/0009-3084(87)90016-8. [DOI] [PubMed] [Google Scholar]
- Ceska O, Styles ED. Flavonoids from Zea mays pollen. Phytochemistry. 1984;23:1822–1823. [Google Scholar]
- Crowe JH, Crowe LM, Carpenter JF, Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Membrane phase transitions are responsible for imbibitional damage in dry pollen. Proc Natl Acad Sci USA. 1989;86:520–523. doi: 10.1073/pnas.86.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:570–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Nguyen KHN, Crowe LM. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim Biophys Acta. 1996;1280:187–196. doi: 10.1016/0005-2736(95)00287-1. [DOI] [PubMed] [Google Scholar]
- Dzuba SA, Golovina EA, Tsvetkov YD. Spin-probe EPR study of some sugars in connection with desiccation tolerance in biological objects. Appl Magn Reson. 1993;5:31–37. [Google Scholar]
- Golovina EA, Tikhonov AN. The structural differences between the embryos of viable and nonviable wheat seeds as studied with the EPR spectroscopy of lipid-soluble spin labels. Biochim Biophys Acta. 1994;1190:385–392. doi: 10.1016/0005-2736(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Golovina EA, Tikhonov AN, Hoekstra FA. An electron paramagnetic resonance spin probe study of membrane permeability changes with seed aging. Plant Physiol. 1997;114:383–389. doi: 10.1104/pp.114.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorobets AV, Bandyukova VA, Shapiro DK. Flavonoid composition of the pollen (pollen pellets) from Salix caprea and S. alba. Chem Nat Compounds. 1982;18:747–748. [Google Scholar]
- Hammoudah MM, Nir S, Bentz J, Mayhew E, Stewart TP, Hui SW, Kurland RJ. Interactions of La3+ with phosphatidylserine vesicles binding, phase transition, leakage, 31P-NMR and fusion. Biochim Biophys Acta. 1981;645:102–114. doi: 10.1016/0005-2736(81)90517-4. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Bomal C, Tetteroo FAA (1993) Membrane phase transitions in dry seeds as measured by FT-IR spectroscopy. In D Come, F Corbineau, eds, Proceedings of the Fourth International Workshop on Seeds: Basic and Applied Aspects of Seed Biology, Angers, France, 20–24 July, 1992. ASFIS, Paris, pp 755–762
- Hoekstra FA, Crowe JH, Crowe LM. Effect of sucrose on phase behavior of membranes in intact pollen of Typha latifolia L., as measured with Fourier transform infrared spectroscopy. Plant Physiol. 1991;97:1073–1079. doi: 10.1104/pp.97.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, Crowe JH, Crowe LM. Germination and ion leakage are linked with phase transitions of membrane lipids during imbibition of Typha latifolia pollen. Physiol Plant. 1992a;84:29–34. [Google Scholar]
- Hoekstra FA, Crowe JH, Crowe LM, Van Bilsen DGJL (1992b) Membrane behaviour and stress tolerance in pollen. In E Ottaviano, DL Mulcahy, M Sari-Gorla, GB Mulcahy, eds, Angiosperm Pollen and Ovules. Springer Verlag, New York, pp 177–186
- Hoekstra FA, Crowe JH, Crowe LM, Van Roekel T, Vermeer E. Do phospholipids and sucrose determine membrane phase transitions in dehydrating pollen species? Plant Cell Environ. 1992c;15:601–606. [Google Scholar]
- Hoekstra FA, Van Roekel T. Desiccation tolerance of Papaver dubium L. pollen during its development in the anther. Possible role of phospholipid composition and sucrose content. Plant Physiol. 1988;88:626–632. doi: 10.1104/pp.88.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, Wolkers WF, Buitink J, Golovina EA, Crowe JH, Crowe LM. Membrane stabilization in the dry state. Comp Biochem Physiol. 1997;117A:335–341. [Google Scholar]
- Iwanami Y, Nakamura N. Storage in an organic solvent as a means for preserving viability of pollen grains. Stain Technol. 1972;47:137–139. doi: 10.3109/10520297209116468. [DOI] [PubMed] [Google Scholar]
- Kaplan J, Canonico PG, Caspary WJ. Electron spin resonance studies of spin-labeled mammalian cells by detection of surface membrane signals. Proc Natl Acad Sci USA. 1973;70:66–70. doi: 10.1073/pnas.70.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith AD, Snipes W. Viscosity of cellular protoplasm. Science. 1974;183:666–668. doi: 10.1126/science.183.4125.666. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Kumar JN, Blumenthal R, Flavin M. Interaction of tubulin with phospholipid vesicles. I. Association with vesicles at the phase transition. J Biol Chem. 1981;256:5879–5885. [PubMed] [Google Scholar]
