Cine displacement encoding with stimulated echoes is a motion-encoding MR imaging technique for myocardial strain assessment with high spatial resolution that could be used in clinical practice to help identify subclinical myocardial dysfunction in patients with diabetes mellitus.
Abstract
Purpose:
To determine if cine displacement encoding with stimulated echoes (DENSE) can help to identify and determine the patterns of subclinical myocardial systolic dysfunction in patients with type 2 diabetes mellitus (DM) when compared with cine DENSE in control patients.
Materials and Methods:
After obtaining approval from the institutional ethics committee and written informed consent from the patients, 37 patients with type 2 DM without overt heart disease and 23 age-matched control patients were prospectively included in the study. The patients underwent standard cine magnetic resonance (MR) imaging with two-dimensional cine DENSE acquisitions. Circumferential (Ecc) and radial (Err) systolic strains were measured on short-axis views at basal, mid, and apical left ventricular levels. Longitudinal strain (Ell) was measured on four- and two-chamber views. Statistical testing included the intraclass correlation coefficient and multiple linear regression analysis.
Results:
The intraobserver intraclass correlation coefficient values were 0.85, 0.95, and 0.90, and the interobserver intraclass correlation coefficient values were 0.79, 0.91 and 0.80 for Ecc, Err, and Ell, respectively. The left ventricular ejection fraction was in the reference range and similar between the groups, and the patients with DM showed a decrease in Ecc (−14.4% ± 1.6 vs −17.0% ± 1.6, P < .001), Err (36.2% ± 10.9 vs 44.4% ± 9.9, P = .006) and Ell (−12.9% ± 2.1 vs −15.5% ± 1.6, P < .001) compared with the control patients. Finally, DM was independently associated with Ecc (P < .001), Err (P = .05) and Ell (P = .01) after adjustment for age, sex, hypertension, body mass index, and left ventricular mass.
Conclusion:
Cine DENSE, a motion-encoding MR imaging technique for myocardial strain assessment with high spatial resolution, appears to be useful in the identification of subclinical myocardial dysfunction in patients with DM.
© RSNA, 2012
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.12112571/-/DC1
Introduction
Noninvasive assessment of myocardial function based on strain is of growing interest because of its clinical value in the detection of early myocardial dysfunction (1) and stratifying patient prognosis (2). Cardiovascular MR imaging techniques are considered reference techniques, especially MR tagging, which remains the most widely available and validated cardiovascular MR modality for myocardial strain quantification (3). Cine displacement encoding with stimulated echoes (DENSE) has been proposed as a method that offers increased spatial resolution related to the pixelwise displacement encoding and more direct computation of displacement (4,5). As a result of experimental studies that used phantom and animal models (4,6–12), methodologic and postprocessing implementations have been achieved during the last decade; therefore, cine DENSE seems suitable for use in clinical practice (6–9,13–17). Thus far, clinical applications of cine DENSE have not been established, and preliminary data have involved a limited number of patients (5,6,8,13,16,18–20).
Diabetic cardiomyopathy is an important factor in the increased cardiovascular morbidity and mortality associated with diabetes mellitus (DM) (21). This pathology is defined as an alteration in cardiac structure and function in the absence of coronary artery disease, hypertension, or other potential etiologic factors (21). At a preclinical stage, echocardiographic techniques such as tissue Doppler imaging and speckle tracking imaging have suggested subclinical systolic myocardial dysfunction in patients with type-2 DM who do not have patent cardiomyopathy (22–25). Such subtle systolic alterations are considered to be early signs of diabetic cardiomyopathy (22–25). Although a decrease in longitudinal myocardial strain has been shown in this population, the occurrence of circumferential and radial abnormalities remains controversial, particularly because of the limitations of ultrasonographic (US) techniques (26).
Because of its improved motion encoding and intrinsic ability to allow assessment of myocardial strain in all three directions, cine DENSE may serve as an alternative clinical tool for quantifying myocardial strains in cardiomyopathy of patients with DM. Consequently, we hypothesized that cine DENSE would show an alteration of myocardial circumferential, radial, and longitudinal function in patients with type-2 DM who do not have overt cardiac disease. Therefore, the objective of this study was to evaluate whether the use of cine DENSE can help to identify and determine the patterns of subclinical myocardial systolic dysfunction in patients with DM compared with healthy control patients.
Materials and Methods
No industry gave support for and the authors declare to have no financial conflict of interest related to this study. The study protocol was approved by the local ethics committee of our institution. All patients provided written informed consent after the nature of the procedure was fully explained.
