Abstract
Objective
To investigate the cardioprotective efficacy of remote ischaemic preconditioning (RIPC) in cardiac surgery.
Design
We have performed a systematic search of MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials to identify randomized controlled trials involving RIPC.
Setting
Randomized controlled trials of RIPC in open cardiac surgery patients.
Main outcome measures
Meta-analysis was performed with the primary outcome the standardized mean difference between intervention and control groups in 12 hour postoperative troponin concentration. Heterogeneity was examined by fixed effects meta-regression.
Results
Ten studies with a total of 693 participants were included in the meta-analysis. RIPC reduced troponin levels 12 hours after surgery compared with control. The fixed and random effects differences were 0.35 (95% CI 0.19 to 0.51) and 0.53 (95% CI 0.18-0.88) respectively. However, important heterogeneity was present. Fixed effects meta-regression partially accounted for heterogeneity based on whether studies had full blinding, comprising blinding of patients, surgeons, anaesthetists and investigators. Studies with incomplete or no blinding demonstrated a larger estimate of effect, 0.74 (95% CI 0.47 to 1.00) compared to those with full blinding, 0.13 (95% CI - 0.07 to 0.33).
Conclusions
Although our analysis suggests RIPC may result in cardiac protection during cardiac surgery, the effect was most marked in studies without full blinding, with a smaller and statistically non-significant effect in fully blinded studies. We propose that further double blind randomized controlled trials investigating the cardioprotective effects of RIPC in cardiac surgery are required to resolve the current clinical uncertainty.
Introduction
Remote ischaemic preconditioning (RIPC) describes the phenomenon by which brief periods of ischaemia in one tissue can protect other tissues from subsequent prolonged ischaemic insults. It is simple to perform by inflation of a blood pressure cuff on the upper or lower limb to cause transient limb ischaemia. A number of mechanisms for how this transient ischaemia provides protection to other tissues have been postulated. These primarily operate through humoral or neuronal pathways.1–3 The potential utility of this non-invasive and inexpensive technique during cardiac surgery was first demonstrated in 2006 when RIPC was found to reduce postoperative troponin concentrations in children undergoing open cardiac surgery.4
Other studies have found that RIPC reduces postoperative troponin levels in adult patients following coronary artery bypass graft (CABG) surgery5–8 and percutaneous coronary intervention.9 Such was the enthusiasm for RIPC, that in 2009 it was described as potentially the ‘best hope for myocardial protection in cardiac surgery’.10 Within a year following this statement, however, two comparatively large trials failed to demonstrate significant cardiac protection through the use of RIPC in CABG surgery.11,12
In this systematic review and meta-analysis we investigate the efficacy of RIPC in providing cardiac protection in open cardiac surgery and the factors that might influence any effect.
Methods
Search strategy
Studies investigating the effect of RIPC on postoperative troponin in cardiac surgery patients were identified from the following databases, search date 7th of April 2011: MEDLINE 1950 to present with daily update, MEDLINE pending, OLDMEDLINE, EMBASE and Cochrane Central Register of Controlled Trials via OVID. The following key words were combined by OR: condition$, precondition$, pre-condition$, ischaemi$, ischemi$. The resulting papers were then combined by AND with the keyword remote. Duplicates were removed and the reference lists of all relevant studies were examined.
Inclusion and exclusion criteria
Paper titles, abstracts and, if necessary, the full text were reviewed. For inclusion, studies had to be randomized controlled trials (RCTs) in humans undergoing open cardiac surgery. The participants had to be randomized to receive either RIPC (via the inflation of a blood pressure cuff on the upper or lower limb) prior to expected ischaemic damage during surgery or no RIPC. The outcome measures had to include a measurement of troponin concentration, which was taken at any time following surgery. There was no restriction on language. Conference proceedings in which the authors were not contactable for further information were excluded.
Data extraction
Data was extracted from the text, tables or graphs. Contact was made with authors to request further information if needed. Wagner et al.7 and Rahman et al.12 presented published data as medians, and at our request, supplied means and standard deviation data. If information on blinding of patients, anaesthetists, surgeons and investigators was not completely reported, an attempt was made to contact the authors for clarification. Two authors (JP and MW) independently extracted the data with differences resolved by negotiation.
