Abstract
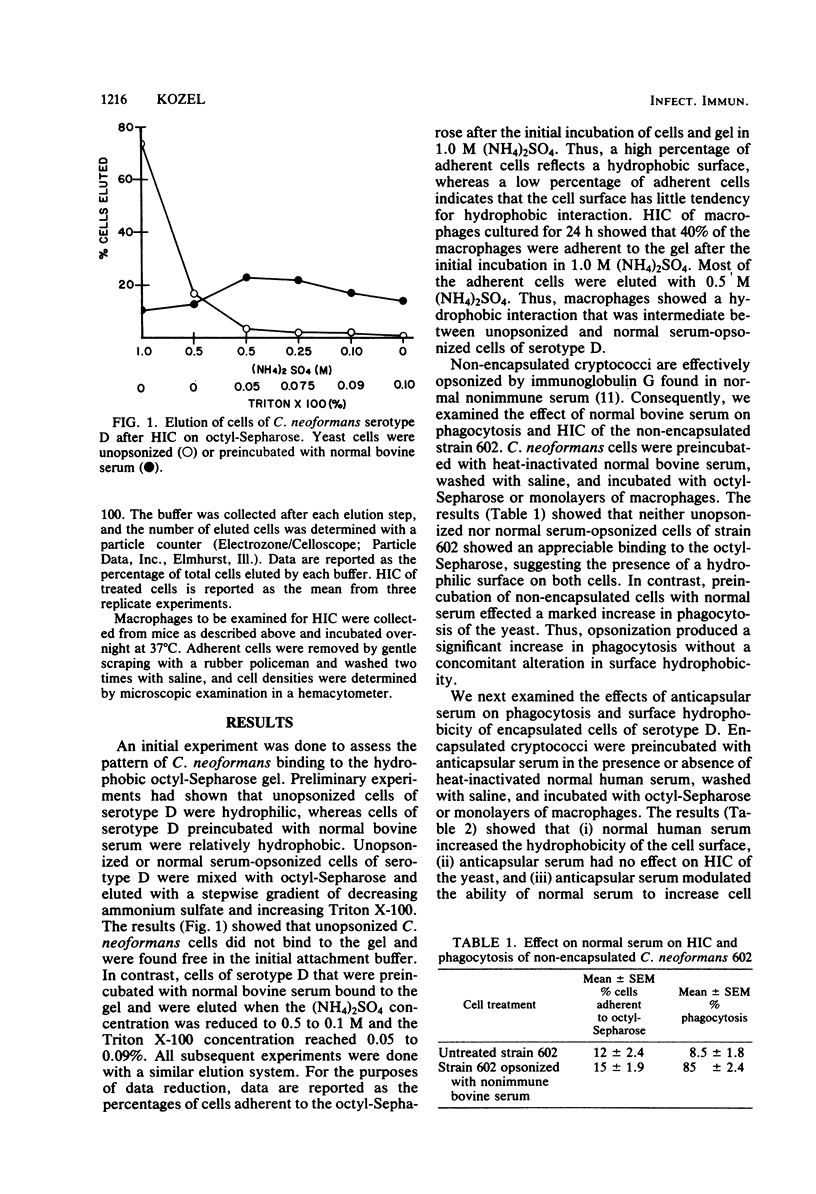
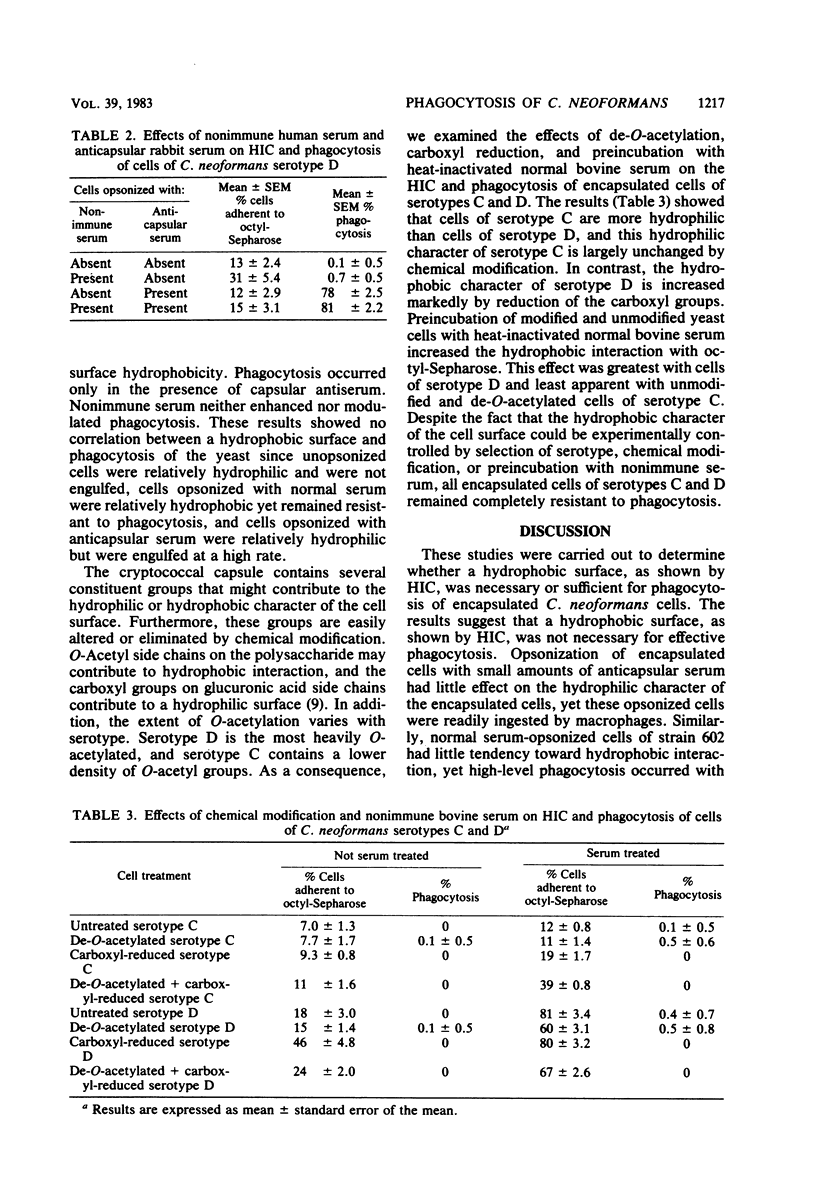
Cryptococcus neoformans is surrounded by a capsular polysaccharide that inhibits phagocytosis of the yeast by macrophages. This capsular polysaccharide also confers several physicochemical properties to the cell surface, including a negative surface charge and a hydrophilic surface. The present study was designed to determine whether a hydrophobic surface was necessary or sufficient for phagocytosis of C. neoformans cells. The hydrophobic nature of the cell surface was measured by hydrophobic interaction chromatography on octyl-Sepharose. Liability to phagocytosis was determined by use of mouse peritoneal macrophages. The surface properties of C. neoformans cells were modified by (i) preincubation of cryptococcal cells with nonimmune serum or immune anticapsular serum, (ii) chemical modification of the carboxyl and O-acetyl groups in the capsular polysaccharide, and (iii) use of various serotypes of C. neoformans with different degrees of O-acetyl and xylosyl substitution. The results showed that it was possible to experimentally vary the surface hydrophobic-hydrophilic characteristics of the cell surface; however, the antiphagocytic character of the capsule remained unchanged. The results further suggest that a hydrophobic surface was neither necessary nor sufficient for phagocytosis of C. neoformans cells by macrophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978 Sep;15(9):673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. The structure of the capsular polysaccharide from Cryptococcus neoformans serotype D. Carbohydr Res. 1979 Aug;73:183–192. doi: 10.1016/s0008-6215(00)85488-9. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Induction of humoral antibody response by soluble polysaccharide of Cryptococcus neoformans. Mycopathol Mycol Appl. 1974 Oct 15;54(1):21–30. doi: 10.1007/BF02055969. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Follette J. L. