Abstract
Objective:
Hygrophila spinosa (Acanthaceae) is traditionally used to treat urinary calculi. The present study aimed to evaluate the antiurolithiatic activity of methanolic extract of Hygrophila spinosa (Acanthaceae) in ethylene glycol induced nephrolithiasic rats.
Materials and Methods:
Methanolic extract of Hygrophila spinosa (HSME) (250 and 500 mg/ kg body weight) was administered orally to male Wistar albino rats. Ethylene glycol (EG) was used to induce nephrolithiasis. The parameters studied included water intake, urinary volume, urinary pH, urinary and kidney oxalate and calcium, urinary magnesium and serum uric acid.
Results:
Ethylene glycol feeding resulted in hyperoxaluria as well as increased renal excretion of calcium and serum uric acid along with decreased excretion of urinary magnesium. Treatment with HSME significantly reduced the elevated urinary oxalate, urinary calcium and serum uric acid with increase in reduced urinary magnesium. Ethylene glycol feeding also resulted in increased levels of calcium and oxalate in kidney which was decreased after the treatment with HSME. The increased deposition of stone forming constituents in the kidneys of ethylene glycol treated rats was significantly lowered by treatment with HSME.
Conclusion:
The results indicate that the aerial parts of Hygrophila spinosa are endowed with antiurolithiatic activity, thereby justifying its traditional claim.
KEY WORDS: Ethylene glycol, Hygrophila spinosa, kidney stones, nephrolithiasis
Introduction
Urinary stones have afflicted humankind since antiquity and can persist with serious medical consequences throughout a patient's lifetime. Kidney stone formation or urolithiasis is a complex process that results from a succession of several physico-chemical events including supersaturation, nucleation, growth, aggregation, and retention within the renal tubule.[1] The incidence of kidney stones has increased worldwide in the last five decades in association with the economic development. Epidemiological data collected during several decades shows that the majority of stones in the urinary system arise from a common component of urine, viz. calcium oxalate (CaOx), representing up to 80% of analyzed stones.[2] Currently, open renal surgery for nephrolithiasis is unusual and rarely performed since the introduction of extracorporeal shockwave lithotripsy (ESWL), which has become the standard procedure for eliminating kidney stones.
However, in addition to the traumatic effects of shock waves, persistent residual stone fragments and the possibility of infection suggest that ESWL may cause acute renal injury, a decrease in renal function and an increase in stone recurrence.[3,4] Though drug treatment has shown effect in many randomized trials, it is not without side effects, which are at times very serious.[5] Therefore, it is worthwhile to look for an alternative by using medicinal plants or phytotherapy. A number of plants have been used globally which claim efficient cure of urinary stones.[6] In the indigenous system of medicine, the seeds of Hygrophila spinosa claim to be useful in the treatment of urinary stones.[7–9] However, no systematic study has been reported so far confirming the antiurolithiatic property of methanolic extract of Hygrophila spinosa. In the present study, an effort has been made to establish the scientific validity for the antiurolithiatic property of Methanolic extract of Hygrophila spinosa (HSME) using ethylene glycol induced nephrolithiasis model in rats.
Materials and Methods
The whole plant Hygrophila spinosa syn. Asteracantha longifolia (Acanthaceae) was collected in month of September from the region of Indore, Madhya Pradesh, India and authenticated at Botanical Survey of India (BSI), Government of India, Ministry of Environment and Forests, Pune, India by Dr. P. G. Diwakar. A voucher specimen of the plant was deposited in the BSI herbarium under the number BSI/WC/Tech/2010/851. The aerial parts were dried in shade for two days and ground to a coarse powder.
Preparation of extract
Powder of dried aerial parts of Hygrophila spinosa was extracted with 80% methanol and concentrated. The concentrated mass was washed with petroleum ether several times to remove the resinous matter. The mass was then diluted with methanol and concentrated by drying to get the powdered form of the extract. The methanolic extract of Hygrophila spinosa (HSME) was stored tightly in a closed glass bottle in the refrigerator at 2 - 8°C.
