Abstract
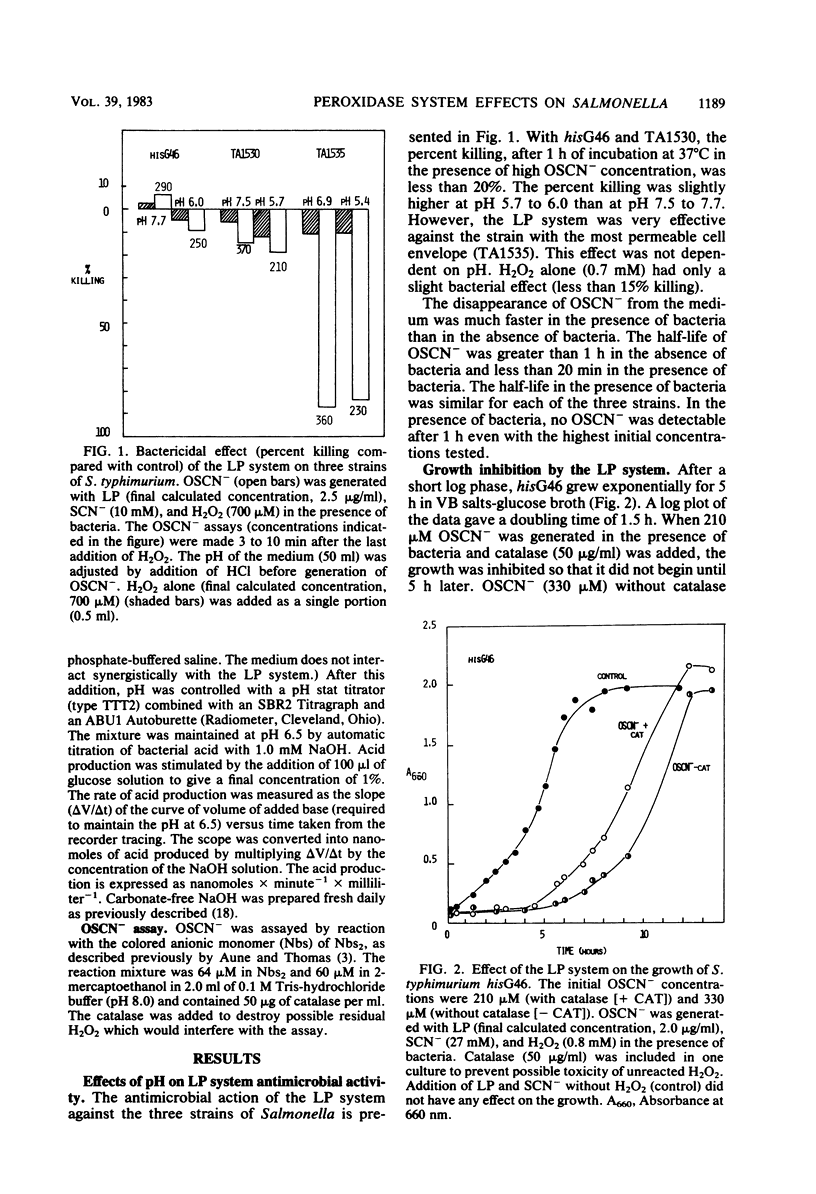
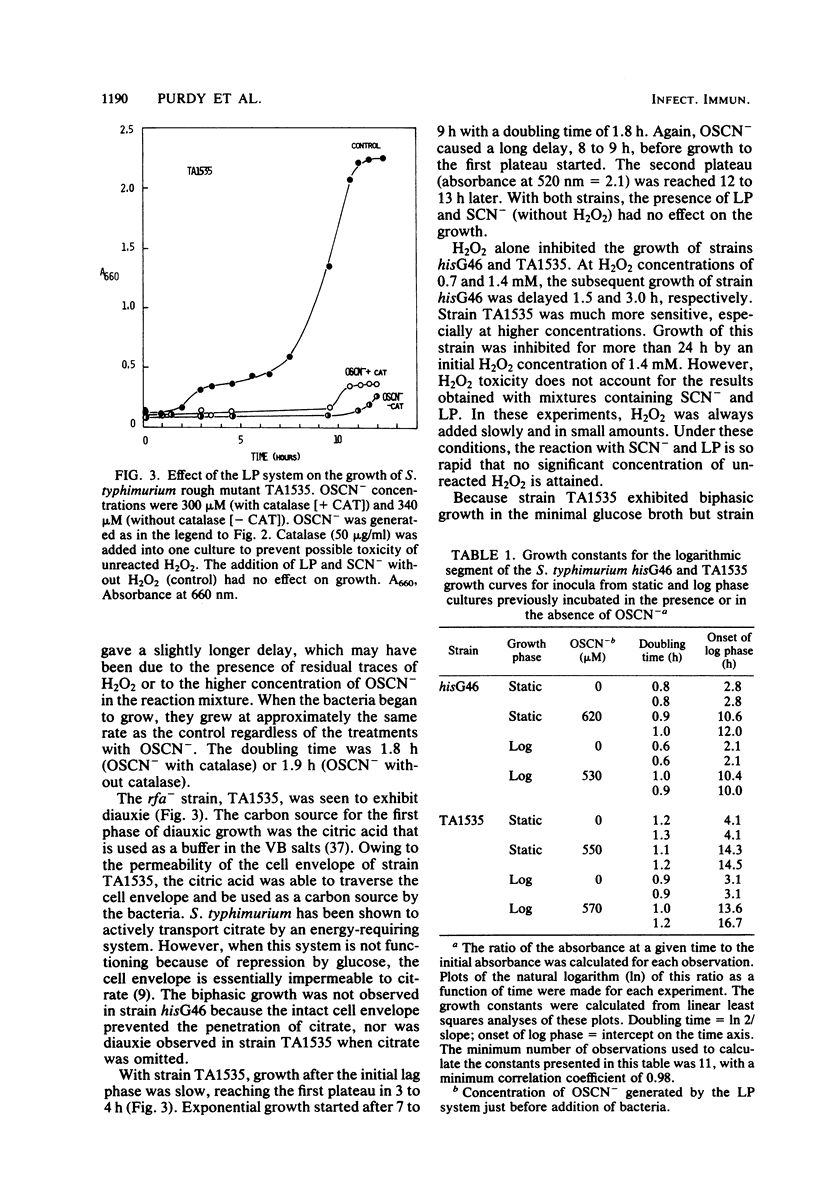
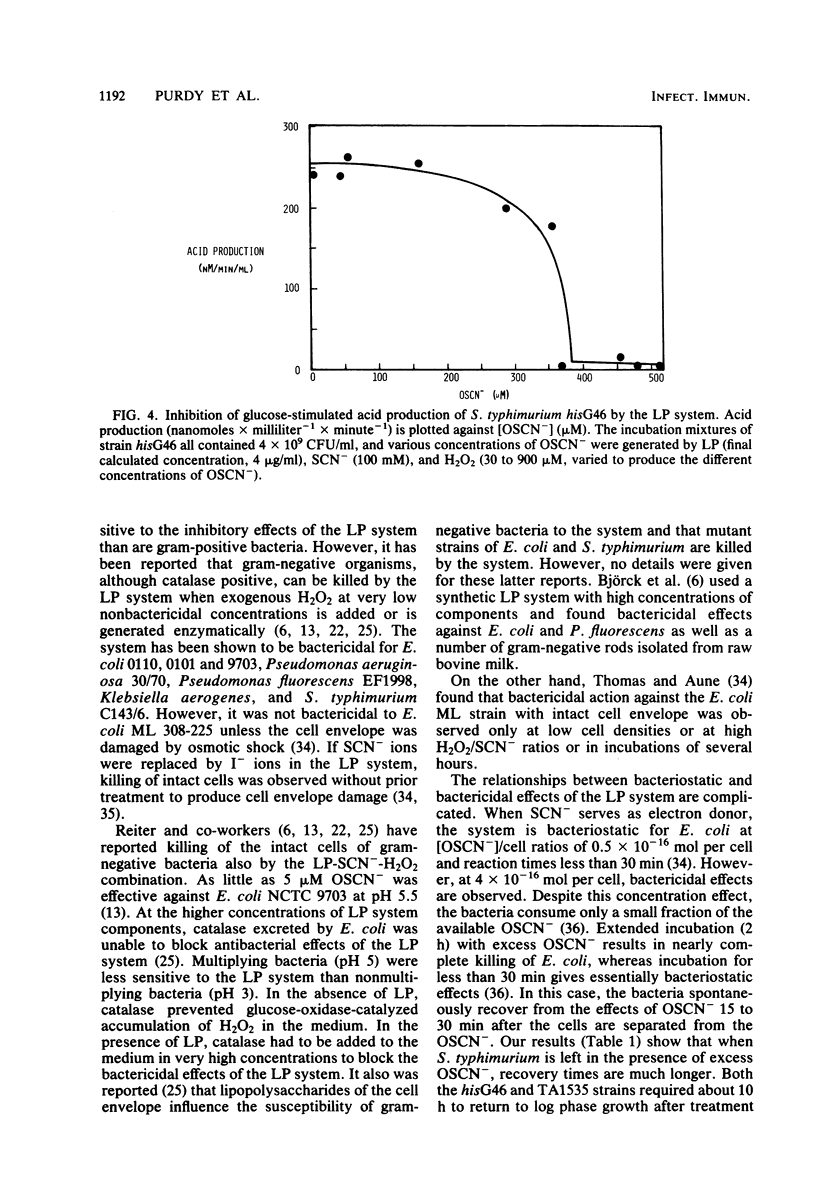
The lactoperoxidase-thiocyanate-hydrogen peroxide system was found to have both bacteriostatic and bactericidal activities against strains of Salmonella typhimurium. The bactericidal activity was clearly dependent on the permeability of the bacterial cell envelope. The deep rough mutant TA1535, with the most permeable cell envelope, was killed both at neutral and acid pH, whereas very little or no killing was observed with the intact cells of the parent strain hisG46. The delta gal mutant, TA1530, representing an intermediate in cell envelope permeability, was inhibited to a much lesser extent than TA1535. Bacteria in log phase of growth were more sensitive to the bactericidal effects than were those in stationary phase. Growth phase had little influence on the bacteriostatic effects. The hisG46 strain produced significant quantities of acid in the presence of glucose. This acid production was inhibited by the lactoperoxidase-thiocyanate-hydrogen peroxide system, and, in contrast to results obtained with several strains of streptococci, this inhibition was not reversed by addition of a reducing agent (2-mercaptoethanol).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry. 1978 Mar 21;17(6):1005–1010. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- Björck L., Rosén C., Marshall V., Reiter B. Antibacterial activity of the lactoperoxidase system in milk against pseudomonads and other gram-negative bacteria. Appl Microbiol. 