Study Population
We prospectively enrolled 60 patients (mean age, 49.9 years; range, 29–66 years) between March 2008 and August 2010. Mean age for the men was 50 years (range, 29–66 years) and for the women, 49.7 years (range, 40–55 years) (P = .48, χ2 test). Among the 60 patients, we included 37 patients with type-2 DM with a mean age of 50.1 years (range, 38–66 years). Mean age of men (50.3 years; range, 38–66 years) and women (49.8 years; range, 46–53 years) with diabetes was not different (P = .69). There was no statistically significant difference between the sexes in patient age (P = .69). The 60 patients also included 23 age-matched normoglycemic control patients with a mean age of 49.0 years (range, 29–66 years), with 47.0 years (29–66 years) and 49.5 years (40–55 years) for men and women, respectively. (P = .98).
Patients with type-2 DM were recruited in the outpatient Department of Endocrinology of our institution if they met the following inclusion criteria: age 35–65 years; oral antidiabetic or insulin treatment; no signs, symptoms, or history of heart disease (cardiomyopathy, coronary artery disease or valvular heart disease); sinus rhythm; left ventricular (LV) ejection fraction greater than 55%; absence of regional LV motion abnormalities assessed by means of cine MR imaging, standard resting echocardiographic results in the reference range, and the absence of contraindications to MR imaging. The exclusion criteria were severe renal failure, defined as a glomerular filtration rate estimated as a modification of diet in renal disease formula result of less than 30 mL/min/1.73 mm2 (n = 2); severe, uncontrolled DM, defined as glycated hemoglobin of greater than 12% or glycemia greater than 3 g/L (n = 1); uncontrolled blood pressure at rest, defined as systolic blood pressure greater than 180 mm Hg and/or diastolic blood pressure greater than 100 mm Hg (n = 2). In addition, all patients underwent a maximal exercise electrocardiogram (≥ 85% of maximum predicted heart rate), or stress echocardiography or myocardial perfusion scintigraphy within 1 month before inclusion. Patients were excluded from the study if silent ischemia was present, defined as a positive stress test.
A group of 23 normoglycemic patients was selected to constitute an age-matched control group. In addition, patients were eligible if they met the following criteria: no signs, symptoms, or history of heart disease; LV ejection fraction greater than 55%; absence of regional LV motion abnormalities; standard echocardiographic results in the reference range; sinus rhythm; blood pressure at rest less than or equal to 140/90 mm Hg; and the absence of contraindications to MR imaging. All patients of this cohort underwent two-dimensional cine DENSE MR imaging at inclusion.
MR Imaging Protocol
Cardiovascular MR studies were performed by using 1.5-T whole-body MR imagers (Magnetom Avanto and Sonata; Siemens Medical Solutions, Erlangen, Germany). Cine MR imaging acquisitions were performed to assess LV mass, volume, and ejection fraction. We used breath-hold electrocardiogram-gated steady-state free precession pulse cine sequences in both long- and short-axis views in standard angulations (repetition time, 3 msec; echo time, 1.6 msec; section thickness, 7 mm; at least 20 phases; matrix size, 256 × 184). The short axis imaging covered the whole LV with 8–12 contiguous sections.
Subsequently, a two-dimensional cine DENSE pulse sequence with a short echo train echo-planar imaging readout (breath hold, 15–20 seconds; 20 phases; flip angle, 6 degrees; voxel size, 2 × 2 × 7 mm; echo train length, six; displacement-encoding sensitivities of 0.63–0.84 rad/mm) was acquired in short-axis views at the basal, mid, and apical levels, as well as in two long-axis views (four- and two-chamber views). The short-axis locations were selected on an end-diastolic, two-chamber, and long-axis cine view (16).
Postprocessing
All analyses were carried out independently by two observers (L.E. and P.C., with 5 and 21 years of experience, respectively) who were blinded to all clinical and biologic data (27).
LV volume, mass, and ejection fraction were assessed at standard cine MR imaging by using dedicated software (Argus VA50A; Siemens, Erlangen, Germany). The first frame in each series was defined as the end-diastolic frame, and the image with the smallest ventricular volume was defined as the end-systolic frame. For LV ejection fraction calculation, the endocardial and epicardial contours of the end-diastolic and end-systolic frames of the LV were traced.
By using a 17-segment model of the LV (28), we measured the circumferential (Ecc), radial (Err) and longitudinal (Ell) myocardial strains with cine DENSE acquisitions. Err and Ecc were assessed on short-axis views at the basal, mid, and apical levels, and Ell was assessed on the two long-axis views. The apical segment was excluded, and the analysis was performed on the remaining 16 LV segments for Ecc and Err and on 12 segments for Ell.
