Abstract
Laparoscopic surgery for colonic cancer is a safe and established alternative to traditional open colectomy. The potential advantages of shorter length of stay, faster recovery and fewer operative complications are well documented. The last 5 years has seen an increase in the number of laparoscopic colorectal operations as more surgeons learn this technique. Short and medium term results have been encouraging with respect to oncological outcomes. However, laparoscopic surgery for rectal cancer remains a contentious issue. The increased complexity of operating within the confines of the pelvis and the greater risk of oncological compromise, have led to some surgeons urging caution. We present the challenges associated with laparoscopic rectal cancer surgery and explain that appropriate patient selection, surgical planning and laparoscopic experience are the key to successful outcomes.
Laparoscopic surgery for cancer of the colon is a safe and well established technique in selected patients, when performed by trained and suitably experienced surgeons. Although several studies have documented the benefits of laparoscopic surgery compared with conventional open colectomy such as reduced blood loss, decreased hospital stay and less post-operative pain,1,2 it is the equivalent oncological outcomes which have led to acceptance of a minimal access approach. However, laparoscopic resection for rectal cancer (defined as carcinoma within 15 centimetres of the anal verge) has not been as thoroughly evaluated and remains controversial.3 The main concern is fear of oncological compromise and that tumour clearance, and lymph node yields, as markers of surgical success may not be comparable with those achieved at open surgery. There is also a perceived risk of technical compromise due to the inflexibility of the instruments used during laparoscopy. This paper aims to highlight the potential benefits and challenges associated with laparoscopic rectal cancer surgery.
Methods
A search of Medline, Embase, Cochrane Library and Google was undertaken using search names ‘rectal cancer’, ‘laparoscopy’, ‘anterior resection’, and ‘magnetic resonance imaging (MRI)’. There were no language or date restrictions. The search results included original articles, case series, review articles and case reports. In addition to journal articles, book chapters were also included where appropriate. The authors also drew upon their own experience on dealing with this condition and those of their colleagues.
Anatomical considerations and the importance of MRI
The rectum lies within the lymphovascular envelope of the mesorectum. This surrounds the rectum and is key to the pathological spread of cancer. The bony pelvis within which the rectum lies is a confined space and contains other structures which can be affected by tumour spread. Even the minimal disturbance of these adjacent structures can lead to profound physiological and functional consequences. The complexity of the rectum's anatomical relations is compounded by the pathological spread of rectal cancer. Tumour spread is both longitudinal and radial, or circumferential. Quirke identified that circumferential margin (CRM) involvement of the specimen by cancer following surgery correlated with the development of local recurrence.4
High spatial resolution MRI has allowed surgeons to better understand the anatomy of rectal cancer, which is invaluable when planning the feasibility of locally curative surgery. Appreciation of lateral margin of the tumour is an important factor when deciding on neodjuvant therapy in the form of radiotherapy and/or chemoradiotherapy. Figure 1 shows the intimate relationship between the rectum and surrounding viscera as seen on MRI.
Figure 1.
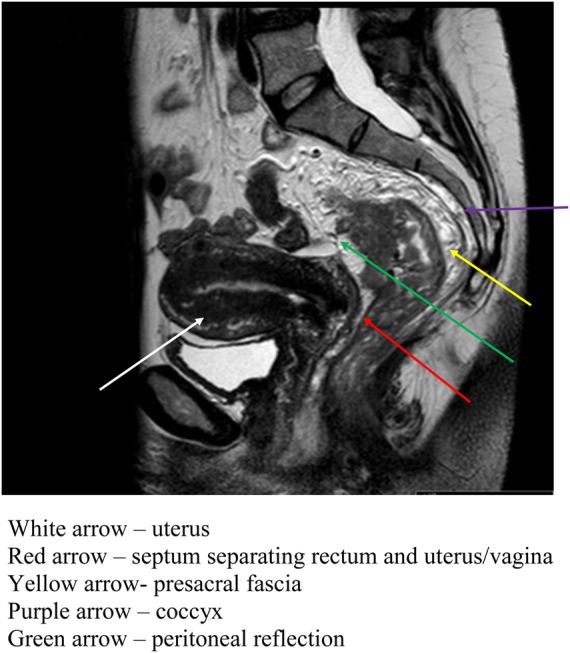
MRI image showing the rectum and surrounding structures of the pelvis
(This figure is available in colour in the online edition at http://jrsm.rsmjournals.com/)
MRI has become an integral part of the staging process in rectal cancer in the UK and in many other parts of the world. In comparison with endoluminal techniques, such as endoluminal ultrasound, MRI offers detailed assessment of all tumours including bulky and stricturing tumours. Furthermore, it allows assessment of the entire mesorectum and accuracy in depth of invasion, tumour sub-type (e.g. mucinous) and extra-mural venous invasion. This is particularly important when deciding the appropriateness of laparoscopic resection. Figure 2 shows a rectal carcinoma as seen on MRI.