Statistical methods
The primary outcome variable was the troponin concentration 12 hours after surgery. Secondary outcome measures were postoperative troponin concentration at 6–8 hours and 24 hours, the area under the curve troponin to 72 hours after surgery, and mortality.
Meta-analysis used the inverse variance weighting method for standardized mean difference with bias correction for small samples.13 Homogeneity statistics and the I-squared statistic were calculated for each analysis.14 Publication bias was examined through funnel plots and formal tests of publication bias. The standardized mean difference was chosen as the metric for meta-analysis because of the variable concentration of expected troponin release with different types of cardiac surgery,15,16 as well as incomparability between differing troponin assays.17 The standardized mean difference can be interpreted as the number of standard deviations difference between active and control treatment. Fixed effects meta-regression18 was planned, if statistically significant heterogeneity was identified, based on the following study level co-variates: CABG versus other types of cardiac surgery, adult versus infant surgery, measurement of troponin I versus troponin T, and full blinding versus incomplete blinding. Full blinding was defined as blinding of patients, anaesthetists, surgeons and investigators. All other studies were defined as incompletely blinded, including those that did not report any blinding. For completeness we calculated random effects estimates although our intention was to explain, if possible, heterogeneity through the fixed effects meta-regression.
Results
Systematic review
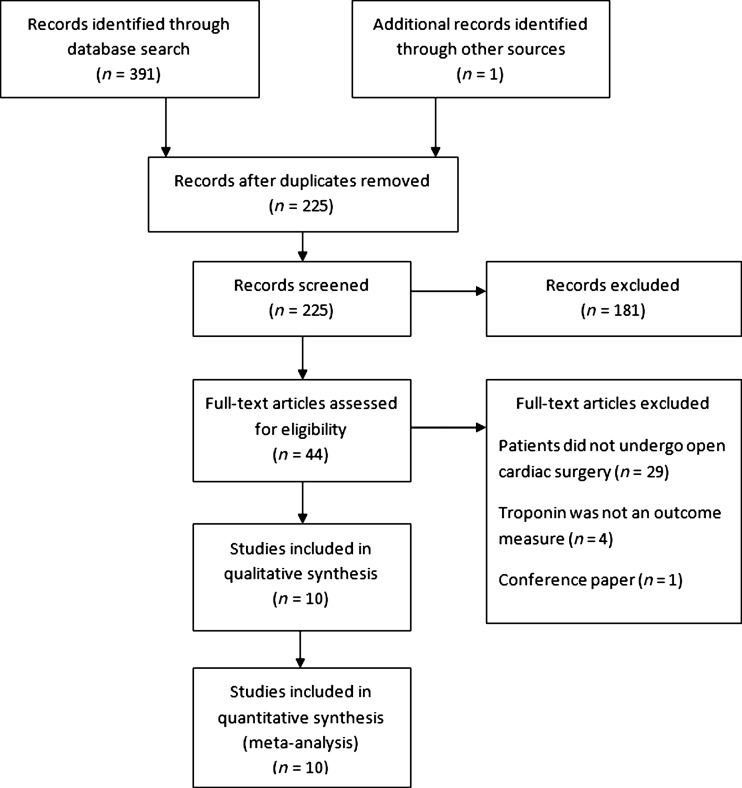
The OVID search identified 224 papers of which nine met the criteria for inclusion in the meta-analysis (Figure 1).4–8,11,12,19,20 An additional paper,21 not available via OVID, was sourced by examining the reference lists of relevant papers, and translated from Chinese to English. A total of 693 participants were included in the 10 papers (Table 1). All studies reported randomization of their participants, with five describing this to be computer generated6,7,11,12,19 and one via a random numbers table.5 Three studies described exclusion of participants following randomization.7,8,11 The majority of studies involved CABG surgery,5–8,11,12 of which two included concurrent valve surgery.6,7 Two studies investigated RIPC in valve replacement without CABG19,21 and two involved paediatric cardiac surgery.4,20 With the exception of Hong et al.,11 all studies involved cardiopulmonary bypass. Various forms of cardioprotection including cross clamp fibrillation, cold blood cardioplegia, and crystalloid cardioplegia were used in the included studies. Authors chose to time blood tests in relation to hours after cardiopulmonary bypass,4 cross clamp removal,19,21 reperfusion12 and end of surgery.5–8,11,20
Figure 1.