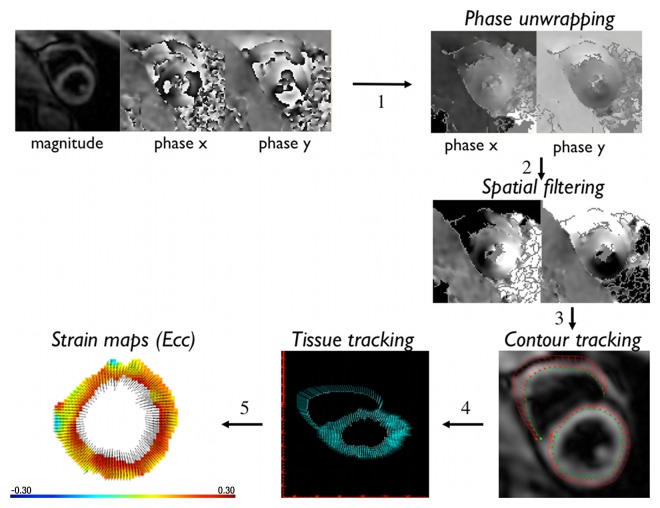
Cine DENSE postprocessing was performed on a laptop computer by using adaptive phase unwrapping and spatial filtering techniques (16) (Fig 1). A step-by-step semiautomated software program was used (DENSEview; National Institutes of Health, Bethesda, Md). In the first step, the initial multichannel complex images were combined into three cine sets encoded in the three Cartesian directions. The operator then manually defined the myocardial wall on the last cine frame by drawing the endocardial and epicardial borders of the LV. In later steps, all phase maps were unwrapped with an adaptive technique, converted to displacement vectors, and spatially filtered. In the next step of tissue tracking, the myocardial pixels were followed over time with displacement vectors. Through this process, manual segmentation of the last cine frame was extended to the other frames. Strain maps and the mean values of strain in each segment were obtained in a text file. The average maximal systolic strain at the three LV levels (base, mid, apex) and the whole-heart mean value for each patient were subsequently calculated.
Figure 1:
Illustration of the steps of cine DENSE postprocessing.
Intraobserver and Interobserver Variability
To define the variability of the strain measurements, 14 patients (seven patients with DM and seven control patients) were randomly selected. In these patients, cine DENSE analyses were repeated 3 months later by the same observer, and the analyses were performed by a second blinded observer.
Biochemistry
Blood samples were obtained from patients with type-2 DM for biochemical analysis of renal function; electrolytes; triglycerides; total, low density, and high density lipoprotein cholesterol; and glycated hemoglobin. Microalbuminuria was also measured in all DM patients. All patients had fasted and refrained from smoking for 6 hours.
Statistical Analysis
The statistical analysis was performed by using SPSS 17.0 (SPSS, Chicago, Ill). Normally distributed data were expressed as the mean ± standard deviation, and categorical variables were expressed as numbers and percentages. Baseline data in the two groups were compared by using the unpaired t test for the continuous variables and the χ2 test for the categorical variables. Univariate and two-way analysis of variance were used to determine the effects of disease and LV levels on strain values and to further assess second-order interaction terms between the independent variables.
Feasibility was determined as the number of segments in which the image quality and the signal-to-noise ratio were sufficient to calculate the strain value; otherwise, no strain value was obtained. Intraobserver and interobserver variability were assessed by using a Bland-Altman analysis (29) and two-way random intraclass correlation coefficients.
Finally, independent associations between DM and systolic strain values were studied by using a linear regression with multivariable adjustments for potentially confounding factors (eg, age, sex, history of hypertension, body mass index, LV mass, and LV ejection fraction). In a second model, history of hypertension was replaced by systolic blood pressure during the MR imaging examination. Variables associated with a P value less than .10 in simple linear regression were selected to enter the backward selection model. The same analysis was performed in the DM patients to analyze the associations between systolic strain and DM duration, DM imbalance (HbA1C), renal function, cholesterol levels, and medications.
All statistical analysis was performed on the average strain values obtained from the patients (with a total number of 60 points) except intraobserver and interobserver variability, which were assessed on the basis of independent segmental strain values. A P value of less than .05 was considered to indicate statistical significance.
Results
Characteristics of the Study Population
The DM patients had a higher body mass index than the control patients did (Table E1 [online]). The systolic blood pressure levels were higher in the DM patients than those in the control patients, although all the levels were in the reference range. A standard cine MR imaging analysis showed a normal LV ejection fraction that was similar between the two groups. LV mass was higher in the patients with DM than that in the control patients. The detailed characteristics of patients with type-2 DM are provided in Table E2 (online).
Feasibility and Reproducibility
The average postprocessing time was 5 minutes per section analysis. Strain calculations were feasible with cine DENSE in 909 of the 976 segments (93%) for Ecc, in 819 of the 976 segments (84%) for Err, and in 521 of the 732 segments (71%) for Ell. In the remaining segments (n = 67; [7%], 157 [16%], and 211 [29%] for Ecc, Err, and Ell, respectively), image quality and/or the signal-to-noise ratio were insufficient for calculation of the strain value (ie, no strain value was calculated by DENSEview software).