Figure 2.
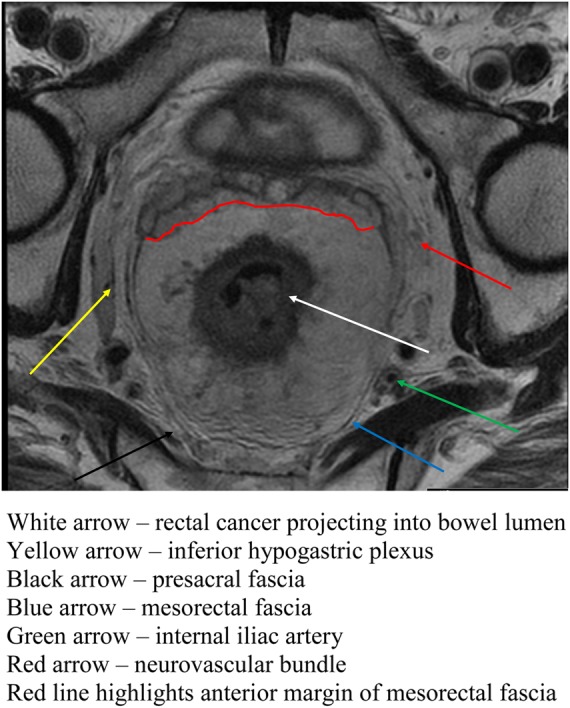
Axial section of rectum on MRI showing rectal cancer (arrow)
(This figure is available in colour in the online edition at http://jrsm.rsmjournals.com/)
Surgical technique
The concept of total mesorectal excision (TME) during rectal resection was introduced and popularized by Heald5 and is now generally accepted as optimal care for operable rectal cancer. TME involves excision of the rectum with its associated lymphovascular envelope – the mesorectum. This fascial plane of the mesorectum provides a natural barrier of tumour spread and confines local cancer spread if the fascia is not breached by the tumour or iatrogenically at surgery. Resection of the rectum with an intact mesorectum is recognized as a key factor in minimizing pelvic recurrence. Figure 3 shows the relationship between a rectal cancer and the mesorectal fascia which is also the site of the circumferential resection margin following surgery. In addition to the oncological benefits of TME, identification and preservation of the pelvic autonomic nerves (responsible for normal bladder and sexual function) has been one of the functional successes of this technique. This surgical technique is the single-most important reason for the well documented reduction in local recurrence of rectal cancer over the past three decades. However, there is still a wide variation in the local recurrence rates and quality of resection specimens.6,7 Obtaining a tumour-free resection margin in the confines of a narrow pelvis is one of the most challenging aspects of colorectal cancer surgery. The need for such a major pelvic dissection, with a low colorectal or coloanal anastomosis, when a restorative resection is performed, is associated with considerable morbidity. Anastomotic leak rates of 15% have been reported and it is relatively routine to perform a temporary defunctioning stoma to reduce the consequences of anastomotic leakage.8
Figure 3.
