Pure water without traditional contrast media can be used as an effective in vivo MR imaging contrast agent by enhancing its MR signal with dynamic nuclear polarization (DNP) hyperpolarization under continuous flow.
Abstract
Purpose:
To assess the feasibility of a perfusion magnetic resonance (MR) imaging technique that uses Overhauser dynamic nuclear polarization (DNP) to provide contrast during the continuous delivery of hyperpolarized water in rats.
Materials and Methods:
Protocols approved by the local institutional animal care and use committees were followed. Twelve male Wistar rats were anesthetized and prepared by placing injection tubing in the subcutaneous layer (n = 3), peritoneum (n = 3), aorta (n = 3), or carotid artery (n = 3). Water was hyperpolarized by means of Overhauser DNP in the 0.35-T fringe field of a 1.5-T MR imaging magnet by using a custom-built system to continuously deliver radical-free hyperpolarized water to the subject. Fast gradient-echo and spoiled gradient-recalled-echo MR imaging sequences were used. The signal-to-noise ratio (SNR) of the images was calculated and compared.
Results:
Images showed greatly altered SNR and enhanced flow contrast at all injection locations. For subcutaneous and intraperitoneal injections, the water perfusion trajectory was observed for approximately 5 seconds after injection. Flow through a 4.2-cm length of artery was seen during intra-aortic injection. The right hemisphere of the brain was seen during injection into the right carotid artery. Images with hyperpolarized water had greatly altered SNR compared with images without injection or with the injection of nonhyperpolarized water, with a range of 13%–27% for the carotid and 444%–2900% for the other regions.
Conclusion:
Perfusion contrast for MR imaging can be obtained by continuously infusing hyperpolarized water, providing localized angiography or brain perfusion information in vivo for rat models.
© RSNA, 2012
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.12111804/-/DC1
Introduction
The ability to monitor flow and perfusion is important in imaging for a wide range of biomedical problems. Magnetic resonance (MR) imaging techniques allow noninvasive spatial differentiation of flowing and static matter by using nonionizing radiofrequency radiation (1–3). Although these techniques are currently used in clinical practice, there is still a need for higher reader sensitivity and enhanced contrast on images (4). In addition, there are concerns about the safety of gadolinium-based contrast agents for certain groups of patients (5,6). These needs and problems motivate researchers to explore and develop new methods for imaging flow and perfusion that are relevantly applicable to human MR imaging. One such method is hyperpolarization, which creates substances with dramatically increased nuclear spin polarization (and thus, greatly enhanced MR signal intensity) to enhance the information and contrast available on an MR image. Currently, dissolution dynamic nuclear polarization (DNP) is the most popular and only commercially available hyperpolarization method for MR imaging applications and can be used for imaging the flow of discrete injections of hyperpolarized compounds (7). However, the time scale of dissolution DNP (lifetime of enhanced hyperpolarized carbon 13 [13C] signal, 5–60 seconds; repetition time, approximately 1 hour) usually limits its in vivo uses to the study of cellular metabolism with slow-relaxing 13C or nitrogen 15 (15N) nuclei (7–14). Dissolution DNP can be used to hyperpolarize water, but the relatively fast relaxation rate of water makes the injection of a single batch of hyperpolarized water an unattractive approach for perfusion imaging. Parahydrogen-induced polarization can also hyperpolarize the 13C signal of small molecules (repetition time, 5 minutes) (15–17), but this technique is unable to hyperpolarize water.