The intraobserver limits of agreement were −4.5% to 4.3% for Ecc, −12.6% to 11.8% for Err, and −3.1% to 3.5% for Ell. The interobserver limits of agreement were −5.3% to 5.1% for Ecc, −17.5% to 16.9% for Err, and −4.7% to 4.9% for Ell. The corresponding intraclass correlation coefficient values were 0.85, 0.95, and 0.90 for the intraobserver analysis and 0.79, 0.91, and 0.80 for the interobserver analysis for Ecc, Err, and Ell, respectively.
Myocardial Systolic Strain Assessment
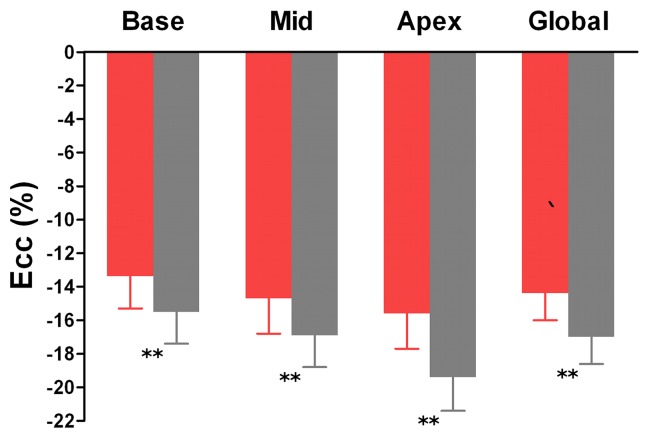
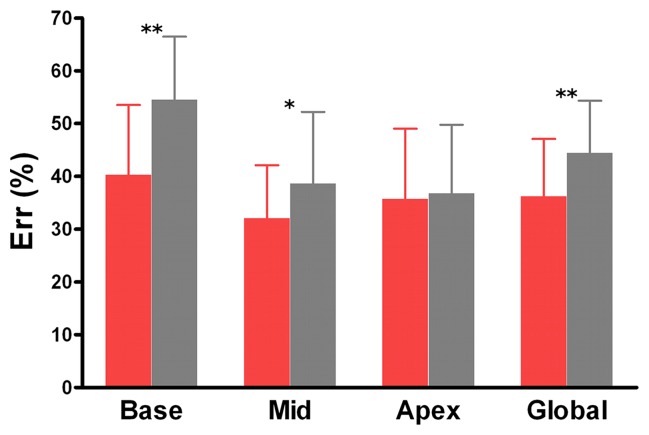
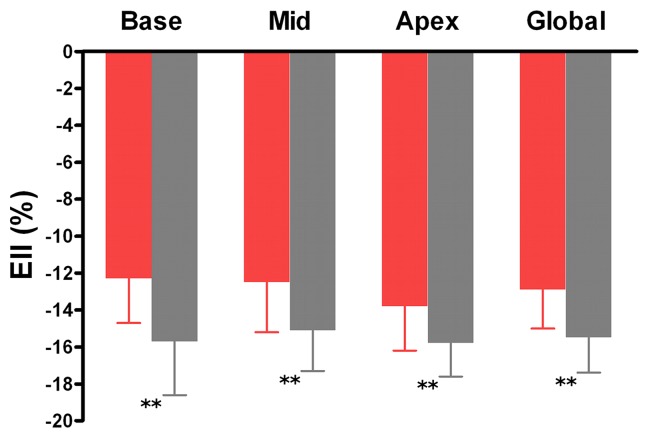
As shown in Figures 2 and 3, the patients with DM showed a decrease in global Ecc (−14.4% ± 1.6 vs −17.0% ± 1.6; P < .001), Err (36.2% ± 10.9 vs 44.4% ± 9.9, P = .006) and Ell (−12.9% ± 2.1 vs −15.5% ± 1.6, P < .001) compared with the control patients. Ecc, Err, and Ell were all significantly lower in the patients with type-2 DM than in the control patients at the three LV levels, except at the apical level for Err.
Figure 2a:
Graphs show mean values of maximal systolic strain assessed at cine DENSE MR imaging at basal, mid, and apical levels of LV and in the whole heart (global) in patients with type-2 DM (red bars) and control patients (gray bars). For comparisons of patients with type-2 DM and control patients, P < .05 (*) and P < .01 (**). Error bars = standard deviation.