Preliminary phytochemical screening
Preliminary phytochemical screening[10] revealed the presence of phenolic compounds, steroids, alkaloids, flavonoids, and triterpenoids in the extract.
Animal selection
For acute toxicity studies, healthy female Wistar rats (150 - 250 g) were selected and healthy adult male Wistar rats (150-250 g) were selected for the antiurolithiatic activity. The animals were acclimatized to standard laboratory conditions (temperature: 25 ± 2°C) and maintained on 12 hour light/dark cycle. They were provided with regular rat chow (Amrut Feed, Sangali, India) and water ad libitum. The animal care and experimental protocols were approved by the Institutional Animal Ethical Committee (IAEC).
Acute toxicity studies
The acute oral toxicity study was carried out as per the guideline 423 set by Organization for Economic Cooperation and Development (OECD) received from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). One-tenth of the median lethal dose (LD50) was taken as an effective dose[11].
Ethylene glycol induced urolithiasis model
Male rats of the Wistar strain (150 - 250 g) were housed in metabolic cages 3 days prior to the start of the experiment for acclimatization. They were fed regular chow and had free access to tap water ad libitum. The animals were then divided into five groups of six rats each. Group I was used as control which received distilled water. Ethylene glycol (EG) 0.75% was added in drinking water of all groups except control for 28 days to induce a chronic low grade hyperoxalluria and generate CaOx deposition into kidneys. Group II, was given ethylene glycol (EG) 0.75% only, and was used as untreated nephrolithiasic rats. Groups III and IV were used as treated nephrolithiasic rat groups which received HSME at dose of 250 mg and 500 mg per kg body weight, respectively, for 28 days along with EG 0.75%. Group V received a standard antiurolithiatic drug, aqueous solution of cystone (750 mg/kg body weight ) obtained from the market and prepared in distilled water along with EG 0.75% for 28 days.[12,13] All extracts were given once daily by oral route. At the end of the experimental study, blood was withdrawn by retro-orbital route under ether anesthesia. After blood withdrawal, animals were sacrificed by cervical decapitation, the kidneys were harvested from the animals and fixed with formalin.
Assessment of antiurolithiatic activity
Collection and analysis of urine
All animals were kept in individual metabolic cages and 24 hour urine samples were collected in presence of sodium azide as an antibacterial agent on the 7th, 14th, 21st and 28th day. Animals had free access to drinking water during the study period. Urinary components, like water intake, urinary volume and pH were noted. Urine was analyzed for calcium, magnesium and oxalate content. Urinary oxalate was estimated according to the method described by Hodgkinson et al. with some modifications.[12] Briefly, 1 ml of urine was acidified with concentrated Nitric acid (HNO3) to solubilize crystals and then adjusted to pH 7 by Sodium hydroxide (NaOH) in the presence of color indicator, the bromothymol blue. About 2 ml of saturated Calcium sulfate (CaSO4) and 14 ml of pure ethanol were added to precipitate oxalate overnight. The samples were centrifuged at 450 × g for 10 minutes and then filtered. The precipitate obtained was solubilized in 10 ml of water acidified by 2 ml of concentrated sulfuric acid. The samples were titrated by a solution of Potassium permanganate (KMnO4).
Serum analysis
Serum was separated by centrifugation at 3000 × g for 10 minutes and analyzed for serum uric acid.[14]
Kidney homogenate analysis
The abdomen was cut open to remove both the kidneys from each animal. Isolated kidneys were cleaned and preserved in 10% neutral formalin. The kidneys were then dried at 80° C in a hot air oven. A sample of 100 mg of the dried kidney was boiled in 10 ml of 1 N hydrochloric acid for 30 minutes and homogenized. The homogenate was centrifuged at 2000 × g for 10 minutes and the supernatant was separated. The calcium and oxalate content in the kidney homogenate was determined.[12,15]
Data analysis
Results were expressed as mean ± Standard Error of Mean (SEM). Differences among mean of the groups were determined using one-way Analysis of Variance (ANOVA) followed by Dunnett's multiple comparison test. p < 0.05 was considered statistically significant.
