Abstract
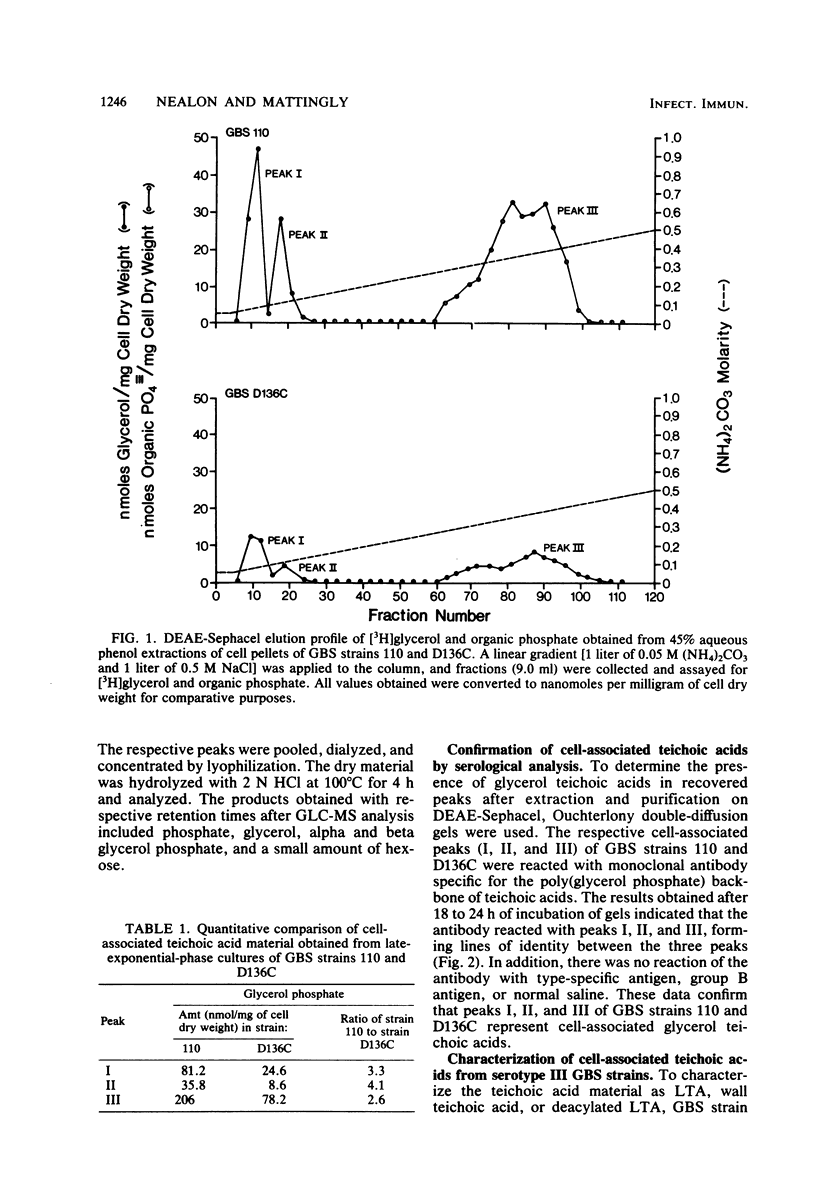
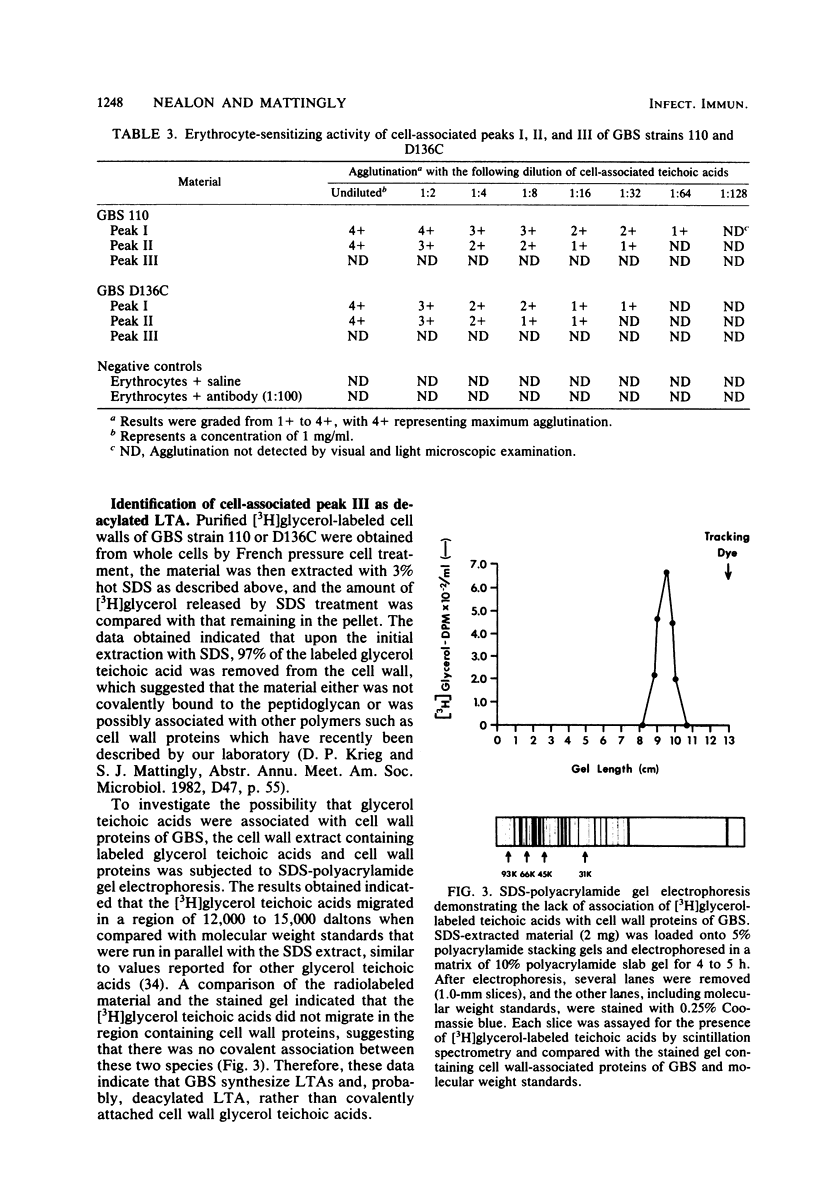
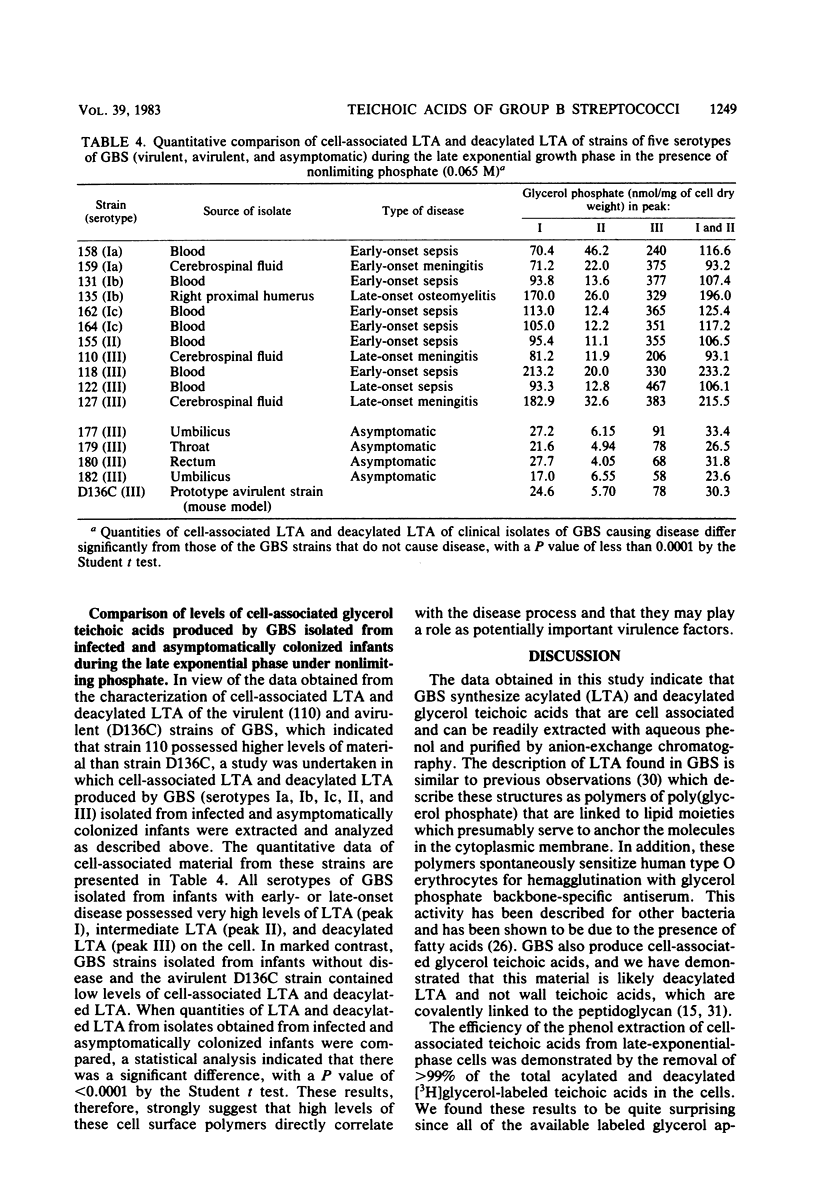
Cell-associated lipoteichoic acids (LTAs) from late-exponential-phase cultures (serotypes Ia, Ib, Ic, II, and III) of group B streptococci isolated from infected and asymptomatically colonized infants were quantitated and characterized by growing the organisms in a chemically defined medium containing [3H]glycerol and [14C]acetate. Cell pellets were extracted with 45% aqueous phenol and chloroform-methanol and subjected to DEAE-Sephacel anion-exchange chromatography. Elution profiles resolved three major peaks, I, II, and III, with glycerol and phosphate present in a 1:1 molar ratio in each peak, and results obtained by Ouchterlony immunodiffusion analysis confirmed the presence of poly(glycerol phosphate). Saponification indicated that [14C]acetate was incorporated into fatty acids of peaks I and II only, suggesting that these were cell-associated LTAs. Peak II was of small molecular weight (less than 10,000) and probably represented another species of LTA. Peaks I and II were further demonstrated to be LTA by their ability to sensitize human type O erythrocytes. Peak III lacked fatty acids and was shown to probably be deacylated LTA. Quantitation of cell-associated teichoic acid material produced by the group B streptococcal strains indicated that the clinical isolates from infants with early- or late-onset disease possessed significantly higher levels than did the asymptomatic (clinical isolates from infants without symptoms of disease) group B