PRISMA statement for inclusion of studies for meta-analysis
Table 1.
Characteristics of the studies included in meta-analysis
| Paper (Reference) | n | Surgery description | RIPC | Blinding of surgeon, anaesthetists, investigator and patient | Troponin assay measured | Demonstration of a significant reduction in troponin at any time point following surgery | ||
|---|---|---|---|---|---|---|---|---|
| Limb | Number of cycles and their length | Timing in relation to anaesthetic induction | ||||||
| Venugopal (6) | 45 | CABG on cardiopulmonary bypass with or without concurrent valve | Upper | 3 × 5 minutes | Following anaesthetic induction | No | T | Yes |
| Hausenloy (5) | 57 | Upper | 3 × 5 minutes | Following anaesthetic induction | No | T | Yes | |
| Rahman (12) | 162 | Upper | 3 × 5 minutes | Following anaesthetic induction | Yes | T | No | |
| Wagner (7) | 66 | Upper | 3 × 5 minutes | 18 hours prior to surgery | No | I | Yes | |
| Thielmann (8) | 53 | Upper | 3 × 5 minutes | Following anaesthetic induction | No | I | Yes | |
| Hong (11) | 130 | CABG without cardiopulmonary bypass | Upper | 4 × 5 minutes | Following anaesthetic induction | Yes | I | No |
| Li (19) | 53 | Valve replacement with cardiopulmonary bypass | Lower | 3 × 4 minutes | Following anaesthetic induction | Yes | I | No |
| Liu (21) | 30 | Upper | 2× 3 minutes | Within 10 minutes of aortic clamping | No | I | Yes | |
| Cheung (4) | 37 | Infant surgery with cardiopulmonary bypass | Lower | 4 × 5 minutes | Completion of RIPC 5–10 minutes prior to initiation of cardiopulmonary bypass | Yes | I | Yes |
| Zhou (20) | 60 | Upper | 3 × 5 minutes | 24 hours and 1 hour prior to surgery | No | I | Yes | |
CABG: Coronary artery bypass grafting, RIPC: Remote ischaemic preconditioning
Study and patient characteristics hypothesized to affect the efficacy of RIPC and/or postoperative troponin are shown in the online supplement tables S1 and S2. Liu et al.21 reported a significant difference between treatment and control groups, with cardiopulmonary bypass time following aortic cross clamp release in their RIPC group to be 43 minutes compared to 66 minutes in their control group (P < 0.05). The mean concentrations of troponin varied by assay (as demonstrated in online supplement table S3).
The studies had varied exclusion criteria regarding cardiac ischaemia prior to surgery (demonstrated in online supplement S1). Six studies excluded patients who had unstable angina.5–8,11,12 Other exclusion criteria involved a history of myocardial infarction in the seven days,7,11 four weeks6 or 30 days prior to surgery.12 Thielmann et al.8 excluded all subjects with myocardial infarction in the previous two weeks or a baseline troponin I measurement of over 0.1 ng/mL, to avoid the inclusion of patients with unrecognized acute coronary syndrome. All of the other studies involving adult patients measured baseline troponin, and did not report levels as indicative of preoperative acute coronary syndrome.5–7,11,12,19,21
