Abstract
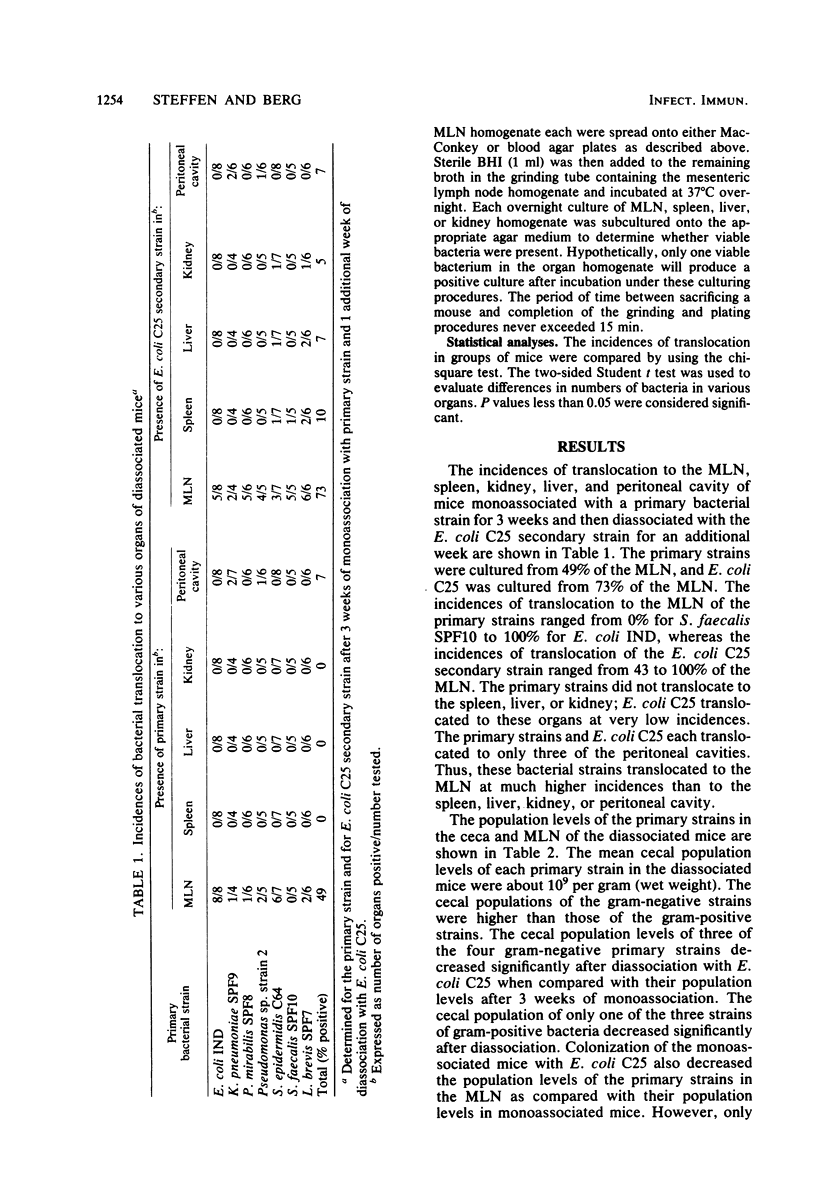
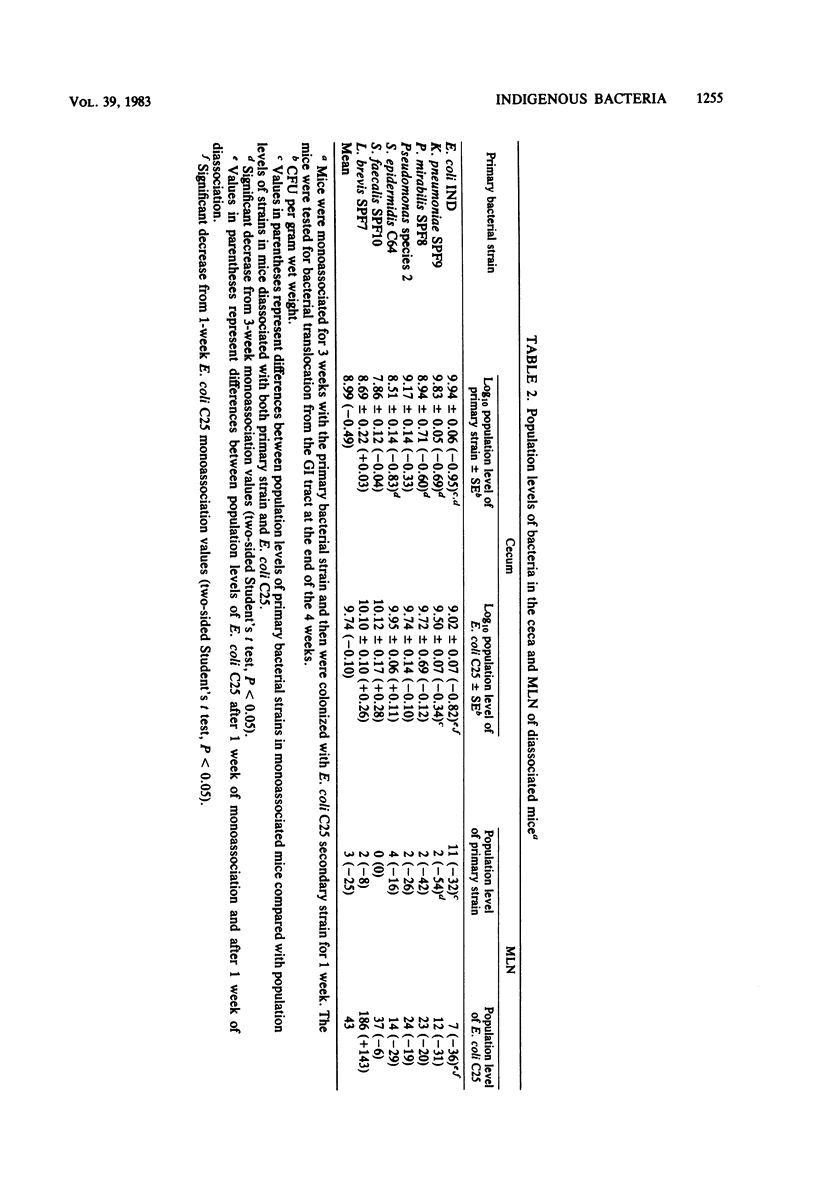
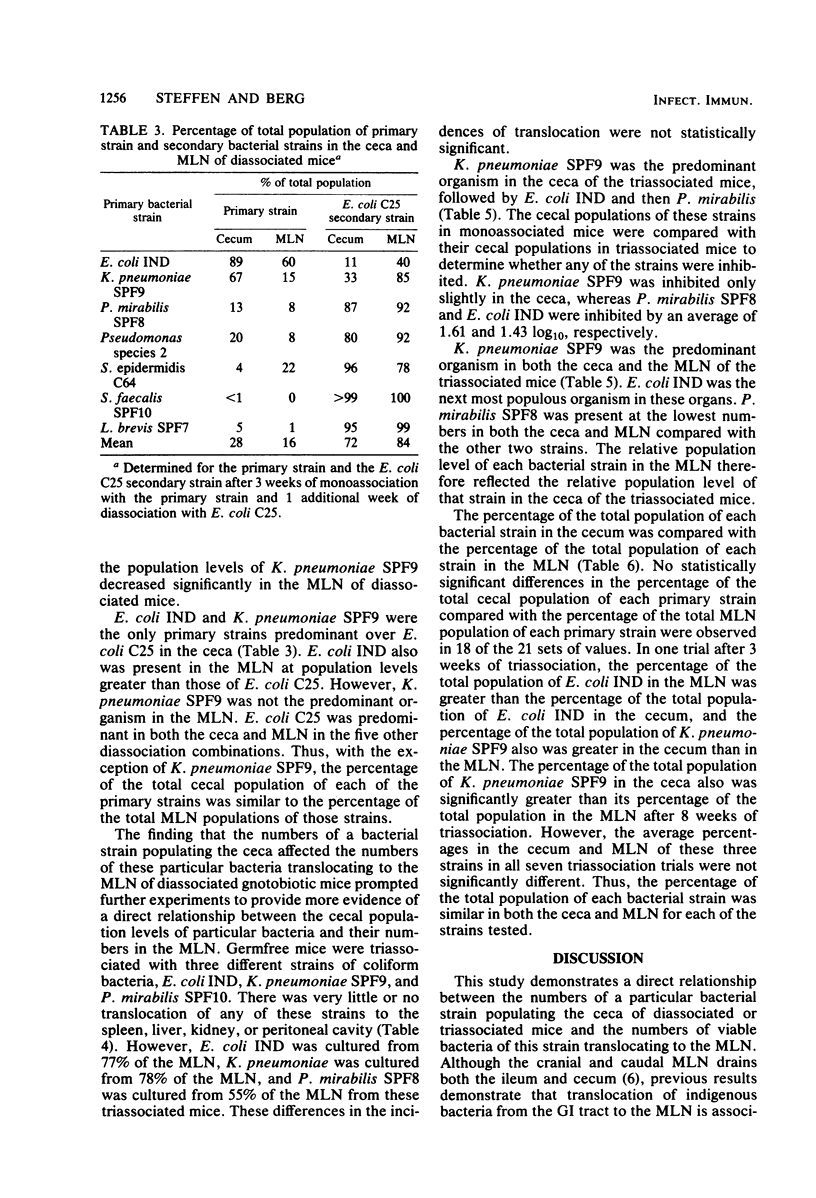
Translocation is defined as the passage of viable bacteria from the gastrointestinal tract to the mesenteric lymph nodes (MLN) and other organs. The extent of translocation of certain indigenous, oxygen-tolerant bacteria from the cecum to the MLN, spleen, liver, kidney, and peritoneal cavity were determined in diassociated or triassociated gnotobiotic mice. Minimal bacterial translocation occurred to the spleen, liver, kidney, or peritoneal cavity. However, most bacterial strains readily translocated to the MLN. The percentage of the total population of each bacterial strain in the ceca was compared with the percentage of the total population of that strain in the MLN. There was a direct relationship between the numbers of a particular bacterial strain populating the ceca of diassociated or triassociated mice and the numbers of viable bacteria of this strain present in the MLN. Thus, the cecal population level of a particular bacterial strain determined the numbers of viable bacteria of this strain translocating to the MLN. The translocation of these bacterial strains from the gastrointestinal tract is an important first step in the pathogenesis of infection caused by members of the normal intestinal microflora.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Inhibition of Escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobiotic or antibiotic-decontaminated mice. Infect Immun. 1980 Sep;29(3):1073–1081. doi: 10.1128/iai.29.3.1073-1081.