streptococcal strains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bab I. A., Sela M. N., Ginsburg I., Dishon T. Inflammatory lesions and bone resorption induced in the rat periodontium by lipoteichoic acid of Streptococcus mutans. Inflammation. 1979 Sep;3(4):345–358. doi: 10.1007/BF00913493. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F. Transmission of group B streptococci among parturient women and their neonates. J Pediatr. 1973 Dec;83(6):919–925. doi: 10.1016/s0022-3476(73)80524-4. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Simpson W. A., Evans J. D., Knox K. W., Ofek I., Wicken A. J. Erythrocyte binding properties of streptococcal lipoteichoic acids. Infect Immun. 1979 Mar;23(3):618–625. doi: 10.1128/iai.23.3.618-625.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigan V. M., Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei: D-alanyl-lipophilic compounds as intermediates. J Bacteriol. 1981 Apr;146(1):239–250. doi: 10.1128/jb.146.1.239-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox F. Prevention of group B streptococcal colonization with topically applied lipoteichoic acid in a maternal-newborn mouse model. Pediatr Res. 1982 Oct;16(10):816–819. doi: 10.1203/00006450-198210000-00003. [DOI] [PubMed] [Google Scholar]
- Doran T. I., Straus D. C., Mattingly S. J. Factors influencing release of type III antigens by group B streptococci. Infect Immun. 1981 Feb;31(2):615–623. doi: 10.1128/iai.31.2.615-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICKHOFF T. C., KLEIN J. O., DALY A. K., INGALL D., FINLAND M. NEONATAL SEPSIS AND OTHER INFECTIONS DUE TO GROUP B BETA-HEMOLYTIC STREPTOCOCCI. N Engl J Med. 1964 Dec 10;271:1221–1228. doi: 10.1056/NEJM196412102712401. [DOI] [PubMed] [Google Scholar]