Four studies blinded the patient, investigators, surgeon and anaesthetist.4,11,12,19 Blinding can be performed by inflation of the blood pressure cuff next to the patient's limb on the operating table in the control group, visually obscured by surgical drapes. In the six studies classified as incompletely blinded, this was due to lack of blinding of the anaesthetist,8 anaesthetist and investigators,5,6 patients,7 parents of infants undergoing surgery20 and lack of reporting of any blinding.21
Primary outcome
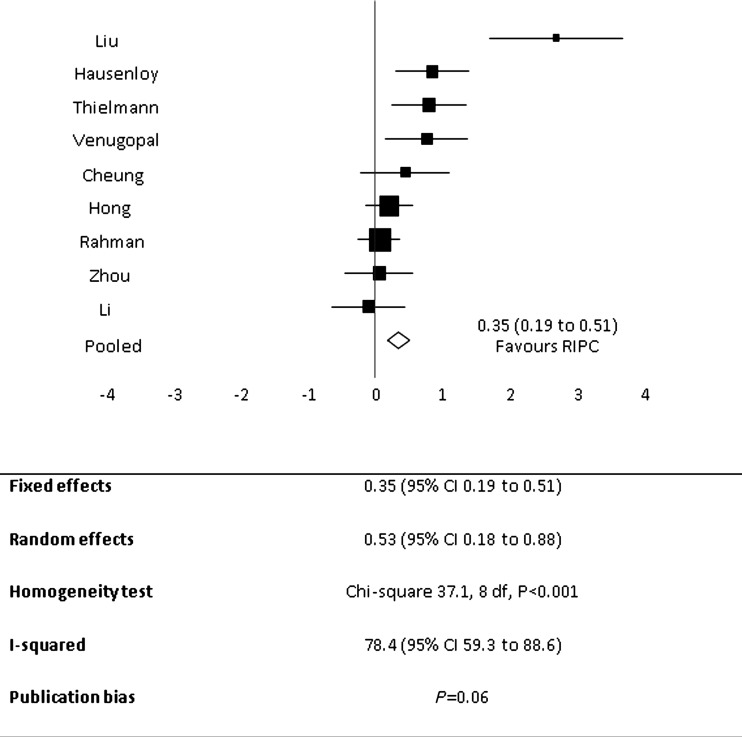
RIPC significantly reduced the concentration of postoperative troponin at 12 hours after surgery (Figure 2). Fixed effect and random effect standardized mean differences with RIPC were, respectively, 0.35 (95% CI 0.19 to 0.51) and 0.53 (95% CI 0.18 to 0.88). The homogeneity statistics were significant at P < 0.001 and the I-squared statistic was correspondingly large: 78.4 (95% CI 59.3 to 88.6).
Figure 2.
Forest plot for mean postoperative troponin at 12 hours following surgery demonstrating pooled analysis favours remote ischaemic preconditioning. Individual trial and pooled estimates with the size of the boxes on the forest plot inversely proportional to the size of the variance of the study estimates so that more precise studies have larger boxes
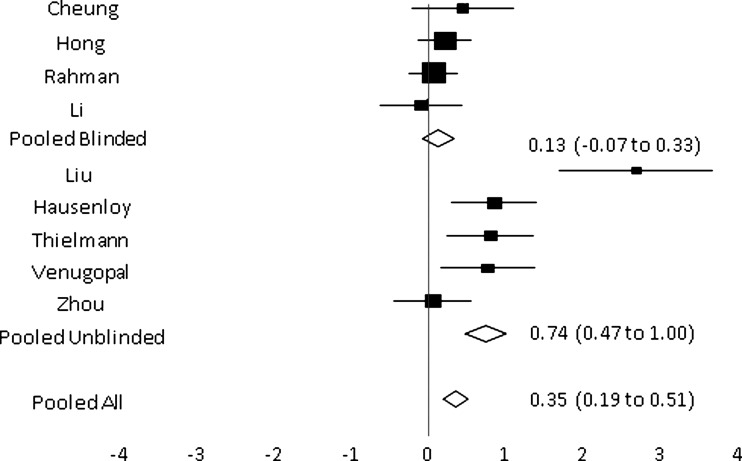
The fixed effects meta-regression found heterogeneity was mostly explained by the difference between incompletely blinded (n = 5) and fully blinded trials (n = 4), P < 0.001 (Figure 3). The point estimate for fully blinded trials was 0.13 (95% CI -0.07 to 0.33) and for partially blinded trials was 0.74 (95% CI 0.47 to 1.0). The blinded trials were homogenous (P = 0.58), however there was evidence of heterogeneity within the group of unblinded trials (P < 0.001), with the very large estimate of effect for the Liu et al. study21 contributing substantially to the heterogeneity. Heterogeneity was not explained by fixed effects meta-regression for CABG versus non-CABG surgery, troponin T versus I measurement, or child versus adult (Table 2). There was weak evidence of publication bias, P = 0.06, on formal testing, although inspection of the funnel plot suggested this was due to the outlying effect of the study by Liu et al.21
Figure 3.
