Abstract
A 43 year old male patient, known case of multidrug resistant tuberculosis, was prescribed antitubercular drugs: kanamycin, levofloxacin, ethionamide, terizidone, Para-Aminosalicylate Sodium (PAS), pyrazinamide and pyridoxine. After 4 months of treatment, the patient developed a lump in the right breast which was approximately around 3 × 3 cm in size, tender on palpation, and not fixed to the underlying tissues. Ultrasonography (USG) revealed a hypoechoic mass of size 2.5 × 0.92 × 2.6 cm in the right breast region behind the nipple without any infiltration to the deeper structures. Gynecomastia due to ethionamide was suspected and the patient was advised anti-inflammatory drugs for 5 days without any change in drug therapy. The pain subsided; however, the nodule remained. Treatment was continued without any change till the patient stopped using the drugs on his own and without doctor's consent. Within a week of stopping of treatment the nodule also disappeared.
KEY WORDS: Adverse drug reaction, Ethionamide, Gynecomastia
Introduction
Second line antitubercular drug ethionamide is 2-ethylpyridine-4-carbothioamide, a congener of thioisonicotinamide. The drug is a prodrug and mycobacterial Etha A, a nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) -specific, flavin adenine dinucleotide (FAD)-containing monooxygenase, converts it to a sulfoxide, and then to 2-ethyl-4-aminopyridine. Ethionamide inhibits mycobacterial growth by inhibiting the activity of the inhA gene product, the enoyl- acyl-carrier-protein (ACP) reductase of fatty acid synthase II. This is the same enzyme that activates isoniazid inhibits. This results in inhibition of mycolic acid biosynthesis and consequently impairs cell wall synthesis. Ethionamide is a part of the World Health Organization (WHO) regime for multidrug resistant tuberculosis (MDR-TB) along with ethambutol, prothionamide, ofloxacin, pyrazinamide, aminoglycoside and capreomycin for six months followed by ethambutol, ethionamide, prothionamide, ofloxacin and pyrazinamide for further 12 to 18 months. ‘Its use is increasing with an increase in the prevalence of resistant tuberculosis. The most common adverse effects associated with this drug are anorexia, nausea and vomiting, gastric irritation and a variety of neurologic symptoms. Severe postural hypotension, mental depression, drowsiness and asthenia are common and convulsions and peripheral neuropathy are rare.[1] One of the rarest adverse effects of this drug is gynecomastia. Most standard text books and reference books of pharmacotherapeutics have not mentioned about it,[2] and to the best of our search, we could not find any case reports available from a search made in Pubmed. In this case report, we have presented the case of unilateral painful Gynecomastia, which on the WHO causality assessment scale is probably/likely associated with the use of ethionamide.[3]
Case Report
A 43 year old male patient presented to the Department of Pulmonary Medicine of Dr. R. P. Government Medical College, Kangra in the month of August 2010 with a history of consumption of Category I antituberculosis treatment from 19.12.2008. This was followed by Category II antituberculosis treatment from 10.03.2009, as Category I failed. Patient relapsed and was again put in Category II from 16.04.2010. The drug treatment failed again and the patient was referred to department of Pulmonary Medicine. Culture and sensitivity testing for Mycobacterium tuberculosis revealed resistance to streptomycin, isoniazid, rifampicin and ethambutol. Pretreatment evaluation was done and patient was put on Injection kanamycin 750 mg intramuscular (IM) daily 6 days a week, tablet levofloxacin 750 mg once daily, tablet ethionamide 250 mg twice daily, capsule terizidone 250 mg twice daily, gr. PAS 10 G once at bed time, tablet pyrazinamide 1500 mg once daily and tablet pyridoxine 100 mg once at bed time from 28.10.2010. At the end of one month of treatment, patient became sputum smear and culture negative. On 24.02.2011, the patient complained of pain in the right breast due to friction by clothes since one week, and noticed a swelling of the nipple which was tender on palpation. Physical examination revealed a 3 × 3 cm tender nodule behind the right nipple that was not fixed to underlying tissues [Figure 1]. The local temperature was slightly raised without much change of color. Ultrasonography (USG) revealed a hypoechoic mass of size 2.5 × 0.92 × 2.6 cm in the right breast region behind the nipple, without any infiltration to the deeper structures. Gynecomastia due to ethionamide was suspected. The relevant investigations were found to be within the normal range. Patient was advised diclofenac sodium 50 mg once daily for 5 days and for another 5 days if required; without any change in the antituberculosis regime. Patient was asked to report if the pain did not subside. On the next follow up, pain was absent. The nodule was of the same size; however, without the accompanying tenderness. The treatment was continued. The patient then stopped the treatment on his own on completion of one year of therapy without doctor's consent and informing the doctor. Within a week of stopping the treatment he reported that the nodule had also disappeared.
Figure 1.