Results
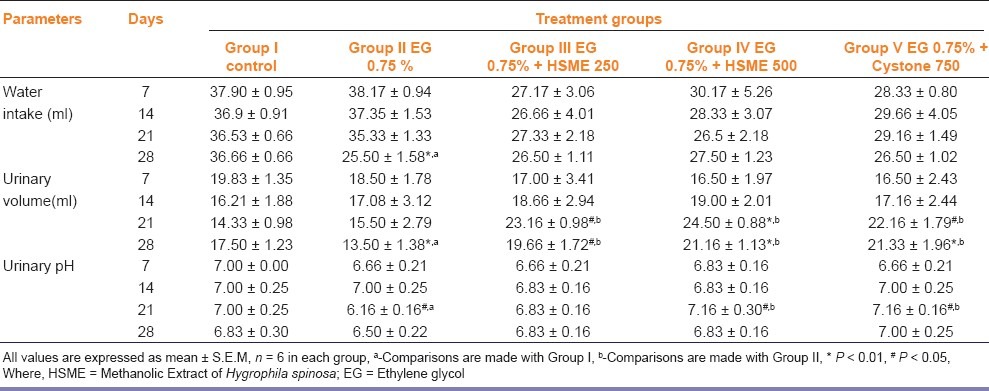
From the acute toxicity study, the LD50 cut-off dose was found to be 2000 mg/kg body weight for HSME. Hence, the therapeutic dose was taken as 200 mg/kg body weight for the extract. The values reported in Table 1 showed that water intake, urinary volume and pH were similar in all the groups at the beginning of the experiment. During the experiment, the values remained mostly constant for control group but increased for the groups receiving EG only, EG plus HSME and EG plus cystone. Both the water intake and urinary volume were significantly decreased in untreated nephrolithiasic rats (group II), as compared with control (group I) on day 28 (p < 0.01). Group III, IV and V showed a significant increase in urinary volume on days 21 and 28 as compared to Group II (P < 0.05). Urinary pH remained constant for control group throughout the experiment, while group II showed significant decrease as compared to control on day 21 (P < 0.05). Treatment with HSME 500 mg/kg and cystone 750 mg/kg elevated the decreased urinary pH to normal.
Table 1.
Effect of methanolic extract of Hygrophila spinosa (HSME) on parameters of urinary excretion in control and experimental animals
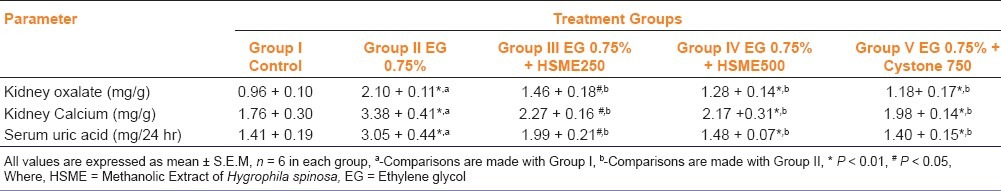
In the present study, chronic administration of 0.75% (v/v) ethylene glycol aqueous solution for 28 days resulted in hyperoxaluria in male Wistar rats. Oxalate and calcium excretion were significantly increased (P < 0.01), whereas magnesium decreased in urine and kidney of EG treated animals group II as compared to group I [Tables 2 and 3]. However, supplementation with HSME at 250 and 500 mg/kg body weight and cystone 750 mg/kg significantly (P < 0.05) lowered the elevated levels of oxalate and calcium in urine and kidney as compared to the untreated group II animals [Tables 2 and 3]. Magnesium level in the standard and test group IV came close to normal and was comparable to the levels in the rats belonging to the untreated group II [Table 2]. Serum uric acid was significantly increased in calculi-induced animals (group II) indicating marked renal damage [Table 3]. The treatment with HSME (Group III and IV) and cystone (Group V) significantly (P < 0.01) lowered the elevated serum level of uric acid as compared to group II [Table 3].
Table 2.