Combined forest plot for mean postoperative troponin at 12 hours following surgery demonstrating pooled results from the grouping of trials as fully blinded and incompletely blinded. The size of the boxes on the forest plot is inversely proportional to the size of the variance of the study estimates so that more precise studies have larger boxes
Table 2.
Meta-regression to explain potential sources of heterogeneity
| Potential heterogeneity source | 6–8 hour | 12 hour | 24 hour | |
|---|---|---|---|---|
| Fully blinded versus non-fully blinded | Significance | Yes (P < 0.001) | Yes (P < 0.001) | Yes (P < 0.001) |
| Heterogeneity within groups | No (P = 0.37 and 0.2 for fully blinded and partially blinded trials respectively) | Yes (P = 0.001) | Yes (P < 0.001) | |
| CABG versus non-CABG | Significance | Yes (P = 0.037) | No (P = 0.91) | No (P = 0.29) |
| Heterogeneity within groups | Yes (P = 0.009) | N/A | N/A | |
| Troponin T versus I | Significance | Yes (P = 0.013) | No (P = 0.96) | No (P = 0.17) |
| Heterogeneity within groups | Yes (P = 0.019) | N/A | N/A | |
| Child versus adult | Significance | No (P = 0.37) | No (P = 0.23) | No (P = 0.38) |
| Heterogeneity within groups | N/A | N/A | N/A |
N/A: Not applicable
Secondary outcomes
RIPC significantly reduced the troponin at the other time points following surgery (for figures refer to S4, S5 and S6 of the online supplement). At 6–8 hours after surgery fixed effect and random effect standardized mean differences with RIPC were, respectively, 0.42 (95% CI 0.26 to 0.59) and 0.56 (95% CI 0.27 to 0.86). At 24 hours after surgery, fixed effect and random effect standardised mean differences with RIPC were, respectively, 0.29 (95% CI 0.13 to 0.45) and 0.59 (95% CI 0.16 to 1.01). For the 72 hour area under the curve, postoperative troponin fixed effect and random effect standardized mean differences with RIPC were, respectively, 0.45 (95% CI 0.24 to 0.67) and 0.49 (95% CI 0.23 to 0.75). Homogeneity statistics were significant for troponin at 6–8 hours (P = 0.003) and 24 hours (P < 0.001), but not for area under the curve to 72 hours (P = 0.21).
The fixed effects meta-regression for the 6–8 and 24 hour postoperative troponin concentrations are presented in Table 2. Whether studies were fully or incompletely blinded explained heterogeneity. In the 6–8 hour troponin measurements the resulting fully blinded and incompletely blinded groups had no heterogeneity within them.
Recorded mortality rates were low with only one death reported in one study.12 No deaths to 30 days were reported in three studies,8,12,19 no deaths were reported during the postoperative course in one study11 and no deaths within six months of discharge in another.20 Mortality was not reported in the other studies.
Other outcome measures
Zhou et al.20 found postoperative creatinine phosphokinase (CK), CK-MB and lactate dehydrogenase (LDH) to be statistically significantly reduced with RIPC. Liu et al.21 also reported a significant reduction in postoperative CK-MB with RIPC. There are two studies of RIPC in open cardiac surgery that did not select postoperative troponin concentration as an outcome measure. They were therefore ineligible for meta-analysis. The first study involved eight patients undergoing CABG surgery with cardiopulmonary bypass and reported that postoperative CK and CK-MB were not significantly different between control and treatment groups.22 It was found that LDH was significantly increased in patients with RIPC, which the authors suggested could be due to protection by increased anaerobic glycolysis. The second study, involving 100 patients, demonstrated that CK-MB was significantly reduced by RIPC at 8, 16, 24 and 48 hours following CABG surgery with cardiopulmonary bypass.23
Inotrope support was also used as an outcome measure in some of the studies. It was found to be statistically significantly reduced following RIPC in the two infant studies.4,20 It was also investigated in the studies by Theilmann,8 Rahman12 and Li et al.,19 in which no significant differences between the control and treatment groups were reported.
The impact of RIPC on postoperative renal function has also been investigated. Theilmann et al.8 found RIPC patients to have lower postoperative peak creatinine, however there was no significant difference detected in individual measurements taken at various points over the 72 hours following surgery. Rahman et al.12 were unable to detect any impact on creatinine, dialysis requirements, urine albumin-creatinine ratio or alpha microglobulin levels.