- Leprince O, Hoekstra FA (1998) The responses of cytochrome redox state and energy metabolism to dehydration support a role for cytoplasmic viscosity in desiccation tolerance. Plant Physiol 118: in press [DOI] [PMC free article] [PubMed]
- Liao MC, Liu YL, Xiao PG. Studies on the flavonoids from the pollens of Typha davidiana, T. latifolia and T. angustata (pu huang) Acta Bot Sin. 1989;31:939–947. [Google Scholar]
- MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu LR. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Maher P, Singer SJ. Structural changes in membranes produced by the binding of small amphipathic molecules. Biochemistry. 1984;23:232–240. doi: 10.1021/bi00297a010. [DOI] [PubMed] [Google Scholar]
- Marsh D. Electron spin resonance: spin labels. In: Grell E, editor. Membrane Spectroscopy, Molecular Biology, Biochemistry and Biophysics, Vol 31. Berlin: Springer Verlag; 1981. pp. 51–142. [DOI] [PubMed] [Google Scholar]
- Miller RW. Osmotically induced removal of water from fungal cells as determined by a spin probe technique. Plant Physiol. 1978;62:741–745. doi: 10.1104/pp.62.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley DA (1986) Seed Aging: Implications for Seed Storage and Persistence in Soil. Comstock, Ithaca, NY
- Rizk AM, Ismail SI, Azzam SA, Wood G. Constituents of green beans Phaseolus vulgaris (lipids and flavonoids) Qatar Univ Sci J. 1992;12:69–72. [Google Scholar]
- Rowe ES, Zhang F, Leung TW, Parr JS, Guy PT. Thermodynamics of membrane partitioning for a series of n-alcohols determined by titration calorimetry: role of hydrophobic effects. Biochemistry. 1998;37:2430–2440. doi: 10.1021/bi9721602. [DOI] [PubMed] [Google Scholar]
- Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radical Biol Med. 1995;19:481–486. doi: 10.1016/0891-5849(94)00240-k. [DOI] [PubMed] [Google Scholar]
- Shimshick EJ, McConnell HM. Lateral phase separation in phospholipid membranes. Biochemistry. 1973;12:2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Shinitzky M. Membrane fluidity and cellular functions. In: Shinitzky M, editor. Physiology of Membrane Fluidity, Vol 1. Boca Raton, FL: CRC Press; 1984. pp. 1–51. [Google Scholar]
- Simon EW. Phospholipids and plant membrane permeability. New Phytol. 1974;73:377–420. [Google Scholar]
- Slade L, Levine H. Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Crit Rev Food Sci Nutri. 1991;30:115–360. doi: 10.1080/10408399109527543. [DOI] [PubMed] [Google Scholar]
- Terao J, Piskula M, Yao Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch Biochem Biophys. 1994;308:278–284. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- Tetteroo FAA, de Bruijn AY, Henselmans RNM, Wolkers WF, van Aelst AC. Characterization of membrane properties in desiccation-tolerant and -intolerant carrot somatic embryos. Plant Physiol. 1996;111:403–412. doi: 10.1104/pp.111.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T, Taylor LP. Flavonol 3-O-glycosyltransferases associated with petunia pollen produce gametophyte-specific flavonol diglycosides. Plant Physiol. 1995;108:903–911. doi: 10.1104/pp.108.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PF, Zheng RL. Inhibitions of the autoxidation of linoleic acid by flavonoids in micelles. Chem Phys Lipids. 1992;63:37–40. doi: 10.1016/0009-3084(92)90019-l. [DOI] [PubMed] [Google Scholar]
- Wiermann R, Vieth K. Outer pollen wall, an important accumulation site for flavonoids. Protoplasma. 1983;118:230–233. [Google Scholar]
- Ylstra B, Touraev A, Benito Moreno RM, Stöger E, Van Tunen AJ, Vicente O, Mol JNM, Heberle-Bors E. Flavonols stimulate development, germination and tube growth of tobacco pollen. Plant Physiol. 1992;100:902–907. doi: 10.1104/pp.100.2.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerback R, Bokel M, Geiger H, Hess D. A kaempferol 3-glucosylgalactoside and further flavonoids from pollen of Petunia hybrida. Phytochemistry. 1989;28:897–899. [Google Scholar]