1975 Aug;30(2):199–204. doi: 10.1128/am.30.2.199-204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn H., Piessens J. P., Scholtes W., Stoddard L. A. Hypothiocyanite ion; the inhibitor formed by the system lactoperoxidase-thiocyanate-hydrogen peroxide. I. Identification of the inhibiting compound. Caries Res. 1977;11(2):77–84. doi: 10.1159/000260252. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J., LUEBKE R. G. THE ANTILACTOBACILLUS SYSTEM OF SALIVA. ROLE OF SALIVARY PEROXIDASE. Proc Soc Exp Biol Med. 1965 Feb;118:483–486. doi: 10.3181/00379727-118-29882. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Cameron M. Citrate transport in Salmonella typhimurium. Arch Biochem Biophys. 1978 Sep;190(1):270–280. doi: 10.1016/0003-9861(78)90276-x. [DOI] [PubMed] [Google Scholar]
- Kersten H. W., Moorer W. R., Wever R. Thiocyanate as a cofactor in myeloperoxidase activity against Streptococcus mutans. J Dent Res. 1981 Apr;60(4):831–837. doi: 10.1177/00220345810600041201. [DOI] [PubMed] [Google Scholar]
- Marshall V. M., Reiter B. Comparison of the antibacterial activity of the hypothiocyanite anion towards Streptococcus lactis and Escherichia coli. J Gen Microbiol. 1980 Oct;120(2):513–516. doi: 10.1099/00221287-120-2-513. [DOI] [PubMed] [Google Scholar]
- Modrzakowski M. C., Cooney M. H., Martin L. E., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes. Infect Immun. 1979 Mar;23(3):587–591. doi: 10.1128/iai.23.3.587-591.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu Z., Tenovuo J., Mestecky J., Pruitt K. M. Human milk peroxidase is derived from milk leukocytes. Biochim Biophys Acta. 1982 Sep 17;718(1):103–108. doi: 10.1016/0304-4165(82)90014-9. [DOI] [PubMed] [Google Scholar]
- Möller A. J. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr. 1966 Dec 20;74(5 Suppl):1–380. [PubMed] [Google Scholar]