In infants, RIPC was demonstrated to have an effect on airway resistance,4 lung compliance and respiratory index.20 Rahman et al.12 found no difference in partial pressure of oxygen to fraction of inspired oxygen ratio following RIPC in adults.
Key excluded studies of interest and unpublished data
Zhou et al.20 reported an analysis of unpublished data of RIPC in adult patients undergoing valve replacement surgery in which ‘heart protective effects’ were not detected. A conference proceeding from 2009 reported an RCT of RIPC involving 40 patients undergoing elective CABG.24 Troponin T was found to be significantly reduced at 6, 24 and 48 hour following surgery with RIPC. Standard deviations or other measures of variability were not reported and we were unable to contact the authors.
Discussion
Main findings
This systematic review and meta-analysis has shown that RIPC significantly decreased postoperative troponin concentration following open cardiac surgery. However, there was statistical heterogeneity between studies which was partly explained by the degree of blinding. Blinded studies demonstrated a smaller effects size and no statistically significant effect of RIPC on troponin concentrations. As a result, uncertainty persists regarding the presence or magnitude of any protective effect of RIPC in cardiac surgery.
The use of troponin as an outcome measure
The majority of RIPC studies in cardiac surgery have used postoperative troponin as their primary outcome measure.5–8,11,12,19,21 In support of this approach, the magnitude of troponin release following cardiac surgery has been associated with an elevated risk of mortality at 30 days and six months following CABG surgery.16,25 The magnitudes of troponin release following valvular16 and paediatric surgery26 have also been independently associated with mortality. In addition, raised troponin has been associated with increased major adverse cardiac events (MACE).27 However, the minimum clinically important increase in troponin after cardiac surgery has not been clearly established, particularly as troponin concentrations following cardiac surgery vary with both the type of surgery15,16 and troponin assay used.17 This creates difficulty in interpreting how the magnitude of the reduction in troponin observed in our study might influence other clinically important outcomes such as morbidity and mortality.
Factors influencing outcomes
There was statistical evidence of heterogeneity in the analysis of troponin measurements at 6–8, 12 and 24 hours after surgery. Blinding in the studies was the only factor we identified that at least partly explained heterogeneity at 6–8, 12 and 24 hours following surgery. This is consistent with previous work demonstrating that in RCTs blinding results in smaller estimates of treatment effect.28 It is unknown how blinding could influence an outcome based on an automated measurement of troponin; however, it may represent a surrogate measure of other aspects of study quality. For example, unblinded studies may have had other systematic attributes associated with clinical care, which inadvertently favoured the treatment group.
We examined the study level co-variates of adult versus child surgery, troponin I versus troponin T measurement and CABG versus non-CABG surgery, and were unable to demonstrate that heterogeneity was explained by these factors. A number of other factors that could confound the association between RIPC and outcome could not be analyzed in this meta-analysis due to lack of individual patient data. These include factors that may affect RIPC efficacy, such as age,29 hypertension,29 preoperative cardiac ischaemia,30 or medications such as glibenclamide31 or volatile anaesthetics.32 Factors affecting troponin, including the European System for Cardiac Operative Risk Evaluation Score (EUROSCORE),33 type of surgical procedure15,16 and cardiopulmonary bypass time 33 could also influence the trial results.
Outcome could also relate to RIPC methodology, including the number of cycles, length of cycles, application to the upper verses lower limb and the timing of RIPC prior to surgery. A number of studies did not document the time between RIPC and events where cardiac injury is likely, such as cardiopulmonary bypass or cross clamp removal. There is clinical evidence to suggest there are two potential windows for preconditioning, with an initial window of protection within hours and a second within days.34 This late effect was investigated by Wagner et al.7 who demonstrated a reduction in postoperative troponin concentration following administration of RIPC 18 hours prior to surgery.
Authors had variable definitions for when the timing of postoperative bloods were taken, ranging from hours after completion of cardiopulmonary bypass4 to the end of surgery.5–8,11,20 We have reported all of these measures as ‘time from surgery’ in this review, and acknowledge that variation in the time point at which they were taken from may have caused variability in results.