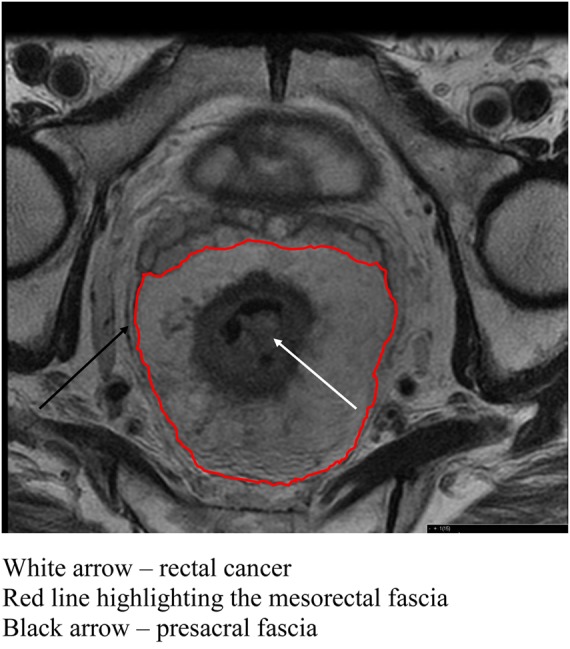
Axial section of rectum showing rectal cancer and mesorectal fascia
(This figure is available in colour in the online edition at http://jrsm.rsmjournals.com/)
Unsurprisingly, there remains the question as to whether laparoscopic resection is appropriate in such a potentially hazardous scenario. The main clinical measure of success is comparable oncological results such as local recurrence rates and survival. However it is important to also take into account the importance of functional outcomes such as sexual function, bowel habit, recovery and quality of life. Laparoscopic rectal cancer resection must prove to be at minimum comparable, and ideally have potential to improve on all these factors. Unfortunately, much of the published literature regarding laparoscopic rectal cancer surgery does not emphasize such functional outcomes.
Patient selection
Patient selection and appropriate work-up are crucial components of optimal cancer management, whether this is by open or laparoscopic surgery. There are some factors that are particularly relevant in patient selection for successful laparoscopic surgery. Patients with significant co-morbidity, high BMI, locally advanced cancers or those who have undergone preoperative long course chemo-radiotherapy, all present distinct challenges with regard to rectal cancer surgery. These factors may preclude laparoscopic rectal cancer surgery for some surgeons.9,10 Appropriate selection will mean less likelihood of conversion and this may be related to co-morbidity as well as technical factors associated with the patient and/or the tumour. Tumour size must also be a consideration, not just overall stage. Although there is no suggested figure with regards to an upper limit on tumour size, the operating surgeon must bear this in mind when deciding the appropriateness of laparoscopy.
Oncological safety
The main opposition to laparoscopic rectal resection is compromise from an oncological point of view without clear, definitive benefits. The quality of the resection specimens are now assessed with great scrutiny and detail.11 The reports in the literature suggest that local recurrence, lymph node harvest and oncological clearance are not being compromised.12 However, the quality of some studies remain debatable, particularly with scant information on the tumour size and location, technique and short follow-up periods.13 Furthermore, it is difficult to know whether the tumours resected where particularly favourable to laparoscopy.
Bretagnol and colleagues reported a prospective series of 144 laparoscopic TME procedures with low colorectal or coloanal anastomosis for mid and low rectal cancers of stage T3 N1 or less.14 Clear distal and circumferential margins were achieved in 98% and 94% respectively. Only 2 patients developed local recurrence and the 3-year overall and disease-free survival rates were 89% and 77%, respectively. Another study by Zhou et al. reported excellent results of laparoscopic resection for rectal cancer.15 Their overall morbidity rate was 6.1% and anastomotic leakage rate was 1.2%. However, they provided no details on method of randomization or definition and rate of conversion, nor whether the analysis was performed on an intention-to-treat basis. Lujan et al. have reported a single centre randomized controlled trial in which 204 patients with mid and low rectal cancers were randomized to either open and laparoscopic resection.16 Patients in the laparoscopic group had earlier return of gut function and shorter hospital stay at a mean of 2.8 days and 8.2 days, respectively. There were fewer anastomotic leaks in the laparoscopic group, 4% of patients had a positive resection margin, 5-year local recurrence was 4.8%, disease-free survival was 84.8% and overall survival 72.1%. Overall, the results demonstrated oncological equivalence but with significantly faster return of gut function, decreased transfusion requirements and shorter hospital stay following laparoscopic resection.
A meta-analysis comparing short and medium term outcomes of over 1400 laparoscopic versus 1755 open TMEs concluded that there were no reported oncological differences between laparoscopic and open resections for treatment of primary rectal cancer.12 Laurent et al. emphasized the importance of specialization in the principles and practice of TME in their series of 238 laparoscopic TME procedures with 5-year follow up.17 More than 80% of their rectal resections were for mid and low tumours, and all patients were treated with curative intent. The conversion rate was 15% and the local recurrence rate was 3.8% in laparoscopic completed and equivalent in laparoscopic cases converted to open surgery (3.5%). Both local recurrence figures were lower than the 5.5% observed in their open TME surgery experience. Additionally, a notable observation by Laurent et al. was that the overall survival at 5 years was better in the laparoscopic group than in the open group, especially in Stage III cancers. This potential beneficial impact of laparoscopic surgery on survival requires further investigation.