- Pruitt K. M., Adamson M., Arnold R. Lactoperoxidase binding to streptococci. Infect Immun. 1979 Jul;25(1):304–309. doi: 10.1128/iai.25.1.304-309.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. M., Tenovuo J., Andrews R. W., McKane T. Lactoperoxidase-catalyzed oxidation of thiocyanate: polarographic study of the oxidation products. Biochemistry. 1982 Feb 2;21(3):562–567. doi: 10.1021/bi00532a023. [DOI] [PubMed] [Google Scholar]
- Pruitt K. M., Tenovuo J., Fleming W., Adamson M. Limiting factors for the generation of hypothiocyanite ion, an antimicrobial agent, in human saliva. Caries Res. 1982;16(4):315–323. doi: 10.1159/000260614. [DOI] [PubMed] [Google Scholar]
- Pruitt K. M., Tenovuo J. Kinetics of hypothiocyanite production during peroxidase-catalyzed oxidation of thiocyanate. Biochim Biophys Acta. 1982 Jun 4;704(2):204–214. doi: 10.1016/0167-4838(82)90147-9. [DOI] [PubMed] [Google Scholar]
- Reiter B., Marshall V. M., BjörckL, Rosén C. G. Nonspecific bactericidal activity of the lactoperoxidases-thiocyanate-hydrogen peroxide system of milk against Escherichia coli and some gram-negative pathogens. Infect Immun. 1976 Mar;13(3):800–807. doi: 10.1128/iai.13.3.800-807.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter B. Review of nonspecific antimicrobial factors in colostrum. Ann Rech Vet. 1978;9(2):205–224. [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Myeloperoxidase-Cl--H2O2 bactericidal system: effect of bacterial membrane structure and growth conditions. Infect Immun. 1978 Mar;19(3):1110–1112. doi: 10.1128/iai.19.3.1110-1112.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sips H. J., Hamers M. N. Mechanism of the bactericidal action of myeloperoxidase: increased permeability of the Escherichia coli cell envelope. Infect Immun. 