However, hyperpolarization by means of Overhauser DNP is well suited for continuous-flow enhancement of water, because this method rapidly (< 1 sec) transfers polarization from unpaired electrons to nuclei in the liquid state, increasing the MR signal intensity of the target nuclei by up to two orders of magnitude. The appendix (online) includes a glossary and detailed introduction to Overhauser DNP. Overhauser DNP of water can be used to enhance in vivo imaging in two different ways. One is the direct hyperpolarization of water inside the subject after the in vivo injection of nitroxide radicals, known as proton-electron double resonance imaging (18–20). This approach tracks the perfusion of radicals, not that of water, and requires the use of very low fields for hyperpolarization (< 20 mT) because electron excitation must be applied directly to the subject—with minimal heating. Alternately, Overhauser DNP can be performed outside the subject, followed by the injection of hyperpolarized water (21–23), allowing the use of standard clinical MR imaging (1.5 T). The fast polarization times allow for continuous delivery of hyperpolarized water, which is essential for monitoring flow and perfusion. Because the radicals required for DNP can be immobilized on beads and filtered from the injected water, the only substance injected is pure water in a hyperpolarized state, which retains all the physicochemical properties of water and thus, is safe to inject in moderate volumes. In addition, the hyperpolarization of water reaches a steady state in vivo, which eliminates the need for fast MR imaging sequences. This technique, known as remotely enhanced liquids for imaging contrast, has been previously shown in vitro (24,25). Thus, our purpose was to assess the feasibility of a perfusion MR imaging technique that uses Overhauser DNP to provide contrast during the continuous delivery of hyperpolarized water in rats.
Materials and Methods
Animal Surgery
All experiments were conducted according to protocols approved by our respective institutional animal care and use committees. Twelve male Wistar rats (weight, 200–400 g) were used, three in each group. Rats were anesthetized with 1% isoflurane via face mask. For subcutaneous injections, a 1-inch (1 inch = 2.54 cm) length of polymer tubing with 0.0625-inch (0.16 cm) outer diameter and a 0.010-inch (0.03 cm) inner diameter was inserted into the dorsum of each rat. For intraperitoneal injections, tubing and flow connectors were inserted into the abdomen. The subcutaneous and intraperitoneal injection groups were allowed to recover after the procedure. In experiments requiring vascular access, we used an MTV1 28-gauge catheter (Braintree Scientific, Braintree, Mass). A cannula was inserted into the abdominal aorta after blunt dissection of the retroperitoneal space through a midsagittal abdominal incision. Puncture was achieved with a 25-gauge needle, through which the catheter was advanced in an antegrade fashion; the needle was removed and the catheter was secured with suture. Similarly, a catheter was inserted in the right common carotid artery through a U-shaped incision of the ventral neck. After vascular access experiments, we euthanized the anesthetized rats. For all injection locations, water was injected at 1.5 milliliter per minute during the acquisition of two images (with and without DNP) and, in addition, for approximately 15 seconds before the start of each acquisition to reach a steady state. The amount of water injected for each image pair depended on the acquisition time: subcutaneous, 9 mL; intraperitoneal, 17 mL; aorta, 3.25 mL; carotid, 5.6 mL.
Hyperpolarization Technique
The equipment for these experiments has been described in detail elsewhere (24), so here we only give a brief overview. More details about the technique and equipment for Overhauser DNP are included in the Appendix (online).
Although water protons are by far the most sensitive nuclei for MR imaging, the sensitivity is still surprisingly low, because only approximately one millionth of the possible nuclear signal is used in a standard MR experiment. Here, we overcame the standard limit through hyperpolarization, for which a higher proportion of water protons is recruited to provide MR signal. This is done through a mechanism known as Overhauser DNP, which relies on the through-space dipolar interaction between an unpaired electron (usually a stable organic radical) and the nuclei of interest (here, the hydrogen 1 [1H] of water), mediated by molecular motions in the liquid state (26,27). The MR transition of the unpaired electron is continuously saturated with on-resonant radiation, resulting in the transfer of polarization from the electrons to the nuclei if efficient electron-nuclear cross relaxation is present. The increased DNP performance and relatively simple, readily available microwave components at 9.8 GHz led to the choice of 0.35 T for hyperpolarization (24). Imaging was conducted in a standard clinical 1.5-T MR imaging unit, and hyperpolarization was performed in the 0.35-T fringe field of the same magnet.