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Owens W. E. Inhibition of translocation of viable Escherichia coli from the gastrointestinal tract of mice by bacterial antagonism. Infect Immun. 1979 Sep;25(3):820–827. doi: 10.1128/iai.25.3.820-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect Immun. 1981 Sep;33(3):854–861. doi: 10.1128/iai.33.3.854-861.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Stevens W. Gut-associated lymphoepithelial tissue: bidirectional transport of tracer by specialized epithelial cells associated with lymphoid follicles. J Reticuloendothel Soc. 1977 Apr;21(4):245–254. [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- FRETER R. In vivo and in vitro antagonism of intestinal bacteria against Shigellaflexneri. II. The inhibitory mechanism. J Infect Dis. 1962 Jan-Feb;110:38–46. doi: 10.1093/infdis/110.1.38. [DOI] [PubMed] [Google Scholar]
- Fuller R., Jayne-Williams D. J. Resistance of the fowl (Gallus domesticus) to invasion by its intestinal flora. II. Clearance of translocated intestinal bacteria. Res Vet Sci. 1970 Jul;11(4):368–374. [PubMed] [Google Scholar]
- KELLER R., ENGLEY F. B., Jr Fate of bacteriophage particles introduced into mice by various routes. Proc Soc Exp Biol Med. 1958 Jul;98(3):577–580. doi: 10.3181/00379727-98-24112. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F. Functional characteristics of Peyer's patch lymphoid cells. IV. Effect of antigen feeding on the frequency of antigen-specific B cells. J Immunol. 1977 Mar;118(3):992–997. [PubMed] [Google Scholar]