1981 Jan;31(1):11–16. doi: 10.1128/iai.31.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo J. Formation of the bacterial inhibitor, hypothiocyanite ion, by cell-bound lactoperoxidase. Caries Res. 1979;13(3):137–143. doi: 10.1159/000260393. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Mansson-Rahemtulla B., Pruitt K. M., Arnold R. Inhibition of dental plaque acid production by the salivary lactoperoxidase antimicrobial system. Infect Immun. 1981 Oct;34(1):208–214. doi: 10.1128/iai.34.1.208-214.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo J., Moldoveanu Z., Mestecky J., Pruitt K. M., Rahemtulla B. M. Interaction of specific and innate factors of immunity: IgA enhances the antimicrobial effect of the lactoperoxidase system against Streptococcus mutans. J Immunol. 1982 Feb;128(2):726–731. [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Cofactor role of iodide in peroxidase antimicrobial action against Escherichia coli. Antimicrob Agents Chemother. 1978 Jun;13(6):1000–1005. doi: 10.1128/aac.13.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Susceptibility of Escherichia coli to bactericidal action of lactoperoxidase, peroxide, and iodide or thiocyanate. Antimicrob Agents Chemother. 1978 Feb;13(2):261–265. doi: 10.1128/aac.13.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibria between oxidized forms of thiocyanate. Biochemistry. 1981 May 26;20(11):3273–3280. doi: 10.1021/bi00514a045. [DOI] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: effect of exogenous amines on antibacterial action against Escherichia coli. Infect Immun. 1979 Jul;25(1):110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