Figure 3:
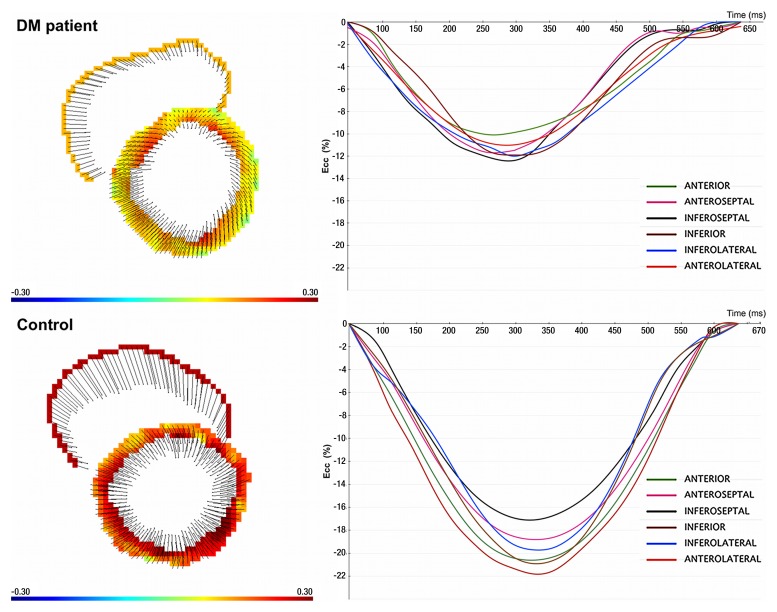
Strain maps (left) and strain curves (right) show measurement of Ecc in a 50-year-old man with type-2 DM and a 50-year-old man from the control group on short-axis view at midventricular level.
Figure 2b:
Graphs show mean values of maximal systolic strain assessed at cine DENSE MR imaging at basal, mid, and apical levels of LV and in the whole heart (global) in patients with type-2 DM (red bars) and control patients (gray bars). For comparisons of patients with type-2 DM and control patients, P < .05 (asterisk) and P < .01 (double asterisk). Error bars = standard deviation.
Figure 2c:
Graphs show mean values of maximal systolic strain assessed at cine DENSE MR imaging at basal, mid, and apical levels of LV and in the whole heart (global) in patients with type-2 DM (red bars) and control patients (gray bars). For comparisons of patients with type-2 DM and control patients, P < .05 (asterisk) and P < .01 (double asterisk). Error bars = standard deviation.
A significant difference between the three LV levels was found for Ecc and Err values in both the patients with DM (P < .001 for Ecc and P = .001 for Err) and control patients (P < .001 for Ecc and Err), with a significant decrease of this difference in the patients with DM (DM*level interaction term: P = .02 for Ecc and P = .001 for Err). No difference was found between the three LV levels in Ell in the patients with DM (P = .24) or in the control patients (P = .06).
Segmental and individual values of Ecc, Err, and Ell in both groups are described in Figures E1 and E2 (online). Twelve of the 16 LV segments showed a decrease in Ecc values in the patients with DM compared with those of the control patients. Err was mainly decreased in the basal segments. Finally, Ell was altered in all segments except for the inferoseptal segments.
Multivariable Analysis
By multivariable analysis, DM was independently associated with Ecc (β = .017, P < .001), Err (β = ₋.058, P = .05), and Ell (β = .014, P = .01) after adjustment for age, sex, history of hypertension, body mass index, LV mass, and LV ejection fraction. When the same model included systolic blood pressure level instead of history of hypertension, the results remained essentially unchanged for Ecc (β = .017, P < .001), Err (β = ₋.058, P = .05) and Ell (β = .015, P = .006).
In addition, a multivariable analysis of the DM patients showed that Ecc was not associated with DM duration, HbA1C, cholesterol levels, renal function, microalbuminuria, retinopathy, or medications, although it was associated with the use of β blockers (β = −.024, P = .01). Neither Err nor Ell was associated with DM duration, HbA1C, cholesterol levels, renal function, microalbuminuria, retinopathy, or medications.
Discussion
In this study, by using cine DENSE, we showed alterations in the three components of myocardial function (ie, Ecc, Err, Ell) in patients with type-2 DM compared with those in control patients. Myocardial dysfunction assessed by using cine DENSE was independently associated with DM after adjusting for age, sex, history of hypertension, body mass index, and LV mass. The results of this study show that cine DENSE is feasible and reproducible for identifying decreases in myocardial systolic strain in patients with DM.