Effect of methanolic extract of Hygrophila spinosa (HSME) on urinary parameters in control and experimental animals
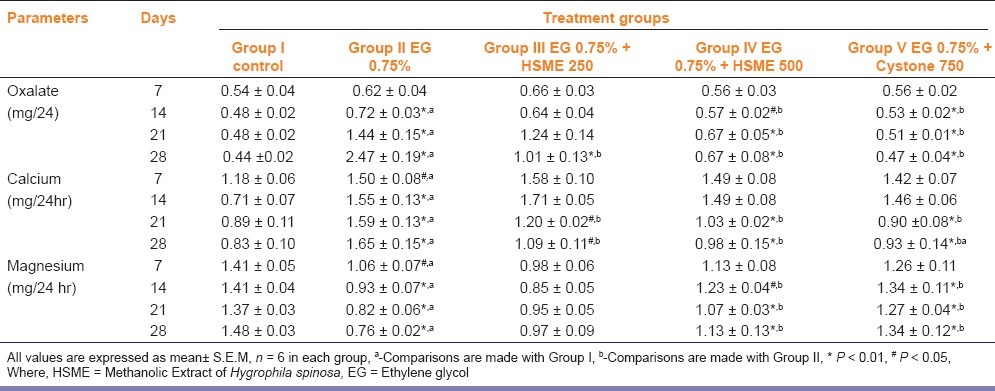
Table 3.
Effect of methanolic extract of Hygrophila spinosa (HSME) on kidney and serum parameters in control and experimental animals
Discussion
In the present study, male rats were selected to induce urolithiasis because the urinary system of male rats resembles that of humans, and previous studies have shown that the amount of stone deposition in female rats was significantly less.[16] Urinary supersaturation with respect to stone-forming constituents is generally considered to be one of the causative factors in kidney stones. Previous studies have observed that young male albino rats form renal calculi composed mainly of calcium oxalate in response to 14 day period of ethylene glycol (0.75%, v/v) administration.[12,16] The biochemical mechanisms for this process are related to an increase in the urinary concentration of oxalate. Stone formation in ethylene glycol and ammonium oxalate fed animals is caused by hyperoxaluria, which causes increased renal retention and excretion of oxalate.[16–18]
In the present study, oxalate and calcium excretion are progressively increased in calculi-induced animals (Group II). Since it is accepted that hyperoxaluria is a far more significant risk factor in the pathogenesis of renal stones than hypercalciuria,[19] the changes in urinary oxalate levels are relatively much more important than those of calcium. Increased urinary calcium is a factor favouring the nucleation and precipitation of CaOx or apatite (calcium phosphate) from urine and subsequent crystal growth.[18] However, HSME lower the levels of oxalate and calcium excretion, and restores the magnesium levels as well, thus reducing the risk of stone formation.
In urolithiasis, the glomerular filtration rate (GFR) decreases due to the obstruction to the outflow of urine by stones in the urinary system. Due to this, the waste products, particularly nitrogenous substances such as uric acid get accumulated in blood. Also, increased lipid peroxidation and decreased levels of antioxidant potential have been reported in the kidneys of rats supplemented with a calculi-producing diet. In this context, oxalate has been reported to induce lipid peroxidation and to cause renal tissue damage by reacting with polyunsaturated fatty acids in cell membrane.[11] In calculi-induced rats (Group II), marked renal damage was seen as indicated by the elevated serum levels of uric acid. Urinary volume decreased in rats receiving EG on day 28. The treatment with Hygrophila spinosa causes increase in urine volume in both test groups III and IV at week 3. Overall, the data presented in this current paper indicates that the administration of Hygrophila spinosa extract to experimentally CaOx-induced nephrolithiasic rats reduced the deposition of crystals into kidneys, confirming its antilithiatic effect. It can be concluded from the present study that HSME possesses significant antilithiatic activity, and it may prove to be effective for the treatment of kidney stones. Further studies are necessary to clarify the mechanism underlying this effect.
Footnotes
Source of Support: Nil.
Conflict of Interest: No.