Potential benefits of laparoscopic rectal cancer surgery
Improvements in image quality and the development of newer instruments have allowed surgeons to expand the horizons of laparoscopic colorectal surgery. Rectal resection for cancer is no exception. Optimal laparoscopy may allow for a more precise anatomical TME dissection. High definition laparoscopes with increased viewing angles, means that the abdominal and pelvic cavity can be inspected with more precision. The surgeon is able to view areas of the pelvis which are inaccessible to the naked eye at open surgery. For example, dissection in a narrow male pelvis is often difficult to complete under direct vision at open surgery and can require several position changes to complete the operation safely. Furthermore, the laparoscopic view is also well illuminated, and magnified, making it easier to follow surgical planes and anticipate and preempt bleeding.
Laparoscopic surgery has the potential to reduce the trauma to the specimen with less inadvertent handling, but one must be mindful not to use the laparoscopic instruments overly aggressively which also have the ability to traumatize tissue. Laparoscopic operating involves displacing the rectum and mesorectum gently from side to side. The camera can operate in a very confined space in the pelvis with illumination of the field of view. In contrast, considerable traction is required to obtain adequate light and a clear view low down in the pelvis in conventional open surgery. Such handling and retraction of the cancer bearing specimen can cause tears into the mesorectum or even rupture into the lumen. Once a tear begins, it tends to progress because traction on the specimen is difficult to avoid during open surgery. Rates of ‘R0’ – tumour free, resection theoretically might be higher following laparoscopic resection.
Laparoscopic resection offers the potential for reduced blood transfusion requirements, reduced surgical trauma, a less marked inflammatory response, earlier return of gut function and shorter hospital stay compared with conventional open surgery and contribute to faster recovery in the majority of patients.
Ongoing challenges with laparoscopic colorectal surgery and risks of morbidity
A conventional cross stapled anastomosis is more difficult to achieve laparoscopically than in open surgery because the available staplers for laparoscopic use are not able to flex sufficiently to allow easy placement of a staple line across the rectum at right angles. Frequently it is necessary to use a number of firings of the stapler to transect the rectum along a more oblique line than intended. Precise definition of the intersphincteric plane posteriorally, often necessitates division of muscle fibres of pubococcygeus, as a clear intersphincteric plane posteriorally is a requirement if the puborectal sling is not to be damaged.
Indeed, Leroy et al., in an attempt to explain their 17% clinical leak rate, hypothesized that such a long staple line increases the risk of leakage.18 They suggested that refinement of the staplers and the technique of stapling were required in order to enable the stapling device to be applied perpendicular to the bowel. This would result in a short staple line and a potential reduction in the leak rate. Brannigan et al. examined the technique of laparoscopic rectal stapling following TME using a virtual model and simulation of laparoscopic stapling concluding that the minimal angulation of the stapler head required for successful transverse stapling of the rectum was 62–68°.19
For adequate clearance below a low rectal cancer, a transverse rather than an oblique transaction of the rectum is optimal. In order to compensate for the limited angulation of current laparoscopic staplers it is necessary to employ special techniques to ensure adequate clearance and safe anastomosis for very low rectal cancers. These include perineal pressure to render the lowest part of the rectum more accessible for staple-transection, dissection into the pelvic funnel in the intersphincteric plane to allow the somewhat oblique staple line to adequately clear the low neoplasm. An alternative is transanal division of the rectum and hand-sutured coloanal anastomosis.