There was some evidence of publication bias in formal tests, suggesting that the meta-analysis may be biased in favour of RIPC because of publication of small positive studies. The formal test used for publication bias was a correlation test which was strongly influenced by the outlying study of Liu et al.21 and may have been unreliable in this setting. Simple inspection of the funnel plots did not suggest strong evidence of publication bias. Overall, however, the estimate of the effect of RIPC is likely to be biased upwards.
We limited the analyses to 6–8, 12, 24 hour measurements, and 72 hour area under the curve. This may have still had the effect of inflating the Type I error rate for individual analyses. If the individual patient data were available, a pooled analysis based on the area under the curve may give a more useful summary of the overall effect of the treatment as well as increase the statistical power to detect heterogeneity in this particular outcome variable.
Implications for future trials
Further RCTs are warranted. It is important that, where practical, double-blind study design is implemented in future trials of RIPC in cardiac surgery and other surgical or medical situations. In addition the potential influence of factors such as statin use, cardiopulmonary bypass time and timing of RIPC application should be considered in the design and analysis of future trials.
Conclusions
This systematic review and meta-analysis demonstrated a statistically significant reduction in 12 hour postoperative troponin concentration following RIPC in open cardiac surgery. However, there was heterogeneity partly explained by whether studies were blinded or not, with partially or non-blinded studies having a considerably higher estimate of effect, while fully blinded studies had a smaller estimate of effect which was not statistically significant. We propose that further double-blind RCTs investigating the cardioprotective effects of RIPC in cardiac surgery are required to resolve the current clinical uncertainty.
DECLARATIONS
Conflict of interest
None declared
Funding
The Capital and Coast District Health Board and Medical Research Institute of New Zealand Remote Ischaemic Preconditioning research project has been supported by funding from the Heart Foundation and Lotteries Commission in New Zealand and the Wellington Medical Research Foundation
Ethical approval
Ethical approval was not required for this study. It is a systematic review and meta-analysis only
Guarantor
Janine M Pilcher
Contributorship
Janine M Pilcher: Study development, data extraction, manuscript write up and review, guarantor of paper. Paul Young: Study development, manuscript write up and review. Mark Weatherall: Data extraction, statistical analysis, manuscript write up and review. Ishtiaq Rahman: Manuscript write up and review. Robert S Bonser: Manuscript write up and review. Richard W Beasley: Study development, supervision, manuscript write up and review
Acknowledgments
None
Reviewer
Mark Field and Caroline Hing
References
- 1.Hausenloy DJ, Yellon DM Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 2008;79:377–86 [DOI] [PubMed] [Google Scholar]
- 2.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury-a review. J Surg Res 2008;150:304–30 [DOI] [PubMed] [Google Scholar]
- 3.Walsh SR, Tang T, Sadat U, Dutka DP, Gaunt ME Cardioprotection by remote ischaemic preconditioning. Br J Anaesth 2007;99:611–16 [DOI] [PubMed] [Google Scholar]
- 4.Cheung MMH, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 2006;47:2277–82 [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575–79 [DOI] [PubMed] [Google Scholar]
- 6.Venugopal V, Hausenloy DJ, Ludman A, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart 2009;95:1567–71 [DOI] [PubMed] [Google Scholar]
- 7.Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg 2010;11:758–62 [DOI] [PubMed] [Google Scholar]
- 8.Thielmann M, Kottenberg E, Boengler K, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 2010;105:657–64 [DOI] [PubMed] [Google Scholar]
- 9.Hoole SP, Heck PM, Sharples L, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation 2009;119:820–27 [DOI] [PubMed] [Google Scholar]
- 10.Rahman I, Bonser RS Remote ischaemic preconditioning: the current best hope for improved myocardial protection in cardiac surgery? Heart 2009;95:1553–55 [DOI] [PubMed] [Google Scholar]