To hyperpolarize water with Overhauser DNP, the water must contact the stable radicals hosting an unpaired electron. Although the radicals can be simply dissolved in water, here we used radicals immobilized to solid beads so that water could flow over the beads during hyperpolarization and then be separated from the beads by a filter, producing radical-free hyperpolarized water. This provides several advantages. First, even though low concentrations of nitroxide radicals may not display great toxicity (28), injecting a radical solution could alter the observed contrast and would not permit the visualization of pure injected water. Second, the T1 of radical solutions is much shorter than that of pure water, leading to the rapid loss of hyperpolarized signal intensity during transfer from hyperpolarization to the imaging location. Most importantly, in this form, the method is directly applicable to future clinical studies because only pure water is injected, and the only relevant adverse health effects are due to the hypotonic nature of pure water. The immobilized radicals were synthesized according to a previous protocol (24) and given high DNP enhancement levels, comparable to those achieved with radical solutions.
The 0.35-T hyperpolarization location was 1.5 m from the imaging location, and water traveled this distance in approximately 1.3 seconds at the flow rate of 1.5 mL/min. Degassed deionized water was used for all experiments. Each trial was conducted by sequentially acquiring images with no injection (static), the injection of water, and the injection of hyperpolarized water.
MR Image Acquisition
All images were acquired with an unmodified GE 1.5-T 9.1 LX clinical MR imaging system (GE Healthcare, Waukesha, Wis). A GE 4-channel wrist coil was used for signal reception and the standard body coil was used for transmission. Because hyperpolarized water is continuously injected and reaches a steady state in the subject, fast imaging sequences are not required; instead, standard fast gradient-echo (Figs 1, 2) and spoiled gradient-echo recalled (Figs 3, 4) sequences were used. Figure 1 was acquired with a repetition time (TR) of 500 msec, an echo time (TE) of 2.4 msec, a 30-degree flip angle, and 3-mm section thickness. In the frequency direction, 256 points, and in the phase direction, 160 points were acquired with two acquisitions per phase encoding step (number of signals acquired, two), leading to an acquisition time of 2.7 min per image. At the injection rate of 1.5 mL/min, an enhanced and unenhanced image pair required the injection of 9 mL of water. Figure 2 had the same settings as those for Figure 1 except for: TE, 2.7 msec; section thickness, 2 mm; number of signals acquired, four, acquisition time, 5.4 minutes; and injection volume, 17 mL. In Figure 3, MR imaging parameters were: TR, 300 msec; TE, 3.1 msec; flip angle, 30-degree; section thickness, 1 mm; points, 256 × 160; number of signals acquired, one; acquisition time per image, 50 seconds; and injection volume, 3.25 mL. In Figure 4 the same settings were used as those from Figure 3, but with section thickness, 3.25 mm; and number of signals acquired, two; leading to a doubled acquisition time and injection volume.
Figure 1:
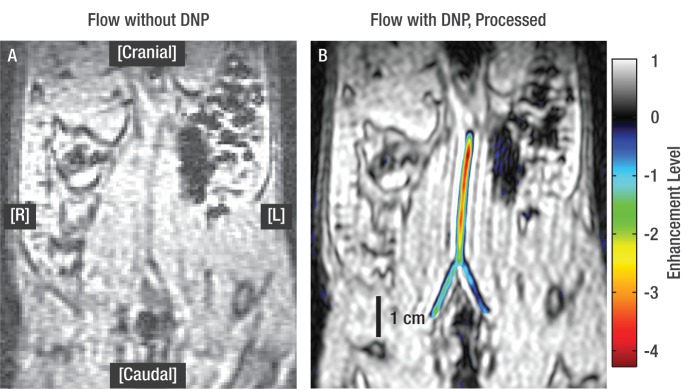
Sagittal fast gradient-echo images in rat model. A, Spin-density image acquired during injection of nonhyperpolarized (bulk) water serves as control image and shows no measureable contrast. B, Spin-density image acquired during injection of hyperpolarized water shows higher signal intensity than that of bulk water. Images A and B are shown at identical signal intensity and contrast levels. C, Processed image of hyperpolarized water injection shows enhanced, inverted signal intensity in color and unenhanced, bulk signal intensity in grayscale. Scale bar represents signal intensity and level of enhancement. Only transient path of water is shown, because hyperpolarized signal decays in approximately 5 seconds.