- Ferrieri P., Cleary P. P., Seeds A. E. Epidemiology of group-B streptococcal carriage in pregnant women and newborn infants. J Med Microbiol. 1977 Feb;10(1):103–114. doi: 10.1099/00222615-10-1-103. [DOI] [PubMed] [Google Scholar]
- Finch R. G., French G. L., Phillips I. Group B streptococci in the female genital tract. Br Med J. 1976 May 22;1(6020):1245–1247. doi: 10.1136/bmj.1.6020.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Rösel P. The alanine ester substitution of lipoteichoic acid (LTA) in Staphylococcus aureus. FEBS Lett. 1980 Oct 6;119(2):224–226. doi: 10.1016/0014-5793(80)80257-2. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., TIPPER D. J., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. IV. THE TEICHOIC ACID-GLYCOPEPTIDE COMPLEX. Biochemistry. 1965 Mar;4:474–485. doi: 10.1021/bi00879a016. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gutiérrez Núez J. J., Harrington P. T. The role of bacterial surface factors in the pathogenesis of infective endocarditis. Bol Asoc Med P R. 1980 Dec;72(12):624–625. [PubMed] [Google Scholar]
- Huff E. Lipoteichoic acid, a major amphiphile of Gram-positive bacteria that is not readily extractable. J Bacteriol. 1982 Jan;149(1):399–402. doi: 10.1128/jb.149.1.399-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyssen F. J., de Boer W. R., Wouters J. T. Effects of carbon source and growth rate on cell wall composition of Bacillus subtilis subsp. niger. J Bacteriol. 