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect Immun. 1981 Mar;31(3):978–984. doi: 10.1128/iai.31.3.978-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., McGaw T. G. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979 Jul;25(1):255–261. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977 Apr;16(1):99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Stendahl O., Tagesson C., Edebo L., Johansson G. The tendency of smooth and rough Salmonella typhimurium bacteria and lipopolysaccharide to hydrophobic and ionic interaction, as studied in aqueous polymer two-phase systems. Acta Pathol Microbiol Scand B. 1977 Jun;85(3):212–218. doi: 10.1111/j.1699-0463.1977.tb01698.x. [DOI] [PubMed] [Google Scholar]
- McGaw T. G., Kozel T. R. Opsonization of Cryptococcus neoformans by human immunoglobulin G: masking of immunoglobulin G by cryptococcal polysaccharide. Infect Immun. 1979 Jul;25(1):262–267. doi: 10.1128/iai.25.1.262-267.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Normann B., Edebo L. Influence of O and K antigens on the surface properties of Escherichia coli in relation to phagocytosis. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):85–91. doi: 10.1111/j.1699-0463.1979.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Stjernström I., Magnusson K. E., Stendahl O., Tagesson C. Liability to hydrophobic and charge interaction of smooth Salmonella typhimurium 395 MS sensitized with anti-MS immunoglobulin G and complement. Infect Immun. 1977 Nov;18(2):261–265. doi: 10.1128/iai.18.2.261-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Wilkinson B. J., Peterson P. K., Quie P. G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsule: model for the antiphagocytic effect of bacterial cell surface polymers. Infect Immun. 1979 Feb;23(2):502–508. doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K. Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun. 1979 Dec;26(3):1159–1163. doi: 10.1128/iai.26.3.1159-1163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]