Figure 2:
Sagittal fast gradient-echo images of intraperitoneal hyperpolarized water injection in rat. A, Spin-density image of injection of nonhyperpolarized water, and, B-D, processed images of hyperpolarized water injection. Image C is the same section as that shown in A. Arrow marks end of injection tubing, and tubing connector was also placed inside peritoneum and is visible as black rectangle in lower left of each image. After water leaves injection tubing, it travels to enhanced region in section 2, then to sections 1 and 3. Path of water after this point cannot be seen because hyperpolarized signal has decayed (approximately 5 seconds) and becomes indistinguishable from bulk signal. Slice = section.
Figure 3:
Coronal spoiled gradient-recalled images of injection of hyperpolarized water into aorta of rat. A, Image acquired during injection of nonhyperpolarized water. B, Processed image shows injection of hyperpolarized water. Catheter is implanted into aorta at position located near top of image, and water inside catheter is not visible because of its small inner diameter (0.007 inches). Hyperpolarized water becomes visible where it enters aorta at top of enhanced region, then flows down aorta and enters two iliac arteries before continuing to femoral arteries.
Figure 4:
Axial spoiled gradient-recalled images of injection of water in right common carotid artery of rat, where each column represents a different section of the image. Top row shows image sections acquired during nonhyperpolarized water injection. Middle row shows sections acquired during injection of enhanced water and bottom row is subtraction image of top two rows. In C–F, attenuation of signal in right hemisphere of brain on injection of hyperpolarized water is visible (arrows, in C, E). Attenuation is due to dilution of negatively enhanced water with large volume of nonhyperpolarized nuclei. Dark lines in D and white lines in F (arrows) are presumed to be lateral ventricle. Dark appearance of ventricles is likely also due to dilution of enhanced signal.
DNP Contrast Mechanisms
The contrast from Overhauser DNP-enhanced water is manifested in two ways. First, the injected hyperpolarized water has greater signal intensity than bulk water already present in the subject, and thus, a higher signal intensity appears on a standard magnitude image. Second, the signal from hyperpolarized water has a phase opposite to that of bulk water (ie, the signal is inverted), which is most notable if the images are processed to include phase information. In addition, if the enhanced, inverted water is spread across a large region of nonhyperpolarized nuclei (positive phase), the net result is seen as a decrease in the overall signal intensity with no phase inversion.
MR Image Processing
To best show DNP contrast, the images acquired during injection of nonhyperpolarized and hyperpolarized water were presented in different forms. The control image without DNP, where the injection proceeded at 1.5 mL/min but DNP was inactive, was shown as a standard magnitude image (produced by the MR imaging unit). For all injection locations except the carotid artery, the raw data acquired during injection of hyperpolarized water were used to create a “processed” image to show the phase contrast provided by the enhanced, inverted signal. This was done by comparing the enhanced and unenhanced images to create a phase map. The DNP-enhanced magnitude image data were then multiplied by that of the phase map to add phase information. A custom color map from Matlab R2009a (The Mathworks, Natick, Mass) labeled any inverted signal with color that corresponded to intensity, and the images were scaled so that the maximum unenhanced signal equaled one. The processed images were zero-filled to 2048 × 2048 points to improve visual quality. The DNP-enhanced carotid images were processed with the GE imaging software and adjusted to equal intensity and contrast; the subtraction images were calculated in Matlab and again displayed with the GE software. The signal-to-noise ratio (SNR) was calculated from the MR imaging unit’s digital and communications in medicine files in Matlab as SNR = S/σ, where S is the average signal intensity from six adjacent pixels in the region of interest, and σ is the standard deviation of 400 pixels of noise from air adjacent to the subject. The region of interest was chosen to be either the area of highest intensity (subcutaneous, intraperitoneal, aorta) or lowest intensity (carotid) in the target area of the image with hyperpolarized water, and the same region of interest was used in all three images in each trial. Trial 1 is shown in Figures 1–4. All image evaluations were the consensus of the authors. The percentage of change in the SNR is the ratio of the SNR during injection of hyperpolarized water to the SNR with no injection, multiplied by 100.