Large epidemiologic studies have showed that DM is a risk factor for heart failure independent of hypertension, coronary artery disease, or other potential etiologic factors (30,31). Various adverse effects of diabetes that affect the myocardium have been described in experimental studies, including impaired calcium homeostasis, alteration of substrate use, lipotoxicity, glucotoxicity, mitochondrial dysfunction, increased oxidative stress, and activation of the renin-angiotensin-aldosterone system (21,32). Such alterations lead to cardiomyocyte necrosis and apoptosis associated with fibrosis and subsequent myocardial dysfunction, which is called diabetic cardiomyopathy (33). At an early stage, US techniques for myocardial strain assessment such as tissue Doppler imaging (22,34–38) and speckle tracking imaging (23–25) help show decreases in systolic function in DM patients without patent cardiomyopathy, compared with that in normoglycemic patients. Because of technical issues, longitudinal dysfunction (22,34–38) has been well validated by using US techniques. In comparison, the importance of radial dysfunction is more controversial (24,25,34,35), but circumferential function is largely unexplored, with only preliminary and conflicting data (23,24). MR imaging techniques provide reliable assessment of myocardial strain in the circumferential direction, which has the least measurement noise (3). Consequently, the results of our study of the use of the cine DENSE method confirmed that radial function is decreased in DM patients (25), but it also showed that circumferential function is altered in DM patients compared with normoglycemic patients. Furthermore, decreases in Ecc, Err, and Ell strains were independently associated with DM after adjustment for confounding factors, such as age, sex, history of hypertension, body mass index, and LV mass.
To our knowledge, researchers of only one published study investigated myocardial deformation in patients with DM by using MR tagging and have identified decreases in Err and Ell strains (39). However, that study included patients with severe diabetes and diastolic abnormalities, diabetes imbalance (HbA1C, 9.3% ± 1.7), and diabetes complications and did not include an assessment of radial function. In addition, silent ischemia was not excluded in that population (39). In our study, we explored the sensitivity of cine DENSE for identifying myocardial dysfunction and enrolled an unselected group of patients with type-2 DM who did not have overt heart disease. In addition, we excluded patients who had experienced silent ischemia during the month before inclusion on the basis of the results of a noninvasive stress test. We showed an alteration in the circumferential, radial, and longitudinal functions in this DM group compared with those of age-matched normoglycemic control patients. By using cine DENSE, we extended and confirmed previous observations of alterations in longitudinal and radial strains with US techniques in DM patients, whereas previous studies have described the preservation of (24) and increase in radial function (35,36) to compensate for decreased longitudinal function.
MR tagging is currently considered the reference standard technique for myocardial strain assessment (3). However, MR tagging has an intrinsically limited encoding spatial resolution that is based on tag spacing (5–6 mm), which is different from image resolution. Conversely, the spatial resolution of cine DENSE provides an improved encoding spatial resolution compared with that of MR tagging because the latter corresponds with image resolution (pixel-wise, 1.5–2 mm) (16). Cine DENSE is a technique based on the modulation of the phase of each pixel according to its position (ie, by using a stimulated echo with a bipolar gradient for displacement encoding) (4). The phase of the MR signal is proportional to the displacement of the tissue (eg, in the direction of the gradient field). Strain maps can be computed by using the spatial derivative of the measured displacement. A second advantage of this technique is an intrinsic two- or three-dimensional acquisition method (41). In our study, we chose to perform two-dimensional acquisitions to reduce breath-hold durations and to avoid respiratory artifacts. Postprocessing was time consuming when DENSEview software was used, but inline automatic postprocessing is ongoing.
Despite major advantages and the development of the cine DENSE technique, data in humans remain limited (5,6,8,13,16,18–20). In this study, we showed that cine DENSE is both feasible and reproducible in clinical practice. The intraobserver and interobserver variability were low, but there were higher intraobserver and interobserver limits of agreement of strain values in Err direction compared with those of Ecc and Ell, confirming that Err is more susceptible to noise than is Ecc (3). This might be due to the fewer pixels across the wall compared with the numerous pixels available for Ecc and Ell measurements.
Because coronary angiography was not performed in this study, coronary artery disease could not be explicitly excluded in all patients. However, because of the morbidity and mortality associated with this invasive procedure, coronary angiography was considered unethical in asymptomatic patients without overt heart disease. Consequently, all patients underwent noninvasive stress testing to detect silent ischemia. Although detection of coronary artery disease by noninvasive stress tests includes a risk of false negative results, the probability that the patients of our study had a significant coronary stenosis was very low.
Segmental strain values were defined as maximal systolic strain (ie, peak systolic strain) such as those used in previous studies (40). The measurement of peak systolic strain might be susceptible to noise. However, the use of adaptive spatial filtering has been shown to reduce the noise of strain measurements (16).
Finally, the patients with DM and control patients were not matched by sex. However, through a multivariable analysis, we showed that DM was independently associated with decreases in Ecc, Err, and Ell function after adjusting for sex.
In conclusion, cine DENSE is a motion-encoding MR imaging technique for myocardial strain assessment with high spatial resolution that allows identification of subclinical myocardial dysfunction in patients with DM.