Some of the large series have reported leak rates ranging from 10–17% after laparoscopic TME. However, with increasing experience, anastomotic leak rates appear to be declining. In a case-control study comparing laparoscopic and open TME, Breukink et al. reported 9% anastomotic leakage in the laparoscopic group compared with 14% in the open group.20 Lujan et al., in their recently published single centre randomized trial of laparoscopic versus open TME for mid and low rectal cancers, reported anastomotic leak rates of 6% in the laparoscopic group as against 12 % in the open TME group.16 Similarly, Tsang et al. from Hong Kong had only 1 anastomotic leak in their series of 105 laparoscopic rectal cancer resections and Li reported a leak rate of 3.5% in his series of 152 patients.21,22
Laparoscopic TME has the potential to achieve better preservation of the pelvic autonomic nervous system because the magnified operative view allows easier identification of pelvic nerves. Liang et al. studied 98 patients with T3 mid or low rectal cancers undergoing laparoscopic TME following neo-adjuvant chemo-radiotherapy.23 They reported that the majority of their patients retained satisfactory genitourinary function following a laparoscopic TME with autonomic nerve preservation techniques. Similarly, Asoglu et al., in their series of 34 laparoscopic and 29 open TMEs, found that the open technique was associated with a significantly higher incidence of sexual dysfunction, but not bladder dysfunction compared with laparoscopic TME.24
Conversion to open surgery
Conversion rates to open surgery range widely. The most common reasons cited for conversion include technical difficulties secondary to tumour fixity, dense adhesions or inadequate visualization due to obesity, uncertainties regarding the oncological completeness or hemorrhage. It has also been suggested that male sex and stapled anastomosis are independent risk factors for conversion.25 Part of the variation may be in the definition of conversion. Some describe hybrid operations in which the rectum is mobilized by an open technique or where a conventional open stapler is applied through a lower abdominal incision. Others would consider these modifications as conversions. We suggest that requirement of an open technique to mobilize the rectum during any part of the laparoscopic TME should be regarded as a conversion.
The experience of the operating surgeon is a major determinant of the conversion rate. Conversion rates have generally decreased over recent years, attributable to a combination of improved instrumentation, imaging technology, increasing experience and perhaps better patient selection. Tsang et al. had 2 conversions in 105 laparoscopic TME procedures.18 Leroy et al., in their series of 102 laparoscopic TME operations, involving patients with tumours across all T stages, reported a conversion rate of 3%.18,21 The conversion rate in our unit for laparoscopic TME surgery is 3.7 % with satisfactory oncological outcomes.
It is likely that conversion will not increase local recurrence rates provided the decision to convert is made before there is any compromise of resection planes or resection margins (as may have happened in the study quoted above). A misguided laparoscopic ‘trial dissection’ that compromises an oncological resection that would have been straightforward conventionally, may condemn the patient to an R1 resection and local recurrence and should be avoided. It is more important to complete the operation in an oncologically sound and radical manner than to complete it laparoscopically, and at all times an R0 resection is the ultimate aim.
Learning curves
The learning curve for rectal cancer surgery is difficult in open surgery. This is equally if not more challenging in laparoscopic rectal resection. It is of paramount importance for surgeons to have a detailed understanding of the oncological principles involved in order to translate these skills to laparoscopy. Laparoscopic resection of rectal cancer must adhere to these principles and match the local recurrence rates of open surgery. The number of cases required to plateau in terms of speed, morbidity rate, conversion rate and, of course, oncological adequacy is debatable. Supervision and preceptorship by an experienced laparoscopic surgeon is required to gain the necessary confidence in the more challenging pelvic surgery required for rectal cancer.
Laparoscopy also provides a unique teaching opportunity which supersedes the traditional practice of holding retractors and trying to learn simultaneously. It also allows contemporaneous recording and use of such material as teaching videos. This should allow for better pattern recognition and anatomical education.