Results
Images with hyperpolarized water had greatly altered SNR compared with that of images with no injection or injection of nonhyperpolarized water, ranging from 13%–27% for the carotid artery and 444%–2900% for the other injected regions (Table). In addition to altered SNR, the images with hyperpolarized water showed the expected transient path of water immediately after injection according to the contrast mechanisms described in the Methods section. In figures 1-3, images are shown both directly from the spectrometer software (grayscale, spin-density images) and after a custom processing method that combines images with and without DNP to obtain phase information (combined color and grayscale, processed images). For the subcutaneous injection (Fig 1) the hyperpolarized water was observed traveling along the inlet tube and exiting through the incision, and because hyperpolarized water decays in approximately 5 seconds (based on the longevity of enhanced signal in comparable studies [24] and the T1 of blood [29]), we can conclude that water left the subcutaneous area within this time. The intraperitoneal images (Fig 2) show the injected water spreading in the peritoneal space in a dependent fashion, collecting most prominently around the injection site and along the ventral side of the peritoneum. Figure 3 shows the travel of hyperpolarized water through the aorta and iliac and femoral arteries, where the total length of artery shown with DNP-enhanced water (aorta to femoral) was 4.2 cm. On injection of hyperpolarized water into the right common carotid artery (Fig 4), the signal from the entire right hemisphere of the brain was slightly attenuated and the signal from the expected location of the right lateral ventricles was strongly attenuated. This attenuation originated from the negative phase of the enhanced water, which when diluted with a large volume of nonhyperpolarized nuclei (positive phase), resulted in a net decrease of the observed signal. Because the appearance of the entire right hemisphere changed in addition to the delineation of the right lateral ventricle, our images suggested that the hyperpolarized water traveled from the right carotid artery to the peripherally located right cerebral arteries, then through the parenchyma of the right hemisphere to collect in the cerebrospinal fluid of the right lateral ventricle. This happens in a few seconds, relevantly implying that we could use this technique to visualize cerebral vasculature.
SNRs of Images Acquired with and without Hyperpolarized Water
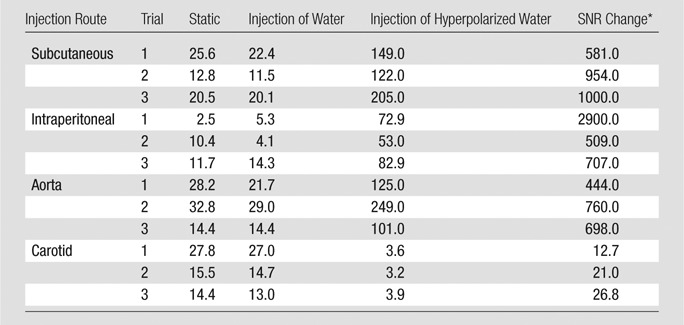
Data are percentages.