- Nielsen M. L., Justesen T., Asnaes S., Stage J. G., Nielsen O. V. Anaerobic and aerobic bacteriological study of the liver and biliary tract in healthy laboratory animals: investigations in rabbits, guinea pigs, mice, and pigs. Dan Med Bull. 1975 Apr;22(4):159–164. [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Owens W. E., Berg R. D. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980 Feb;27(2):461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWEINBURG F. B., SELIGMAN A. M., FINE J. Transmural migration of intestinal bacteria; a study based on the use of radioactive Escherichia coli. N Engl J Med. 1950 May 11;242(19):747–751. doi: 10.1056/NEJM195005112421903. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Tomfohrde K. M., Rhoden D. L., Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol. 1972 Sep;24(3):449–452. doi: 10.1128/am.24.3.449-452.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Volkheimer G. Passage of particles through the wall of the gastrointestinal tract. Environ Health Perspect. 1974 Dec;9:215–225. doi: 10.1289/ehp.749215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolochow H., Hildebrand G. J., Lamanna C. Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J Infect Dis. 1966 Oct;116(4):523–528. doi: 10.1093/infdis/116.4.523. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk-van der Wees Colonization resistance of the digestive tract and the spread of bacteria to the lymphatic organs in mice. J Hyg (Lond) 1972 Jun;70(2):335–342. doi: 10.1017/s0022172400022385. [DOI] [PMC free article] [PubMed] [Google Scholar]