References
- 1.Laroubi A, Touhami M, Farouk L, Zrara I, Aboufatima R, Benares A, et al. Prophylaxis effect of Trigonella foenum graecum L. seeds on renal stone formation in rats. Phytother Res. 2007;21:921–5. doi: 10.1002/ptr.2190. [DOI] [PubMed] [Google Scholar]
- 2.Gerstenbluth RE, Resnick MI. Medical management of calcium oxalate urolithiasis. Med Clin North Am. 2004;88:431–42. doi: 10.1016/S0025-7125(03)00171-8. [DOI] [PubMed] [Google Scholar]
- 3.Bouanani S, Henchiri C, Migianu-Griffoni E, Aouf N, Lecouvey M. Pharmacological and toxicological effects of Paronychia argentea in experimental calcium oxalate nephrolithiasis in rats. J Ethnopharmacol. 2010;129:38–45. doi: 10.1016/j.jep.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 4.Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician. 2001;63:1329–38. [PubMed] [Google Scholar]
- 5.Sur RL, Preminger GM. Medical treatment: Worthwhile and When? EAU Update Ser. 2005;3:10–6. [Google Scholar]
- 6.Vidya L, Lenin M, Varalakshmi P. Evaluation of the effect of triterpenes on urinary risk factors of stone formation in pyridoxine deficient hyperoxaluric rats. Phytother Res. 2002;16:514–8. doi: 10.1002/ptr.940. [DOI] [PubMed] [Google Scholar]
- 7.Nadkarni AK. Indian Materia Medica. 2nd ed. Mumbai: Popular Prakashan; 2007. pp. 668–9. [Google Scholar]
- 8.Khare CP. Indian Medicinal Plants: An illustrated dictionary. 3rd ed. New York: Springer publication; 2007. pp. 317–8. [Google Scholar]
- 9.Kshirsagar AD, Ingale KG, Vyawahare NS, Thorve VS. Hygrophila spinosa: A comprehensive review. Pharmacogn Rev. 2010;8:167–71. doi: 10.4103/0973-7847.70912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khandelwal KR. Practical Pharmacognosy-Techniques in Experiments. 8th ed. Pune: Nirali prakashan; 2001. pp. 149–53. [Google Scholar]
- 11.Karadi RV, Gadge NB, Alagawadi KR, Savadi RV. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105:306–11. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92:137–40. doi: 10.1046/j.1464-410x.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- 13.Mitra SK, Gopu Madhavan S, Venkataranganna MV, Sundaram R. Effect of cystone a herbal formulation on glycolic acid-induced urolithiasis. Phytother Res. 1998;12:372–4. [Google Scholar]
- 14.Cameron MA, Sakhaee K. Uric acid nephrolithiasis. Urol Clin North Am. 2007;34:335–46. doi: 10.1016/j.ucl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Betanabhatla KS, Khristina AJ, Sundar BS, Selvakumar S, Saravanan KS. Antilithiatic activity of Hibiscus safdaria Linn. on ethelyne glycol induced lithiasis in rats. Nat Prod Rad. 2009;8:43–7. [Google Scholar]
- 16.Selvam R, Kalaiselvi P, Govindaraj A, Murugan V, Sathishkumar AS. Effect of A. lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res. 2001;43:89–93. doi: 10.1006/phrs.2000.0745. [DOI] [PubMed] [Google Scholar]
- 17.Selvam R, Adhirai M. Vitamin E pretreatment prevents cyclosporine A-induced crystal deposition in hyperoxaluric rats. Nephron. 1997;75:77–81. doi: 10.1159/000189503. [DOI] [PubMed] [Google Scholar]
- 18.Muthukumar A, Selvam R. Effect of depletion of reduced glutathione and its supplementation by glutathione monoester on renal oxalate retention in hyperoxaluria. J Nutr Biochem. 1997;8:445–50. [Google Scholar]
- 19.Tisselius HG. Solution chemistry of supersaturation. In: Coe FL, Favus MJ, Pak CY, Parks JH, Preminger GM, editors. Kidney Stones: Medical and Surgical Management. Philadelphia: Lippincott Raven; 1996. p. 33. [Google Scholar]