Discussion
We have shown that pure water, without traditional contrast media, can be used as an effective in vivo MR imaging contrast agent by dramatically enhancing its MR signal intensity with DNP hyperpolarization under continuous flow. In particular, the observation of brain perfusion contrast agent during carotid arterial injection of hyperpolarized water presents great relevant for probing brain perfusion and molecular passage across the blood-brain barrier. Furthermore, the appearance of hyperpolarized water in the interstitial-intracellular space of the posterior right hemisphere implies that the active or passive transport of water between intracerebral compartments, which is known to be affected by stroke, traumatic brain injury, or other ischemic episodes, might now be imaged in real time (30,31). Water is uniquely suited as a contrast agent for these applications because it readily crosses the blood-brain barrier. An additional advantage of the presented technique is its capability to obtain brain perfusion MR imaging contrast without the use of chemical contrast agents, because the known and relevant toxicity of existing contrast agents limits the long-term applicability of MR imaging diagnosis. In principle, this method can provide signal contrast similar in nature to arterial spin labeling but relevantly with higher contrast amplitude, because the MR signal of flowing water is only inverted in arterial spin labeling (2,32) but the signal is inverted and substantially enhanced with DNP.
Another relevant application of hyperpolarized water MR imaging is the study of vascular flow, providing information on anatomy and morphology. Although the lifetime of enhanced 1H polarization is not long enough to observe the entire circulatory system in humans, localized angiography with amplified contrast is certainly possible by using this technique. This may be advantageous for investigating blood flow in a localized area (eg, varicose veins) or for patients who have contraindications for gadolinium-based contrast agents. At current signal enhancement levels, DNP-enhanced signal persists for approximately 5 seconds after injection, and the length of observed flow strongly depends on the geometry and rate of transport.
Most importantly, with this method only water is injected into the subject. This could cause some concern due to the hypotonic nature of pure water; however, these concerns will be minimal if the volume of water is low compared with the blood volume. In addition, we used pure water in this initial study, but our method also works with saline solution, although currently enhancement values are approximately 40% lower with saline than those with pure water (24). The water volume required for each image depends on the image acquisition length and injection rate, and can be optimized in future studies to fit a variety of biologic requirements.
The absolute levels of signal obtained with Overhauser DNP are lower than what is achievable with dissolution DNP. However, each dissolution DNP injection takes more than an hour to prepare and may contain free radicals, requiring fast imaging sequences to capture the entire image before the enhanced signal decays. In comparison, Overhauser DNP can operate in continuous-flow mode, allowing imaging with standard sequences and longer studies, and the water is free of any dissolved radicals. Overhauser DNP should be particularly beneficial for perfusion imaging in open MR imaging systems (typically at 0.3–0.5 T), because the contrast available with this technique becomes comparably greater at lower MR imaging field strengths where the bulk signal of the subject is accordingly lower (24).
This technique in general and our study in particular present several limitations. The main limitation of the technique is the fast signal decay of hyperpolarized water, which results in a limited observation time in vivo and the need to hyperpolarize close to the injection site. Our study was limited by low enhancement levels and flow rates. However, it is possible that further development of Overhauser DNP will lead to immobilized radicals and microwave resonator designs with improved enhancement levels, and increased flow rates could be obtained by operating several hyperpolarization systems in parallel.
Practical applications: The results of this exploratory study showed that radical-free hyperpolarized water provides promising perfusion contrast for a range of in vivo MR imaging studies. More work is necessary to show the clinical utility of hyperpolarized water, both by engineering optimized flow-DNP systems with higher throughput and enhancement and by developing optimal medical applications.
Advances in Knowledge.
• A method for perfusion MR imaging that uses Overhauser dynamic nuclear polarization to continuously deliver contrast agent–free hyperpolarized water can be used in vivo.
• In vivo injection of hyperpolarized water in rats safely allows for perfusion imaging in interstitial spaces, localized angiography, and the visualization of brain perfusion because hyperpolarized water freely crosses the blood-brain barrier.
Disclosures of Conflicts of Interest: M.D.L. No relevant conflicts of interest to disclose. T.A.S. No relevant conflicts of interest to disclose. N.S. No relevant conflicts of interest to disclose. O.A.A. No relevant conflicts of interest to disclose. H.R.C. No relevant conflicts of interest to disclose. B.D.R. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Institution received payment for NCI Workshop. Author received payment for work on NIH committees. Grants/grants pending from NIH, ACS, DoD. Institution holds patent for MRS Alzheimer hyperpolarization. Author receives book royalties. Other relationships: none to disclose. P.B. No relevant conflicts of interest to disclose. S.H. No relevant conflicts of interest to disclose.