- 11.Hong DM, Min JJ, Kim JH, et al. The effect of remote ischaemic preconditioning on myocardial injury in patients undergoing off-pump coronary artery bypass graft surgery. Anaesth Intensive Care 2010;38:924–29 [DOI] [PubMed] [Google Scholar]
- 12.Rahman IA, Mascaro JG, Steeds RP, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation 2010;122:S53–59 [DOI] [PubMed] [Google Scholar]
- 13.Whitehead A Meta-analysis of Controlled Clinical Trials. England: J Wiley & Sons, 2002:341 [Google Scholar]
- 14.Higgins JP, Thompson SG Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58 [DOI] [PubMed] [Google Scholar]
- 15.Swaanenburg JC, Loef BG, Volmer M, et al. Creatine kinase MB, troponin I, and troponin T release patterns after coronary artery bypass grafting with or without cardiopulmonary bypass and after aortic and mitral valve surgery. Clin Chem 2001;47:584–87 [PubMed] [Google Scholar]
- 16.Adabag AS, Rector T, Mithani S, et al. Prognostic significance of elevated cardiac troponin I after heart surgery. Ann Thorac Surg 2007;83:1744–50 [DOI] [PubMed] [Google Scholar]
- 17.Panteghini M Standardization of cardiac troponin I measurements: the way forward? Clin Chem 2005;51:1594–97 [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Bushman B Integrating results through meta-analytic review using SAS software. United States of America: SAS Inc, 1999:400 [Google Scholar]
- 19.Li L, Luo W, Huang L, et al. Remote perconditioning reduces myocardial injury in adult valve replacement: a randomized controlled trial. J Surg Res 2010;164:e21–26 [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Zeng D, Chen R, et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol 2010;31:22–29 [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Yi S, Liao B, Deng M, Chen S, Wang F Clinical study of limb ischemic preconditioning on myocardial ischemia-reperfusion injury during open heart surgery. Chinese J Cardiovas Rehab Med 2007;16:347–9 [Google Scholar]
- 22.Gunaydin B, Cakici I, Soncul H, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res 2000;41:493–6 [DOI] [PubMed] [Google Scholar]
- 23.Ali N, Rizwi F, Iqbal A, Rashid A Induced remote ischemic pre-conditioning on ischemia-reperfusion injury in patients undergoing coronary artery bypass. J Coll Physicians Surg Pak 2010;20:427–31 [PubMed] [Google Scholar]
- 24.Gegouskov V, Pawlik E, Akhyari P, Kamiya H, Karck M, Lichtenberg A Effect of remote ischaemic preconditioning in the human heart during coronary artery bypass surgery. Paper presented at: Interactive Cardiovascular and Thoracic Surgery Conference: 23rd Annual Meeting of the European Association for Cardio-Thoracic Surgery; October 2009; Vienna, Austria
- 25.Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011;305:585–91 [DOI] [PubMed] [Google Scholar]
- 26.Mildh LH, Pettila V, Sairanen HI, Rautiainen PH Cardiac troponin T levels for risk stratification in pediatric open heart surgery. Ann Thorac Surg 2006;82:1643–48 [DOI] [PubMed] [Google Scholar]
- 27.Nesher N, Alghamdi AA, Singh SK, et al. Troponin after cardiac surgery: a predictor or a phenomenon? Ann Thorac Surg 2008;85:1348–54 [DOI] [PubMed] [Google Scholar]
- 28.Schulz KF, Chalmers I, Hayes RJ, Altman DG Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–12 [DOI] [PubMed] [Google Scholar]
- 29.Balakumar P, Singh H, Singh M, Anand-Srivastava MB The impairment of preconditioning-mediated cardioprotection in pathological conditions. Pharmacol Res 2009;60:18–23 [DOI] [PubMed] [Google Scholar]
- 30.Wang YY, Yin BL Pro-inflammatory cytokines may induce late preconditioning in unstable angina patients. Med Hypotheses 2006;67:1121–24 [DOI] [PubMed] [Google Scholar]
- 31.Loukogeorgakis SP, Williams R, Panagiotidou AT, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation 2007;116:1386–95 [DOI] [PubMed] [Google Scholar]
- 32.Heling D, Nickenig G, Skowasch D Letter by Heling et al. regarding article, ‘remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment?’ Circulation 2011;123:e424. [DOI] [PubMed] [Google Scholar]
- 33.Onorati F, De Feo M, Mastroroberto P, et al. Determinants and prognosis of myocardial damage after coronary artery bypass grafting. Ann Thorac Surg 2005;79:837–45 [DOI] [PubMed] [Google Scholar]
- 34.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 2005;46:450–6 [DOI] [PubMed] [Google Scholar]