Conclusion
There is little debate about the appropriateness of laparoscopy in the management of operable colon cancer. However, the surgical treatment of rectal cancer is not immediately comparable to that of the colon. The confined space of the pelvis, the more intimately related surrounding structures and the different pathological spread all contribute to making laparoscopic rectal cancer surgery a formidable task. Yet improved training and technology have led to comparable oncological and functional outcomes. This surgery may not be suitable for all rectal cancers, but laparoscopic rectal resection can be safe and successful in selected patients when performed by suitably experienced surgeons.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
Not applicable
Guarantor
Brendan Moran/ Manish Chand
Contributorship
All authors contributed equally
Acknowledgements
None
References
- 1.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718–26 [DOI] [PubMed] [Google Scholar]
- 2.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet oncol 2005;6:477–84 [DOI] [PubMed] [Google Scholar]
- 3.Chand M, Heald RJ, Laparoscopic rectal cancer surgery. Br J Surg 2011;98:166–7 [DOI] [PubMed] [Google Scholar]
- 4.Quirke P, Durdey P, Dixon MF, Williams NS, Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2:996–9 [DOI] [PubMed] [Google Scholar]
- 5.Heald RJ, A new approach to rectal cancer. Br J Hosp Med (Lond) 1979;22:277–81 [PubMed] [Google Scholar]
- 6.Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 2002;235:449–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009;373:821–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R, Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007;246:207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senagore AJ, Stulberg JJ, Byrnes J, Delaney CP, A national comparison of laparoscopic vs. open colectomy using the National Surgical Quality Improvement Project data. Dis Colon Rectum 2009;52:183–6 [DOI] [PubMed] [Google Scholar]
- 10.Tan PY, Stephens JH, Rieger NA, Hewett PJ, Laparoscopically assisted colectomy: a study of risk factors and predictors of open conversion. Surgical endoscopy 2008;22:1708–14 [DOI] [PubMed] [Google Scholar]
- 11.Quirke P, Training and quality assurance for rectal cancer: 20 years of data is enough. Lancet oncol 2003;4:695–702 [DOI] [PubMed] [Google Scholar]
- 12.Anderson C, Uman G, Pigazzi A, Oncologic outcomes of laparoscopic surgery for rectal cancer: a systematic review and meta-analysis of the literature. Eur J Surg Oncol 2008;34:1135–42 [DOI] [PubMed] [Google Scholar]
- 13.Anthuber M, Fuerst A, Elser F, Berger R, Jauch KW, Outcome of laparoscopic surgery for rectal cancer in 101 patients. Dis Colon Rectum 2003;46:1047–53 [DOI] [PubMed] [Google Scholar]
- 14.Bretagnol F, Lelong B, Laurent C, et al. The oncological safety of laparoscopic total mesorectal excision with sphincter preservation for rectal carcinoma. Surgical endoscopy 2005;19:892–6 [DOI] [PubMed] [Google Scholar]
- 15.Zhou ZG, Hu M, Li Y, et al. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surgical endoscopy 2004;18:1211–5 [DOI] [PubMed] [Google Scholar]
- 16.Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P, Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg 2009;96:982–9 [DOI] [PubMed] [Google Scholar]
- 17.Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E, Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg 2009;250:54–61 [DOI] [PubMed] [Google Scholar]
- 18.Leroy J, Jamali F, Forbes L, et al. Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long-term outcomes. Surgical endoscopy 2004;18:281–9 [DOI] [PubMed] [Google Scholar]
- 19.Brannigan AE, De Buck S, Suetens P, Penninckx F, D'Hoore A, Intracorporeal rectal stapling following laparoscopic total mesorectal excision: overcoming a challenge. Surgical endoscopy 2006;20:952–5 [DOI] [PubMed] [Google Scholar]
- 20.Breukink SO, Grond AJ, Pierie JP, Hoff C, Wiggers T, Meijerink WJ, Laparoscopic vs open total mesorectal excision for rectal cancer: an evaluation of the mesorectum's macroscopic quality. Surgical endoscopy 2005;19:307–10 [DOI] [PubMed] [Google Scholar]
- 21.Tsang WW, Chung CC, Kwok SY, Li MK, Laparoscopic sphincter-preserving total mesorectal excision with colonic J-pouch reconstruction: five-year results. Ann Surg 2006;243:353–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng KH, Ng DC, Cheung HY, et al. Laparoscopic resection for rectal cancers: lessons learned from 579 cases. Ann Surg 2009;249:82–6 [DOI] [PubMed] [Google Scholar]
- 23.Liang JT, Lai HS, Lee PH, Laparoscopic pelvic autonomic nerve-preserving surgery for patients with lower rectal cancer after chemoradiation therapy. Ann Surg Oncol 2007;14:1285–7 [DOI] [PubMed] [Google Scholar]
- 24.Asoglu O, Matlim T, Karanlik H, et al. Impact of laparoscopic surgery on bladder and sexual function after total mesorectal excision for rectal cancer. Surgical endoscopy 2009;23:296–303 [DOI] [PubMed] [Google Scholar]
- 25.Laurent C, Leblanc F, Gineste C, Saric J, Rullier E, Laparoscopic approach in surgical treatment of rectal cancer. Br J Surg 2007;94:1555–61 [DOI] [PubMed] [Google Scholar]