Advances in Knowledge.
• Cine displacement encoding with stimulated echoes (DENSE) MR imaging helps to identify decreases in circumferential, radial, and longitudinal systolic strain in patients with type-2 diabetes mellitus who do not have overt heart disease, compared with age-matched normoglycemic control patients.
• Subclinical circumferential, radial, and longitudinal myocardial dysfunction, as assessed by using cine DENSE, is independently associated with type-2 diabetes mellitus after adjustment for age, sex, hypertension, body mass index, and left ventricular mass.
Implication for Patient Care.
• Cine DENSE is a motion-encoding MR imaging technique to assess for myocardial strain for detection of early myocardial dysfunction; further research is needed to assess whether the detection of subclinical myocardial dysfunction is of prognostic value, especially in patients with diabetes mellitus.
Disclosures of Conflicts of Interest: L.E. Financial activities related to the present article: Received a government agency grant from Programme Hospitalier de Recherche Clinique. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. H.T. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Institution received a grant from the French Society of Cardiology; institution received payment for lecture at the Toshiba symposium in January 2012. Other relationships: none to disclose. C.B. No relevant conflicts of interest to disclose. P.M. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Received payment for consultancy from BMS, GSK, Lilly, and Novo Nordisk; payment to author and institution for board membership of BMS-Astra Zenica. Other relationships: Grants/grants pending from Sanofi, author and institution received payment for lectures from Novo Nordisk and MSD; author receive payment for travel and accomodations from Astra Zenica, MSD, Servier, Solvay, and Takeda; Author was a clinical trial co-investigator for Astra Zenica, GSK, Lilly, Merk Lipha, MSD, Novo Nordisk, Novartis, Olympus, Sanofi, Servier, and Takeda. H.W. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Institution holds a patent on an MR imaging technique; author and institution have received royalties from Siemens and General Electric. Other relationships: none to disclose. G.D. Financial activities related to the present article: received a government agency grant from Programme Hospitalier de Recherche Clinique. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. P.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution has grants/grants pending from ANR; author received payment for lectures from Siemens, Guerbet, and Novartis. Other relationships: none to disclose.
Supplementary Material
Received December 1, 2011; revision requested February 6, 2012; revision received April 8; accepted April 25; final version accepted May 3.
Supported by an institutional grant “Programme Hospitalier Régional de Recherche Clinique” (PHRC 27-9, Interégion Rhône Alpes Auvergne, France).
Funding: H.W. is an employee of National Institutes of Health.
Abbreviations:
- DENSE
- displacement encoding with stimulated echoes
- DM
- diabetes mellitus
- Ecc
- circumferential strain
- Ell
- longitudinal strain
- Err
- radial strain
- LV
- left ventricular
References
- 1.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107(9):1375–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2(5):356–364 [DOI] [PubMed] [Google Scholar]
- 3.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aletras AH, Ding S, Balaban RS, Wen HDENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson 1999;137(1):247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aletras AH, Balaban RS, Wen H. High-resolution strain analysis of the human heart with fast-DENSE. J Magn Reson 1999;140(1):41–57 [DOI] [PubMed] [Google Scholar]
- 6.Aletras AH, Wen H. Mixed echo train acquisition displacement encoding with stimulated echoes: an optimized DENSE method for in vivo functional imaging of the human heart. Magn Reson Med 2001;46(3):523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aletras AH, Arai AE. meta-DENSE complex acquisition for reduced intravoxel dephasing. J Magn Reson 2004;169(2):246–249 [DOI] [PubMed] [Google Scholar]
- 8.Aletras AH, Ingkanisorn WP, Mancini C, Arai AE. DENSE with SENSE. J Magn Reson 2005;176(1):99–106 [DOI] [PubMed] [Google Scholar]
- 9.Gilson WD, Yang Z, French BA, Epstein FH. Measurement of myocardial mechanics in mice before and after infarction using multislice displacement-encoded MRI with 3D motion encoding. Am J Physiol Heart Circ Physiol 2005;288(3):H1491–H1497 [DOI] [PubMed] [Google Scholar]
- 10.Ashikaga H, Mickelsen SR, Ennis DB, et al. Electromechanical analysis of infarct border zone in chronic myocardial infarction. Am J Physiol Heart Circ Physiol 2005;289(3):H1099–H1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 2006;113(15):1865–1870 [DOI] [PubMed] [Google Scholar]