Supplementary Material
Acknowledgments
The support and assistance of Dr Sam Tokuyama is gratefully acknowledged.
Received August 24, 2011; revision requested October 4; revision received December 27; accepted March 27, 2012; final version accepted May 8.
Supported by NSF Faculty Early CAREER Award 20070057 (M.D.L., T.A.S., S.H.), Packard Fellowship for Science and Engineering (M.D.L., T.A.S., S.H.), MRSEC Program of the National Science Foundation under DMR 1121053 (S.H.), L.K. Whittier Foundation (B.D.R.), and Tobacco Related Disease Research Program (16KT-0044) (P.B.).
Funding:This research was supported by the National Institutes of Health (grants NIH 1R21 CA118509 [P.B.], NCI 5R01CA122513 [P.B., H.R.C., B.D.R.], NIH 1R01NS048589 [B.D.R.], and NIH/NIDA K2521112 [N.S.]).
Abbreviations:
- DNP
- dynamic nuclear polarization
- SNR
- signal-to-noise ratio
References
- 1.Grandin CB. Assessment of brain perfusion with MRI: methodology and application to acute stroke. Neuroradiology 2003;45(11):755–766 [DOI] [PubMed] [Google Scholar]
- 2.van Laar PJ, van der Grond J, Hendrikse J. Brain perfusion territory imaging: methods and clinical applications of selective arterial spin-labeling MR imaging. Radiology 2008;246(2):354–364 [DOI] [PubMed] [Google Scholar]
- 3.Hartung MP, Grist TM, François CJ. Magnetic resonance angiography: current status and future directions. J Cardiovasc Magn Reson 2011;13(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters EA, Wickline SA. Contrast agents for MRI. Basic Res Cardiol 2008;103(2):114–121 [DOI] [PubMed] [Google Scholar]
- 5.Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol 2009;4(2):461–469 [DOI] [PubMed] [Google Scholar]
- 6.Thomsen HS. Is NSF only the tip of the “gadolinium toxicity” iceberg? J Magn Reson Imaging 2008;28(2):284–286 [DOI] [PubMed] [Google Scholar]
- 7.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Molecular imaging with endogenous substances. Proc Natl Acad Sci U S A 2003;100(18):10435–10439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golman K, in ’t Zandt R, Thaning M Real-time metabolic imaging. Proc Natl Acad Sci U S A 2006;103(30):11270–11275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res 2006;66(22):10855–10860 [DOI] [PubMed] [Google Scholar]
- 10.Kohler SJ, Yen Y, Wolber J, et al. In vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn Reson Med 2007;58(1):65–69 [DOI] [PubMed] [Google Scholar]
- 11.Gabellieri C, Reynolds S, Lavie A, Payne GS, Leach MO, Eykyn TR. Therapeutic target metabolism observed using hyperpolarized 15N choline. J Am Chem Soc 2008;130(14):4598–4599 [DOI] [PubMed] [Google Scholar]
- 12.Cudalbu C, Comment A, Kurdzesau F, et al. Feasibility of in vivo 15N MRS detection of hyperpo larized 15N labeled choline in rats. Phys Chem Chem Phys 2010;12(22):5818–5823 [DOI] [PubMed] [Google Scholar]
- 13.Albers MJ, Bok R, Chen AP, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res 2008;68(20):8607–8615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher FA, Kettunen MI, Brindle KM. Biomedical applications of hyperpolarized 13C magnetic resonance imaging. Prog Nucl Magn Reson Spectrosc 2009;55(4):285–295 [Google Scholar]
- 15.Golman K, Axelsson O, Jóhannesson H, Månsson S, Olofsson C, Petersson JS. Parahydrogen-induced polarization in imaging: subsecond (13)C angiography. Magn Reson Med 2001;46(1):1–5 [DOI] [PubMed] [Google Scholar]