1980 Oct;144(1):238–246. doi: 10.1128/jb.144.1.238-246.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ne'eman N., Ginsburg I. Red cell-sensitizing antigen of group A streptococci. II. Immunological and immunopathological properties. Isr J Med Sci. 1972 Nov;8(11):1807–1816. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Eyal F., Morrison J. C. Postnatal development of binding of streptococci and lipoteichoic acid by oral mucosal cells of humans. J Infect Dis. 1977 Feb;135(2):267–274. doi: 10.1093/infdis/135.2.267. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rølla G., Oppermann R. V., Bowen W. H., Ciardi J. E., Knox K. W. High amounts of lipoteichoic acid in sucrose-induced plaque in vivo. Caries Res. 1980;14(4):235–238. doi: 10.1159/000260459. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., SLADE H. D. THE CELLULAR LOCATION OF THE STREPTOCOCCAL GROUP D ANTIGEN. J Gen Microbiol. 1964 Dec;37:297–305. doi: 10.1099/00221287-37-3-297. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., GHUYSEN J. M. ON THE LINKAGE BETWEEN TEICHOIC ACID AND THE GLYCOPEPTIDE IN THE CELL WALL OF STAPHYLOCOCCUS AUREUS. Biochem Biophys Res Commun. 1963 Aug 14;12:418–424. doi: 10.1016/0006-291x(63)90117-7. [DOI] [PubMed] [Google Scholar]
- Waltersdorff R. L., Fiedel B. A., Jackson R. W. Induction of nephrocalcinosis in rabbit kidneys after long-term exposure to a streptococcal teichoic acid. Infect Immun. 1977 Sep;17(3):665–667. doi: 10.1128/iai.17.3.665-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981 Jun;45(2):211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]