- 12.Zhong J, Yu X. Strain and torsion quantification in mouse hearts under dobutamine stimulation using 2D multiphase MR DENSE. Magn Reson Med 2010;64(5):1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess AT, Zhong X, Spottiswoode BS, Epstein FH, Meintjes EM. Myocardial 3D strain calculation by combining cine displacement encoding with stimulated echoes (DENSE) and cine strain encoding (SENC) imaging. Magn Reson Med 2009;62(1):77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong X, Helm PA, Epstein FH. Balanced multipoint displacement encoding for DENSE MRI. Magn Reson Med 2009;61(4):981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spottiswoode BS, Zhong X, Hess AT, et al. Tracking myocardial motion from cine DENSE images using spatiotemporal phase unwrapping and temporal fitting. IEEE Trans Med Imaging 2007;26(1):15–30 [DOI] [PubMed] [Google Scholar]
- 16.Wen H, Marsolo KA, Bennett EE, et al. Adaptive postprocessing techniques for myocardial tissue tracking with displacement-encoded MR imaging. Radiology 2008;246(1):229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spottiswoode BS, Zhong X, Lorenz CH, Mayosi BM, Meintjes EM, Epstein FH. Motion-guided segmentation for cine DENSE MRI. Med Image Anal 2009;13(1):105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong X, Spottiswoode BS, Meyer CH, Kramer CM, Epstein FH. Imaging three-dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI. Magn Reson Med 2010;64(4):1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Gilson WD, Kramer CM, Epstein FH. Myocardial tissue tracking with two-dimensional cine displacement-encoded MR imaging: development and initial evaluation. Radiology 2004;230(3):862–871 [DOI] [PubMed] [Google Scholar]
- 20.Sigfridsson A, Haraldsson H, Ebbers T, Knutsson H, Sakuma H. In vivo SNR in DENSE MRI; temporal and regional effects of field strength, receiver coil sensitivity and flip angle strategies. Magn Reson Imaging 2011;29(2):202–208 [DOI] [PubMed] [Google Scholar]
- 21.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115(25):3213–3223 [DOI] [PubMed] [Google Scholar]
- 22.Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol 2003;41(4):611–617 [DOI] [PubMed] [Google Scholar]
- 23.Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr 2009;10(8):926–932 [DOI] [PubMed] [Google Scholar]
- 24.Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009;104(10):1398–1401 [DOI] [PubMed] [Google Scholar]
- 25.Ernande L, Rietzschel ER, Bergerot C, et al. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle-tracking imaging study. J Am Soc Echocardiogr 2010;23(12):1266–1272 [DOI] [PubMed] [Google Scholar]
- 26.Bijnens BH, Cikes M, Claus P, Sutherland GR. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur J Echocardiogr 2009;10(2):216–226 [DOI] [PubMed] [Google Scholar]
- 27.Pohost GM, Kim RJ, Kramer CM, et al. Task Force 12: training in advanced cardiovascular imaging (cardiovascular magnetic resonance [CMR]): endorsed by the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2006;47(4):910–914 [DOI] [PubMed] [Google Scholar]
- 28.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105(4):539–542 [DOI] [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1(8476):307–310 [PubMed] [Google Scholar]
- 30.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34(1):29–34 [DOI] [PubMed] [Google Scholar]
- 31.de Simone G, Devereux RB, Chinali M, et al. Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J Hypertens 2010;28(2):353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asghar O, Al-Sunni A, Khavandi K, et al. Diabetic cardiomyopathy. Clin Sci (Lond) 2009;116(10):741–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res 2000;87(12):1123–1132 [DOI] [PubMed] [Google Scholar]
- 34.Vinereanu D, Nicolaides E, Tweddel AC, et al. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci (Lond) 2003;105(5):591–599 [DOI] [PubMed] [Google Scholar]
- 35.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond) 2004;106(1):53–60 [DOI] [PubMed] [Google Scholar]
- 36.Shim CY, Park S, Choi EY, et al. Is albuminuria an indicator of myocardial dysfunction in diabetic patients without overt heart disease? A study with Doppler strain and strain rate imaging. Metabolism 2008;57(4):448–452 [DOI] [PubMed] [Google Scholar]
- 37.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Schnohr P, Jensen JS. Tissue Doppler echocardiography in persons with hypertension, diabetes, or ischaemic heart disease: the Copenhagen City Heart Study. Eur Heart J 2009;30(6):731–739 [DOI] [PubMed] [Google Scholar]
- 38.Stefanidis A, Bousboulas S, Kalafatis J, et al. Left ventricular anatomical and functional changes with ageing in type 2 diabetic adults. Eur J Echocardiogr 2009;10(5):647–653 [DOI] [PubMed] [Google Scholar]
- 39.Fonseca CG, Dissanayake AM, Doughty RN, et al. Three-dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. Am J Cardiol 2004;94(11):1391–1395 [DOI] [PubMed] [Google Scholar]
- 40.Fernandes VRS, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2006;47(12):2420–2428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.