- 16.Chekmenev EY, Hövener J, Norton VA, et al. PASADENA hyperpolarization of succinic acid for MRI and NMR spectroscopy. J Am Chem Soc 2008;130(13):4212–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya P, Chekmenev EY, Reynolds WF, et al. Parahydrogen-induced polarization (PHIP) hyperpolarized MR receptor imaging in vivo: a pilot study of 13C imaging of atheroma in mice. NMR Biomed 2011;24(8):1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lurie DJ, Mäder K. Monitoring drug delivery processes by EPR and related techniques—principles and applications. Adv Drug Deliv Rev 2005;57(8):1171–1190 [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto KI, Subramanian S, Murugesan R, Mitchell JB, Krishna MC. Spatially resolved biologic information from in vivo EPRI, OMRI, and MRI. Antioxid Redox Signal 2007;9(8):1125–1141 [DOI] [PubMed] [Google Scholar]
- 20.Lurie DJ, Davies GR, Foster MA, Hutchison JM. Field-cycled PEDRI imaging of free radicals with detection at 450 mT. Magn Reson Imaging 2005;23(2):175–181 [DOI] [PubMed] [Google Scholar]
- 21.Vahala E, Ylihautala M, Ehnholm G, et al. A study of the use of Overhauser enhancement to assist with needle and catheter placement during interventional MRI. J Magn Reson 2002;157(2):298–303 [DOI] [PubMed] [Google Scholar]
- 22.Dorn HC, Gitti R, Tsai KH, Glass TE. The flow transfer of a bolus with 1H dynamic nuclear polarization from low to high magnetic fields. Chem Phys Lett 1989;155(2):227–232 [Google Scholar]
- 23.Krummenacker JG, Denysenkov VP, Terekhov M, Schreiber LM, Prisner TF. DNP in MRI: an in-bore approach at 1.5 T. J Magn Reson 2012;215(1):94–99 [DOI] [PubMed] [Google Scholar]
- 24.Lingwood MD, Siaw TA, Sailasuta N, Ross BD, Bhattacharya P, Han S. Continuous flow Overhauser dynamic nuclear polarization of water in the fringe field of a clinical magnetic resonance imaging system for authentic image contrast. J Magn Reson 2010;205(2):247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarney ER, Armstrong BD, Lingwood MD, Han S. Hyperpolarized water as an authentic magnetic resonance imaging contrast agent. Proc Natl Acad Sci U S A 2007;104(6):1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausser KH, Stehlik D. Dynamic nuclear polarization in liquids. Adv Magn Reson 1968;3:79–139 [Google Scholar]
- 27.Lingwood MD, Han S. Solution-state dynamic nuclear polarization. Annu Rep NMR Spectrosc 2011;73:83–126 [Google Scholar]
- 28.Ankel EG, Lai CS, Hopwood LE, Zivkovic Z. Cytotoxicity of commonly used nitroxide radical spin probes. Life Sci 1987;40(5):495–498 [DOI] [PubMed] [Google Scholar]
- 29.Sharma P, Socolow J, Patel S, Pettigrew RI, Oshinski JN. Effect of Gd-DTPA-BMA on blood and myocardial T1 at 1.5T and 3T in humans. J Magn Reson Imaging 2006;23(3):323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus 2007;22(5):E1. [DOI] [PubMed] [Google Scholar]
- 31.Bryan RN, Levy LM, Whitlow WD, Killian JM, Preziosi TJ, Rosario JA. Diagnosis of acute cerebral infarction: comparison of CT and MR imaging. AJNR Am J Neuroradiol 1991;12(4):611–620 [PMC free article] [PubMed] [Google Scholar]
- 32.Wintermark M, Sesay M, Barbier E, et al. Comparative overview of brain perfusion imaging techniques. Stroke 2005;36(9):e83–e99 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.