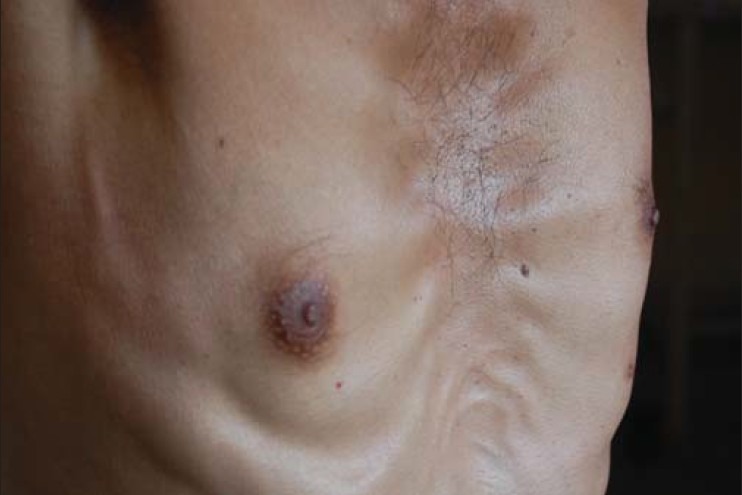
Gynecomastia in a male patient on ethionamide
Discussion
Gynecomastia is defined as a benign proliferation of the male breast glandular tissue. The term comes from the Greek word “gyné” (stem gynaik) meaning “woman” and “mastós” meaning “breast”. Breast prominence can result from hypertrophy of breast tissue, chest adipose tissue (fat) or skin, and is usually a combination of all. Breast prominence solely due to excessive adipose tissue is often termed as pseudogynecomastia. Prevalence of symptomatic gynecomastia is markedly lower than the asymptomatic gynecomastia. The causes of gynecomastia remain uncertain, although it has generally been attributed to an imbalance of sex hormones or lack of tissue responsiveness to them. Further, a root cause is rarely determined for individual cases.[4] One of the most important causes of gynecomastia is drugs. Drug-induced gynecomastia is common and might account for up to a quarter of all cases.[5] A large number of drugs have been incriminated in causing gynecomastia.[6] Most of these have only been implicated by means of case reports, which document a temporal association of the offending drug and occurrence of gynecomastia. The mechanisms by which many of these drugs induce gynecomastia are not yet understood; however, an imbalance between estrogen and testosterone has been suggested.[7]
Amongst the antituberculosis drugs, isoniazid was the first found to be causing gynecomastia. In 1953, a report from France implicated this drug as a cause of gynecomastia.[8] Another report also described painless, bilateral, gynecomastia in a 52 year old man who was receiving 600 mg of isoniazid daily (10 mg/kg/day) for four months.[9] The authors hypothesized that a disturbance in vitamin B6 complex activation in liver could have caused an alteration in estrogen-androgen metabolism. It has also been postulated that isoniazid may act “by means of a re-feeding mechanism in men with tuberculosis”. After isoniazid, thiacetazone has been suggested as a cause of gynecomastia in a single report.[10] A causality assessment using the WHO Uppsala Monitoring Centre scale showed that the present adverse reaction is probably/likely associated with the use of ethionamide. Similar reports are not available in published literature and an awareness of this possible adverse drug reaction due to ethionamide might guide the prescribing doctors in their choice of the antitubercular drugs.
Footnotes
Source of Support: Case Record.
Conflict of Interest: None.
References
- 1.Gumbo T. Chemotherapy of Tuberculosis, Mycobacterium Avium Complex Disease, and Leprosy. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New Delhi: McGraw Hill; 2011. pp. 1549–70. [Google Scholar]
- 2.Sweetman SC, editor. Martindale: The Complete Drug Reference. 36th ed. London: Pharmaceutical Press; 2011. pp. 275–6. [Google Scholar]
- 3.The use of the WHO-UMC system for standardized case causality assessment. [Last accessed on 2012 May 27]. Available from: http://www.WHO-UMC.org/graphics/4409.pdf .
- 4.Braunstein GD. Aromatase and gynecomastia. Endocr Relat Cancer. 1999;6:315–24. doi: 10.1677/erc.0.0060315. [DOI] [PubMed] [Google Scholar]
- 5.Bembo SA, Carlson HE. Gynecomastia: Its features, and when and how to treat it. Cleve Clin J Med. 2004;71:511–7. doi: 10.3949/ccjm.71.6.511. [DOI] [PubMed] [Google Scholar]
- 6.Thompson DF, Carter JR. Drug-induced gynecomastia. Pharmacotherapy. 1993;13:37–45. [PubMed] [Google Scholar]
- 7.McKiernan JF, Hudd D. Breast development in the newborn. Arch Dis Child. 1981;56:525–9. doi: 10.1136/adc.56.7.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guinet P, Garin JP, Morpex A. A case of gynecomastia in pulmonary tuberculosis during the course of treatment with isonicotinic acid hydrazide. Lyon Med. 1953;85:281–4. [PubMed] [Google Scholar]
- 9.Bergogne-Berezin E, Nouhouayi A, Letonturier P, Thibault P, Tourneur R. Gynecomastia caused by isoniazid: Value of determination of inactivation of phenotype. Nouv Presse Med. 1976;5:213–4. [PubMed] [Google Scholar]
- 10.Chunhaswasdikul B. Gynecomastia in association with administration of thiacetazone in the treatment of tuberculosis. J Med Assoc Thai. 1974;57:323–7. [PubMed] [Google Scholar]


